Abstract
Easily available stone dust and alumina were acid activated, mixed in the ratio of 9:1 and studied for fluoride removal capacity. Batch and continuous studies were performed to test the efficiency of the adsorbents. Batch study revealed that the mixed adsorbents can remove up to 19.30 mg fluoride per gram of mixed adsorbent and is best removed at pH 6.5. Adsorption studies showed that the mechanism of adsorption is physical sorption. Flow rate plays a significant role in removing fluoride. Breakthrough study of a continuous operated unit tested at three different flow rates showed that decreasing efficiency took place at higher flow rate. The regeneration study shows that this adsorbent can be desorbed following a simple base–acid rinsing procedure.
Similar content being viewed by others
Introduction
Fluoride in drinking water is known for both beneficial and detrimental effect on health. The presence of fluoride in drinking water at low concentrations is considered essential in guarding against dental caries. However, excess fluoride in drinking water results in many bone diseases, including mottling of teeth and lesions of the endocrine glands, thyroid, liver and other organs (Tang et al. 2009). Excess fluoride is generally encountered in ground water and in rural population of developing countries which use that ground water as their drinking source. Taking the health effect into consideration, World Health Organization (WHO) has set a guideline value of 1.5 mg/L as the maximum permissible level of fluoride for drinking water (WHO 1993). Many countries have regions where water contains fluoride concentration more than the prescribed limit. Cases of endemic fluorosis are reported in parts of Assam state (situated in the north eastern region of India) where supplied water contains fluoride concentration as high as 23.5 mg/L (Chakraborti et al. 2000), The high fluoride levels in drinking water and their impacts on human health have prompted many researchers to study about the importance of defluoridation. In recent years, several methods have been developed to remove fluoride and improve the quality of drinking water. Various fluoride removal technologies based on the principle of adsorption (Ghorai and Pant 2004; Chauhan et al. 2007; Ganvir and Das 2011; Thakre et al. 2010; Wu et al. 2007; Tripathy et al. 2006; Srimurali et al. 1998), precipitation (Larsen and Pearce 1992), electrolysis (Mameri et al. 1998), ion exchange (Varamaa and Lehto 2003), membrane (Joshi et al. 1992), etc. are known. Coagulation methods are generally effective in defluoridation, but are unsuccessful in bringing down fluoride to the desired levels. The electrochemical techniques in general suffer due to high cost factor, both during installation and maintenance. Membrane separations can be performed under isothermal conditions and at low temperatures leading to low energy consumption. But it is relatively expensive to install and operate and prone to fouling and scaling. Also membrane degradation is one of the reasons of its disadvantages. Any particular process of water defluoridation suitable at a particular region may not work at some other region because of presence of different matrix composition of minerals in water (Meenakshi and Maheshwari 2006; Ayoob and Gupta 2007). The adsorption-based technique is one of the approaches for defluoridation of water. Report on the removal efficiency of excess of fluoride from drinking water as well as industrial effluent using quicklime as adsorbent shows positive results, which implies that adsorption technique is suitable to bring down the fluoride concentration in a particular water sample (Islam and Patel 2007)
Cost-effective remedial measures have to be considered for the prevention of fluorosis if fluoride concentration in a water source exceeds the permissible level. In this paper we report a new adsorbent that has been developed by activating the locally available stone dust (SD). The SD is generally generated from stone quarry and is usually considered as waste by the quarry masters because of its small size, low cost and for having fewer applications. The preliminary study on fluoride removal capacity of SD by batch method showed more than 0.91 mg F removed/g of SD. Depending on factors like pH, temperature and, of course, quality of the activated alumina (AA) we could achieve 9 mg F removed/g of AA. However, AA is comparatively expensive and at lower pH presence of aluminum ions in the treated water cannot be ruled out (Hao and Huang 1986). In order to minimize the use of large quantity of AA and to reduce the cost, the idea of using SD and AA adsorbents at a fixed ratio was developed to remove fluoride from water and incorporated in the proposed fluoride removal unit.
Materials and methods
Sodium fluoride, sodium chloride, sodium hydroxide, hydrochloric acid and glacial acetic acid of analytical reagent grade were procured from E. Merck (India) Limited and used as received. SD was procured from a local stone quarry sieved between 150 and 500 μm and alumina was obtained from Loba Chemie Pvt. Ltd, India with size between 70 and 230 μm and weight per ml being 0.9 g (approx.). Doubly distilled water was used throughout for batch study experiments and for preparing solutions.
