Abstract
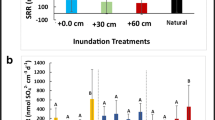
Sea level rise due to climate change will expose Hudson River tidal marshes to chronic shifts in salinity, thus altering habitat conditions and biogeochemical processes. Increased salt intrusion may affect macroinvertebrate and microbial communities that are important in the decomposition of a dominant, invasive plant species Phragmites australis. We hypothesized that increased salinity intrusion will negatively affect macroinvertebrate and microbial litter breakdown processes. Field and laboratory experiments were conducted to test the effect of salinity on Phragmites decomposition. Leaf packets were incubated among tidal wetlands along a salinity gradient and used to compare microbial respiration, fungal biomass, and mass loss. In addition, salinity tolerance of a freshwater isopod (Caecidotea sp.) and heterotrophic microbes were examined using laboratory bioassays. Salinity negatively affected isopod survivorship and microbial activity in controlled laboratory experiments; however, salinity did not significantly account for in situ variation in Phragmites mass loss, microbial respiration, and fungal biomass among wetlands. Future studies need to include litter from additional wetland species and consider alternative controls on decomposition (e.g., variation in temperature or inorganic nutrients) in order to best forecast the long-term impact of sea-level rise and salinity increases among tidal freshwater wetlands.




Similar content being viewed by others
References
Baldy V, Gessner MO, Chauvet E (1995) Bacteria, fungi and the breakdown of leaf litter in a large river. Oikos 74:93–102
Baumann K, Marschner P (2013) Effects of salinity on microbial tolerance to drying and rewetting. Biogeochemistry 112:71–80
Blasius BJ, Merritt RW (2002) Field and laboratory investigations of the effects of road salt (NaCl) on stream macroinvertebrate communities. Environmental Pollution 120:219–231
Brinson MM, Lugo AE, Brown S (1981) Primary productivity, decomposition and consumer activity in freshwater wetlands. Annual Review of Ecology and Systematics 12:123–161
Burdick DM, Konisky RA (2003) Determinants of expansion for Phragmites australis, common reed, in natural and impacted coastal marshes. Estuaries 26:407–416
Chambers RM, Osgood DT, Bart DJ, Montalto F (2003) Phragmites australis invasion and expansion in tidal wetlands: interactions among salinity, sulfide, and hydrology. Estuaries 26:398–406
Collins BM, Sobczak WV, Colburn EA (2007) Subsurface flowpaths in a forested headwater stream harbor a diverse macroinvertebrate community. Wetlands 27:319–325
Costanza R, d’Arge R, de Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’Neill RV, Paruelo J, Raskin RG, Sutton P, van den Belt M (1998) The value of ecosystem services: putting the issues in perspective. Ecological Economics 25:67–72
Findlay S, Howe K, Austin HK (1990) Comparison of detritus dynamics in two tidal freshwater wetlands. Ecology 71:288–295
Findlay SEG, Dye S, Kuehn KA (2002) Microbial growth and nitrogen retention in litter of Phragmites australis compared to Typha angustifolia. Wetlands 22:616–625
Gessner MO (2001) Mass loss, fungal colonisation and nutrient dynamics of Phragmites australis leaves during senescence and early aerial decay. Aquatic Botany 69:325–339
Gessner MO, Chauvet E (1994) Importance of stream microfungi in controlling breakdown rates of leaf litter. Ecology 75:1807–1817
Gessner MO, Newell SY (2002) Biomass, growth rate, and production of filamentous fungi in plant litter. In: C.J. Hurst, R.L. Crawford, G.R. Knudsen, M.J. Mclnerney, L.D. Stetzenbach (eds), Manual of Environmental Microbiology, 2nd edn. American Society for Microbiology, pp 390–408
Graça MAS (2001) The role of invertebrates on leaf litter decomposition in streams- a review. International Review of Hydrobiology 86:383–393
Hemminga MA, de Leeuw J, de Munck W, Koutstaal BP (1991) Decomposition in estuarine salt marshes: the effect of soil salinity and soil water content. Vegetatio 94:25–33
Hieber M, Gessner MO (2002) Contribution of stream detrivores, fungi, and bacteria to leaf breakdown based on biomass estimates. Ecology 83:1026–1038
Jordan TE, Whigham DF, Correll DL (1989) The role of litter in nutrient cycling in a brackish tidal marsh. Ecology 70:1906–1915
Komínková D, Kuehn KA, Büsing N, Steiner D, Gessner MO (2000) Microbial biomass, growth, and respiration associated with submerged litter of Phragmites australis decomposing in a littoral reed stand of a large lake. Aquatic Microbial Ecology 22:271–282
