Abstract
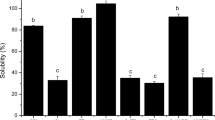
In this study, the effect of high hydrostatic pressure (HHP) on antigenicity, free sulfhydryl group (SH) content, hydrophobicity (Ho), fluorescence intensity and circular dichroism data of soybean β-conglycinin was studied. The antigenicity of soybean β-conglycinin was decreased significantly at pressures 200–400 MPa. The antigenicity inhibition rate of β-conglycinin declined from 92.72 to 55.15%, after being treated at 400 MPa for 15 min. Results indicated that free sulphydryl (SH) groups and surface Ho of β-conglycinin were significantly increased at pressures 200–400 MPa and 5–15 min, whereas these properties decreased at the treatments above 400 MPa and 15 min. The maximum fluorescence intensity was noticed at 400 MPa and 15 min. The circular dichroism data analysis revealed that the amount of β-turns and unordered structure significantly increased, while the content of α-helix1 and β-strand1 noticeably decreased. These results provide evidence that HHP-induced the structural modification of β-conglycinin and could alter the antigenicity of β-conglycinin.




Similar content being viewed by others
References
Ambrosi V, Polenta G, Gonzalez C, Ferrari G, Maresca P (2016) High hydrostatic pressure assisted enzymatic hydrolysis of whey proteins. Innov Food Sci Emerg 38:294–301
Beveridge T, Toma SJ, Nakai S (1974) Determination of sh- and ss-groups in some food proteins using ellman’s reagent. J Food Sci 39(1):49–51
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Cabanillas B, Maleki SJ, Rodríguez J, Cheng H, Teuber SS, Wallowitz ML, Crespo JF (2014) Allergenic properties and differential response of walnut subjected to processing treatments. Food Chem 157(1):141–147
Cabanillas B, Cuadrado C, Rodriguez J, Hart J, Burbano C, Crespo JF, Novak N (2015) Potential changes in the allergenicity of three forms of peanut after thermal processing. Food Chem 183:18–25
Elahi R, Mu TH (2017) High hydrostatic pressure (HHP)-induced structural modification of patatin and its antioxidant activities. Molecules 22(3):438
Galazka VB, Dickinson E, Ledward DA (2000) Influence of high pressure processing on protein solutions and emulsions. Curr Opin Colloid In 5(3–4):182–187
Hancock JD, Cao H, Kim IH, Li DF, Aumaitre A, Lee BD (2000) Effects of processing technologies and genetic modifications on nutritional value of full-fat soybeans in pigs. Asian Austral J Anim 13(4):356–375
He L, Han M, Qiao S, He P, Li D, Li N, Ma X (2015) Soybean antigen proteins and their intestinal sensitization activities. Curr Protein Pept Sc 16(7):613–621
Hu C, Chen H, Gao J, Luo C, Ma X, Tong P (2011) High-pressure microfluidisation-induced changes in the antigenicity and conformation of allergen ara h 2 purified from chinese peanut. J Sci Food Agric 91(7):1304–1309
Hu G, Zheng Y, Liu Z, Deng Y, Zhao Y (2016) Structure and ige-binding properties of α-casein treated by high hydrostatic pressure, UV-c, and far-IR radiations. Food Chem 204:46–55
Kajiyama N, Isobe S, Uemura K, Noguchi A (1995) Changes of soy protein under ultra-high hydraulic pressure. Int J Food Sci Tech 30(2):147–158
Koshiyama I, Fukushima D (1973) Comparison of conformations of 7s and 11s soybean globulins by optical rotatory dispersion and circular dichroism studies. Cereal Chem 50(1):114–121
Krishnan HB, Kim WS, Jang S, Kerley MS (2009) All three subunits of soybean beta conglycinin are potential food allergens. J Agric Food Chem 57:938–943
Li YQ, Chen ZX, Mo HZ (2007) Effects of pulsed electric fields on physicochemical properties of soybean protein isolates. LWT Food Sci Technol 40(7):1167–1175
Li H, Zhu K, Zhou H, Peng W (2012) Effects of high hydrostatic pressure treatment on allergenicity and structural properties of soybean protein isolate for infant formula. Food Chem 132(2):808–814
Li H, Zhu K, Zhou H, Peng W, Guo X (2016) Comparative study of four physical approaches about allergenicity of soybean protein isolate for infant formula. Food Agric Immunol 27(5):1–20
Ma X, He P, Sun P, Han P (2010) Lipoic acid: an immunomodulator that attenuates glycinin-induced anaphylactic reactions in a rat model. J Agric Food Chem 58(8):5086–5092