All the instruments used for the experimental purpose were calibrated as per recommended procedure. The determination of concentration of fluoride was done using ion analyzer (Consort C833) and the fluoride-selective combined electrode. TISAB II was used as a buffer for fluoride measurement. Before the reading was recorded, the solution to be measured was stirred well and allowed to attain thermal equilibrium as described elsewhere (Umlong and Ismail 2005). The pH of the solution was measured by Cyberscan pH 510 meter (Eutech instruments). Scanning Electron Microscope (JEOL 6390LV) was used to scan the surface images, Energy-Dispersive X-ray Analysis (EDAX) was used to analyze the elemental constituents of the adsorbents and Fourier Transform Infrared Spectroscopy (FTIR) and NICOLET Impact I-410 was used to scan the elements. Analyst 200 Atomic Absorption Spectrophotometer (Perkin Elmer) was used to analyze for some trace metal ions (As, Pb, Zn) present in ground water. Other water quality parameters (hardness, sulfate, chloride, Ca, Fe, Mg, etc.) were analyzed using standard methods (Eaton et al. 2005; Bureau of Indian Standards 1968).
Activation of adsorbents was done by washing with 0.1 N NaOH solution followed by water. It was then kept in 0.1 N HCl acid solution overnight and thereafter repeatedly rinsed with excess of water to remove free acid (Ghorai and Pant 2004). The treated adsorbents were dried in an oven at a temperature below 300°C for 4 h.
Batch method was followed to study the effect of dose on the removal capacity of mixed adsorbents. Concentration of fluoride in feed water was maintained at 10 mg/L. Isotherm studies were conducted by varying fluoride concentration (initial fluoride concentration is 7 mg/L) and keeping the sorbent dose constant. Known concentration of fluoride solution was taken and known weight of adsorbent material was added into it and kept in a shaker for 24 h to attain the equilibrium. The filtrate solution was then analyzed for residual fluoride concentration. pH was maintained below 7 (Ghorai and Pant 2004; Tripathy et al. 2006; Oguz 2005). The isotherm parameters were determined non linearly using the optimization procedure employed by the solver add-in with Microsoft’s spreadsheet program, Microsoft excels. The values of coefficients of determination, R2, were maximized by allowing the values of different parameters to change according to the optimization procedure. The isotherm parameters were also evaluated using linear fittings. All experiments were performed at 27 ± 0.1°C.
A simple household scale adsorption unit (Fig. 1) developed for the removal of fluoride consists of mainly four layers which are separated from each other by porous but removable trays: the gravel and coarse sand layer, SD:AA adsorbent layer and fine-sand layer.
The feed water was continuously allowed to pass uniformly through the sorption media by means of an inverted perforated tray. (Some features of the unit are presented in Table 1)
In the continuous investigation, the flow rate was maintained at ~4.28 L/h by passing feed water through the sorption unit and measuring the outflow volume of filtered water against a given time. Groundwater was directly used for the continuous flow experiments. Contamination was done by spiking known concentration of fluoride solution and maintaining the fluoride concentration at 10 mg/L. In each experiment, the fluoride spiked water was passed through the sorption unit and the concentration of fluoride and few other constituents in the filtered as well as in the feed water were measured.
Results and discussion
Selection of component ratio
The removal of fluoride increased up to 82.2% for 9 g SD/100 mL of fluoride solution when SD was used as an adsorbent, as given in Fig. 2a. It can be seen from the figure that after a dosage of 9 g SD/100 mL of fluoride solution, there was no significant change in percentage removal of fluoride. This may be due to the overlapping of active sites at higher dosage and there is decrease in the effective surface area resulting in the conglomeration of exchanger particles (Islam and Patel 2007). Thus, 9 g of SD was selected as one of the component ratios. When AA was used as an adsorbent, the maximum fluoride removal was observed at 1 g AA/100 mL of fluoride solution as shown in Fig. 2b. This amount of AA is taken as the second component ratio. The ratio of SD: AA was thus fixed at 9:1 to achieve fluoride removal to the maximum extent.