Larsen L, Moseman S, Santoro AE, Hopfensperger K, Burgin A (2010) A complex-systems approach to predicting effects of sea level rise and nitrogen loading on nitrogen cycling in coastal wetland ecosystems. In: Kemp PF (ed). EcoDAS VIII Proceedings. p 67–92
Limburg KE, Moran MA, McDowell WH (1986) The Hudson River ecosystem. Springer, New York, pp 6–39
Mendelssohn IA, Sorrell BK, Brix H, Schierup HH, Lorenzen B, Maltby E (1999) Controls on soil cellulose decomposition along a salinity gradient in a Phragmites australis wetland in Denmark. Aquatic Botany 64:381–398
Morris JT, Sundareshwar PV, Nietch CT, Kjerfve B, Cahoon DR (2002) Responses of coastal wetlands to rising sea level. Ecology 83:2869–2877
Muhammad S, Müller T, Joergensen RG (2006) Decomposition of pea and maize straw in Pakistani soils along a gradient in salinity. Biology and Fertility of Soils 43:93–101
Nicholls RJ, Cazenave A (2010) Sea-level rise and its impact on coastal zones. Science 328:1517–1520
Piscart C, Moreteau JC, Beisel JN (2005) Biodiversity and structure of macroinvertebrate communities along a small permanent salinity gradient (Meurthe River, France). Hydrobiologia 551:227–236
Quintino V, Sangiorgio F, Ricardo F, Mamede R, Pires A, Freitas R, Rodrigues AM, Basset A (2009) In situ experimental study of reed leaf decomposition along a full salinity gradient. Estuarine, Coastal and Shelf Science 85:497–506
Reice SR, Herbst G (1982) The role of salinity in decomposition of leaves of Phragmites australis in desert streams. Journal of Arid Environments 5:361–368
Rietz DN, Haynes RJ (2003) Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biology and Biochemistry 35:845–854
Roache MC, Bailey PC, Boon PI (2006) Effects of salinity on the decay of the freshwater macrophyte, Triglochin procerum. Aquatic Botany 84:45–52
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. PNAS 99:2445–2449
Sardinha M, Müller T, Schmeisky H, Joergensen RG (2003) Microbial performance in soils along a salinity gradient under acidic conditions. Applied Soil Ecology 23:237–244
Titus JG (1988) Sea level rise and wetland loss: an overview. Office of Policy Analysis U.S. Environmental Protection Agency 1:1–35
van Bruggen AHC, Semenov AM (2000) In search of biological indicators for soil health and disease suppression. Applied Soil Ecology 15:13–24
Van Ryckegem G, Gessner MO, Verbeken A (2007) Fungi on leaf blades of Phragmites australis in a brackish tidal marsh: diversity, succession, and leaf decomposition. Microbial Ecology 54:600–611
Vermeer M, Rahmstorf S (2009) Global sea level linked to global temperature. PNAS 106:21527–21532
Webster JR, Benfield EF (1986) Vascular plant breakdown in freshwater ecosystems. Annual Review of Ecology and Systematics 17:567–594
Wichern J, Wichern F, Joergensen RG (2006) Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma 137:100–108
Windham L (2001) Comparison of biomass production and decomposition between Phragmites australis (common reed) and Spartina patens (salt hay grass) in brackish tidal marshes of New Jersey, USA. Wetlands 21:179–188
Yozzo DJ, Osgood DT (2013) Invertebrate communities of low-salinity wetlands: overview and comparison between Phragmites and Typha marshes within the Hudson River Estuary. Estuaries and Coasts 36:575–584
Yozzo DJ, Anderson JL, Cianciola MM, Nieder WC, Miller DE, Ciparis S, McAvoy J (2005) Ecological profile of the Hudson River National Estuarine Research Reserve. National Oceanic and Atmospheric Administration, Annandale
Acknowledgments
We are grateful for the financial support from the Hudson River Foundation for Science and Environmental Research, the New York State Department for Environmental Conservation, and the Tibor T. Polgar Fellowship. Partial support for sampling logistics was provided by New York Sea Grant (R/CMC-11). We would like to thank David Fischer, Erica Morgan, Denise Schmidt, and Heather Malcom for their field and laboratory assistance, David Yozzo and Sarah Fernald for providing valuable comments, and Helena Andreyko for logistical support. Lastly, the final version of this manuscript has been much improved by the constructive comments from our reviewers, associate editors, and editor, Dr. Marinus L. Otte.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Connolly, C.T., Sobczak, W.V. & Findlay, S.E.G. Salinity Effects on Phragmites Decomposition Dynamics Among the Hudson River’s Freshwater Tidal Wetlands. Wetlands 34, 575–582 (2014). https://doi.org/10.1007/s13157-014-0526-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-014-0526-1