Meng X, Bai Y, Gao J, Xin L, Chen H (2017) Effects of high hydrostatic pressure on the structure and potential allergenicity of the major allergen bovine β-lactoglobulin. Food Chem 219:290–296
Messens W, Camp JV, Huyghebaert A (1997) The use of high pressure to modify the functionality of food proteins. Trends Food Sci Technol 8(4):107–112
Molina E, Papadopoulou A, Ledward DA (2001) Emulsifying properties of high pressure treated soy protein isolate and 7s and 11s globulins. Food Hydrocolloid 15(3):263–269
Nagano T, Hirotsuka M, Mori H, Kohyama K, Nishinari K (1992) Dynamic viscoelastic study on the gelation of 7s globulin from soybeans. J Agric Food Chem 40(6):941–944
Ogawa T, Bando N, Tsuji H, Nishikawa K, Kitamura K (1995) Alpha-subunit of beta-conglycinin, an allergenic protein recognized by IgE antibodies of soybean-sensitive patients with atopic dermatitis. Biosci Biotechnol Biochem 59(5):831–833
Omi Y, Kato T, Ishida K, Kato H, Matsuda T (1996) Pressure-induced release of basic 7s globulin from cotyledon dermal tissue of soybean seeds. J Agric Food Chem 44(12):3763–3767
Peñas E, Gomez R, Frias J, Baeza ML, Vidal-Valverde C (2011) High hydrostatic pressure effects on immunoreactivity and nutritional quality of soybean products. Food Chem 125(2):423–429
Prieto N, Burbano C, Iniesto E, Rodríguez J, Cabanillas B, Crespo JF, Cuadrado C (2014) A novel proteomic analysis of the modifications induced by high hydrostatic pressure on hazelnut water-soluble proteins. Foods 3(2):279–289
Puppo C, Chapleau N, Speroni F, De Lamballerie-Anton M, Michel F, Añón C, Anton M (2004) Physicochemical modifications of high-pressure-treated soybean protein isolates. J Agric Food Chem 52(6):1564–1571
Puppo MC, Beaumal V, Speroni F, Lamballerie MD, Añón MC, Anton M (2011) β-conglycinin and glycinin soybean protein emulsions treated by combined temperature-high-pressure treatment. Food Hydrocolloid 25(3):389–397
Sen M, Kopper R, Pons L, Abraham EC, Burks AW, Bannon GA (2002) Protein structure plays a critical role in peanut allergen stability and may determine immunodominant IgE-binding epitopes. J Immunol 169(2):882–887
Wang XS, Tang CH, Li BS, Yang XQ, Li L, Ma CY (2008) Effects of high-pressure treatment on some physicochemical and functional properties of soy protein isolates. Food Hydrocolloid 22(4):560–567
Wang JM, Yang XQ, Yin SW, Zhang Y, Tang CH, Li BS, Yuan DB, Guo J (2011) Structural rearrangement of ethanol-denatured soy proteins by high hydrostatic pressure treatment. J Agric Food Chem 59(13):7324
Whitmore L, Wallace BA (2004) Dichroweb, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res 32(Web Server issue):W668–W673
Wilson S, Blaschek K, Mejia E (2004) Allergenic proteins in soybean: processing and reduction of p34 allergenicity. Nutr Rev 63(2):47–58
Zhang H, Li L, Tatsumi E, Kotwal S (2003) Influence of high pressure on Conformational changes of soybean glycinin. Innov Food Sci Emerg 4(3):269–275
Zhang M, Zheng J, Ge K, Zhang H, Fang B, Jiang L, Guo H, Ding Q, Ren F (2014) Glycation of α-lactalbumin with different size saccharides: effect on protein structure and antigenicity. Int Dairy J 34(2):220–228
Zhou H, Wang C, Ye J, Chen H, Tao R, Cao F (2016) Effects of high hydrostatic pressure treatment on structural, allergenicity, and functional properties of proteins from ginkgo seeds. Innov Food Sci Emerg 34:187–195
Acknowledgements
This work was supported by National Natural Science Foundation of China (NSFC, 31671778) and Key Scientific Research Projects of Henan Colleges and Universities under Grant No. (16A550001).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
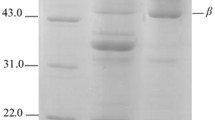
Fig. 1
SDS-PAGE and Western Blot of purified β-conglycinin (A: SDS-PAGE of purified β-conglycinin; B: Western Blot of purified β-conglycinin) (M: Marker; 1: purified β-conglycinin) (TIFF 1272 kb)
Rights and permissions
About this article
Cite this article
Xi, J., He, M. High hydrostatic pressure (HHP) effects on antigenicity and structural properties of soybean β-conglycinin. J Food Sci Technol 55, 630–637 (2018). https://doi.org/10.1007/s13197-017-2972-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-2972-2