Dosage effect
Generally, increasing the adsorbent’s dose increases the percentage removal of adsorbate which is attributed to the increase in surface area and hence more active sites are available. On varying the dose of SD:AA ratio the removal of fluoride was increased to 97.41% for 0.4 g SD:AA ratio/100 mL of fluoride solution as shown in Fig. 2c. On comparing the removal capacity of SD and AA separately, it is seen that AA is a better adsorbent than SD. But when the two adsorbents were mixed at a 9:1 ratio, the mixed adsorbents are at the upper hand for fluoride removal than either of the two adsorbents as shown in Fig. 3. This indicates synergism in adsorption due to mixing, which may be due to exposure of the active sites of AA in the bulk by the porosity of SD which allows solution of fluoride ions to have a direct contact with AA and vice versa, thereby enhancing adsorption capacity of each other.
Characterization studies
Mixed adsorbents before and after treatment with fluoride samples were examined by Scanning Electron Microscope (SEM) and FTIR. SEM photographs before and after treatment with fluoride are given in Fig. 4a and b, respectively. It is clear that the surface morphology of these two samples were different, which confirmed the fluoride sorption onto the SD:AA adsorbents. EDAX analysis of adsorbents before adsorption showed the presence of oxygen, silicon and carbon as principal elements and other minor elements include aluminum, iron and potassium. Percentage elemental analysis of the mixed adsorbents is given in Table 2.
FTIR spectra (Fig. 5a, b) showed peak at 3,430–3,423 cm−1 due to −OH stretching vibrations of the SiOH group, 1,081 and 1,038 cm−1 for Si–O band. Fluoride-containing compound absorbs strongly in the range 1,400–1,000 cm−1. The peak at 525 cm−1 may be due to Si–O–Al(IV) compounded vibrations. There was an overall decrease in the intensity of % transmittance in fluoride-treated SD:AA adsorbents which may be due to the exchange of −OH from its surface (Meenakshi et al. 2008)
Effect of other ions
Many ions such as sulphate, chloride, iodide and hydroxide are present in water and these anions compete with fluoride ions during adsorption. So the effect of these anions was taken into consideration. By fixing the fluoride concentration and varying the concentration of these anion, it was found that the removal efficiency of SD:AA decreases with increasing anions concentration. This trend is also reported in the literature (Tripathy and Raichur 2008). Further, in the present study effects of individual anion was also investigated to ascertain their interference on removal of fluoride. It has been observed that hydroxide ions of low concentration (8 mg/L) have less effect on the removal of fluoride. But as concentration of hydroxide ions is increased (>10 mg/L) the competition with fluoride toward the active site increases thereby decreasing the sorption capacity of fluoride. The effect of anions other than hydroxide (Fig. 6) showed less interference to fluoride sorption on SD:AA and the trend follows hydroxide > iodide > chloride > sulphate ions.
Adsorption studies
Mathematically, adsorption equations can either be used in their original forms (generally non-linear) or can be converted to a linear form and then the coefficient of determination is calculated. But recent reports have indicated the growing discrepancy (between the predictions and experimental data) and disability of the linear method. This variation in error distribution has been attributed to different axial settings which transform the dependent variables to different axial positions. However, the expanding of the non-linear isotherms represents potentially viable and powerful tools which are free from such distribution errors leading to the superior improvement in the area of adsorption study (Parimal et al. 2010; Hadi et al. 2010; Kumar and Sivanesan 2005; Foo and Hameed 2010).
Langmuir isotherm
The adsorption isotherms are generally used to describe how adsorbate interacts with adsorbent at equilibrium and therefore it is critical in optimizing the use of adsorbents. The Langmuir adsorption isotherm (Langmuir 1918) is given by
where qe is the equilibrium quantity adsorbed (mg/g), qm is the maximum capacity of monomer adsorption (mg/g), Ce the equilibrium concentration (mg/L) and b the adsorption equilibrium constant (L/mg) related to the energy of adsorption. Figure 7 shows the experimental data and the non-linear form of the Langmuir model. The non-linear parameters of the Langmuir model qm and b were listed in Table 3 and also given in the table the values of the parameters calculated using linear fittings (in the parentheses).
To predict the adsorption efficiency of the adsorption process the essential characteristics of the Langmuir isotherm can be determined in terms of dimensionless equilibrium parameter or Langmuir isotherm constant parameter (RL) which can be related to b by the following equation (Hall et al. 1966):
where C0 is the initial fluoride ion concentration (mg/L). The value of RL indicates the shape of the isotherms. If the value 0 < RL 1, then the Langmuir isotherm is favorable, if RL =0, it is irreversible, if RL = 1, it is linear and if RL >1, the isotherm is unfavorable. RL at initial concentration was found to be 0.0577 and for other concentrations are given in Table 3.
Freundlich Isotherm
The Freundlich isotherm (Freundlich 1906) is employed to model the multilayer adsorption based on sorption onto heterogeneous surface and is given by
where Kf (mg1−1/n L1/n g−1) is Freundlich constant indicative of the relative adsorption capacity of the adsorbent and n is the empirical parameter representing adsorption intensity of the adsorbent. The values of Kf and n calculated non-linearly are given in Table 3.
The value of n lying between 1 and 10 also indicates the valid conditions for Freundlich adsorption isotherms as well (Treybal 1981). A plot of the experimental data and non-linear form was shown in Fig. 7.
Dubinin–Radushkevich isotherms
The two models discussed above, though widely used, do not give any idea about the adsorption mechanism. However, a Dubinin–Rudushkevich isotherm is applied to distinguish between the physical and chemical adsorption mechanism. A Dubinin–Radushkevich isotherm is generally expressed as (Dubinin and Radushkevich 1947; Radushkevich 1949)
where Qm is the theoretical maximum capacity (mg g−1), ε is the Polanyi potential, K (mol2 kJ−2) is a constant related to the mean free energy of adsorption and ε is defined as
where R is universal gas constant and T is the absolute temperature. Figure 7 shows the experimental data and the non-linear form of the Dubinin–Radushkevich model and the values of K and Qm obtained non linearly are given in Table 3. The mean free energy of adsorption (E) defined as the free energy change when one mol of ion is transferred to the surface of the solid from infinity in solution can be calculated from the following equation (Afzal et al. 1993):
From the calculated value of E one can predict whether the reaction is chemical sorption or physical sorption. If E = 8−16 kJ mol−1 then the adsorption is due to chemisorption, but if E < 8 kJ mol−1, then it is due to physical sorption. The value of E calculated from Eq. 6 was found to be 3.19 kJ mol−1.
The root mean square error (RMSE) of the fitting for Langmuir model was found to be comparatively lesser than that for the Dubinin–Radushkevich model which in turn was lesser than for the Freundlich model (Table 3). Non-linear Chi-square (χ2) analysis also showed that fitting of adsorption data to Langmuir model was better than to the Freundlich adsorption model, but the latter was comparable to Dubinin–Rudeshkevich model.
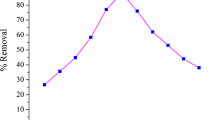
Effect of pH
pH is one of the main factors that control the adsorption of fluoride on the adsorbent in aqueous solution. Figure 8 shows that the adsorption increases with increasing pH till a maximum of 92.88% at pH 6.5 thereafter decreases with further increase in pH. The maximum adsorption at acidic pH may be due to the formation of weak hydrofluoric acid or combined effect of both chemical and electrostatic interaction between the oxide surface and fluoride ion. The removal of fluoride in alkaline medium decreases sharply which may be due to strong competition from hydroxide ions on the active sites (Tripathy et al. 2006).
Effect of flow rate
Batch experiments gave the idea of maximum adsorption of the adsorbents at a given time independent of the flow rate. Continuous studies, on the other hand, are dependent on the flow rate. Reardon and Wang (2000) evaluated the effect of flow rate on effluent levels in order to determine the minimum residence time to attain the maximum contaminant level limit for fluoride using limestone. The effect of flow rate on the removal of fluoride was studied at 4.28, 6 and 9.09 L/h separately. The adsorption of fluoride was found to increase with decreasing flow rate from 9.09 to 4.28 L/h, respectively, as shown in Fig. 9. This may be due to the fact that the availability of reaction sites which is able to capture more ions around or inside the cells at a lower flow rate of 4.28 L/h. In the next stage of the process at the same flow rate, the uptake became less effective which may be due to the gradual occupancy of the active sites. But when flow rate was further increased to 9.09 L/h, adsorbed ion concentration decreased sharply. This may be due to the fact that the residence time of the solute is not enough for adsorption equilibrium to be reached at that flow rate; the fluoride ions leaves the sites before the equilibrium occurs (Ghorai and Pant 2005). When compare the qm value calculated from batch process with qm value calculated from continuous process, it was found that the value obtained from batch process was higher (4.009 mg g−1) than that of continuous process (0.47 mg g−1). The reason may that in batch process the adsorption equilibrium is already reached at a given contact time but not in the case of continuous flow which experienced a minimum contact time.
Regeneration and reuse
To be a cost effective and user-friendly process, the adsorbent under study is regenerated so as to be reused for further adsorption. The fluoride-adsorbed SD:AA can be easily desorbed using 0.1 M NaOH and re-activated with 0.1 M HCl. The experimental data on the regeneration study is given in Table 4. It was observed that the efficiency of the regenerated adsorbent showed that there is a decrease in the removal efficiency at the end of third cycle of regenerations.
Conclusions
The present investigations on fluoride adsorption using SD and AA at a fixed ratio provide information in terms of adsorbent dose, adsorption mechanism, effect of pH, effect of co-ions and flow rate. Fluoride removal capacity of the SD:AA adsorbents were observed up to 97.41% with a minimum dosage of 0.4 g SD:AA per 100 mL of fluoride solution. The equilibrium data were fitted to Langmuir, Freundlich and Dubinin–Radushkevich adsorption models. Langmuir model was best fitted followed by Dubinin–Radushkevich and Freundlich models. The mechanism of adsorption process was found to be physical adsorption. From continuous study, lower flow rate acted as a controlling factor for smooth and steady removal of fluoride. Desorption study shows that it can be easily regenerated simply by base–acid rinsing followed by drying the mixture at 300°C and after the third cycle, the removal capacity is also decreased. This low-cost material can be employed as an adsorbent for fluoride removal from drinking water, in particular in domestic systems where fluoride-related problems exist.
References
Afzal M, Hasany SM, Ahmad H, Mahmood F (1993) Adsorption studies of cerium on lead oxide from aqueous solution. J Radioanal Nucl Chem 170(2):309–319
Ayoob S, Gupta AK (2007) Sorptive response profile of an adsorbent in the defluoridation of drinking water. Chem Eng J 133:273–281
Bureau of Indian Standards (1968) Methods of sampling and test (physical and chemical) for water used in industry. Manak Bhawan, 9 Bahadur Shah Zafar Marg, N. Delhi
Chakraborti D, Chanda CR, Samanta G, Chowdhury UK, Mukherjee SK, Pal AB, Sharma B, Mahanta KJ, Ahmed HA, Singh B (2000) Fluorosis in Assam, India. Curr Sci 78:1421–1423
Chauhan VS, Dwivedi PK, Iyengar L (2007) Investigations on activated alumina based domestic defluoridation units. J Hazard Mater B 139:103–107
Dubinin MM, Radushkevich LV (1947) The equation of the characteristic curve of activated charcoal. Dokl Akad Nauk SSSR 55:327–329
Eaton AD, Clesceri LS, Rice EW, Greenberg AE (2005) Standard methods for the examination of water and wastewater, 21st edn. APHA, Washington
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm system. Chem Eng J 156:2–10
Freundlich HZ (1906) Over the adsorption in solution. J Phys Chem 57A:385–470
Ganvir V, Das K (2011) Removal of fluoride from drinking water using aluminum hydroxide coated rice husk ash. J Hazard Mater 185(2–3):1287–1294
Ghorai S, Pant KK (2004) Investigations on the column performance of fluoride adsorption by activated alumina in a fixed bed. Chem Eng J 98:165–173
Ghorai S, Pant KK (2005) Equilibrium, kinetics and breakthrough studies for adsorption of fluoride on activated alumina. Sep Purif Technol 42:265–271
Hadi M, Samarghandi MR, Mckay G (2010) Equilibrium two-parameter isotherms of acid dyes sorption by activated carbon: study of residual errors. Chem Eng J 160:408–416
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore and solid diffusion kinetics in fixed bed adsorption under constant-pattern conditions. Ind Eng Chem Fund 5(2):212–223
Hao OJ, Huang CP (1986) Adsorption characteristics of fluoride on to hydrous alumina. J Environ Eng Div ASCE 112:1054–1069
Islam M, Patel RK (2007) Evaluation of removal efficiency of fluoride from aqueous solution using quick lime. J Hazard Mater 143:303–310
Joshi SV, Mehta SH, Rao AP, Rao AV (1992) Estimation of NaF using HPLC in reverse osmosis experiments. Water Treat 7:207–211
Kumar KV, Sivanesan S (2005) Prediction of optimum sorption isotherm: comparison of linear and nonlinear method. J Hazard Mater B126:198–201
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Larsen MJ, Pearce EI (1992) Partial defluoridation of drinking water using fluorapatite precipitation. Caries Res 26:22–28
Mameri N, Yeddou AR, Lounici H, Grib H, Belhocine D, Bariou B (1998) Defluoridation of septentrional Sahara water of N Africa by electro-coagulation process using bipolar aluminium electrodes. Water Res 32:1604–1610
Meenakshi S, Maheshwari RC (2006) Fluoride in drinking water and its removal. J Hazard Mater B 137:456–463
Meenakshi S, Sundaram CS, Sukumar R (2008) Enhanced fluoride sorption by mechano-chemically activated Kaolinites. J Hazard Mater 153:164–172
Oguz E (2005) Adsorption of fluoride on gas concrete materials. J Hazard Mater B 117:227–233
Parimal S, Prasad M, Bhaskar U (2010) Prediction of equilibrium sorption isotherm: comparison of linear and nonlinear methods. Ind Eng Chem Res 49(6):2882–2888
Radushkevich LV (1949) Potential Theory of sorption and structure of carbons. Zhurnal Fizicheskoi Khimii 23:1410–1420
Reardon EJ, Wang Y (2000) A limestone reactor for fluoride removal from wastewaters. Environ Sci Technol 34:3247–3253
Srimurali M, Pragathi A, Karthikeyan J (1998) A study on the removal of fluoride from drinking water by adsorption onto low cost material. Environ Pollut 99:285–289
Tang Y, Guan X, Su T, Gao N, Wang J (2009) Fluoride adsorption onto activated alumina: Modeling the effects of pH and some competing ions. Colloids Surf A: Physico-chem Eng Aspects 337:33–38
Thakre D, Jagdap S, Bansiwal A, Setwar NL, Rayalu S (2010) Synthesis of La incorporated chitosan beads for fluoride removal from water. J Fluorine Chem 131:373–377
Treybal RE (1981) Mass transfer operations, 3rd edn. McGraw Hill, New York
Tripathy SS, Raichur AM (2008) Abatement of fluoride from water using manganese dioxide coated activated alumina. J Hazard Mater 153:1043–1051
Tripathy SS, Bersillon J–L, Gopal K (2006) Removal of fluoride from drinking water by adsorption onto alum-impregnated alumina. Sep Purif Technol 50:310–317
Umlong IM, Ismail K (2005) Micellization of AOT in aqueous sodium chloride, sodium acetate, sodium propionate and sodium butyrate media: a case of two different concentration regions of counter ion binding. J Colloid Interface Sci 291:529–536
Varamaa K, Lehto J (2003) Removal of metals and anions from drinking water by ion exchange. Desalination 155:157–170
WHO (1993) Guidelines for drinking-water quality, Vol. 1 Recommendations, 2nd edn. WHO, Switzerland
Wu X, Zhang Y, Dou X, Yang M (2007) Fluoride removal performance of a novel Fe–Al–Ce trimetal oxides adsorbent. Chemosphere 69:1758–1764
Acknowledgments
The authors are thankful to DRDO HQs, New Delhi, for providing necessary financial assistance to carry out the research work. We are also thankful to Dr. Ashim Jyoti Thakur, Associate Professor, Tezpur University and Prof. K. Ismail of North Eastern Hill University, Shillong, for their technical help and valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Umlong, I.M., Das, B., Devi, R.R. et al. Defluoridation from aqueous solution using stone dust and activated alumina at a fixed ratio. Appl Water Sci 2, 29–36 (2012). https://doi.org/10.1007/s13201-011-0019-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-011-0019-9