Abstract
This is a conventional kind of monitoring study. The objective of the study was to assess and monitor the physicochemical parameters in wastewater at inlet and outlet of sewage treatment plant (STP) and also to study the effectiveness of the STPs. The average concentration of parameters at inlet sampling site pH, electrical conductivity, total dissolved solids, are 7.16, 2,169 μS/cm, 766.06 mg/l, and major ions bicarbonate, nitrate, sulphate, phosphate, chloride, sodium, potassium, magnesium and calcium values 515.88, 4.28, 82.85, 15.17, 7.01, 23.08, 29.34, 4.14 and 84.31 mg/l. While the average concentration of these parameters, after treatment shows following values 7.47, 2,161.43 (μS/cm), 695.81, 436.52, 1.25, 99.22, 12.69, 6.83, 23.18, 29.07, 4.40 and 82.65 mg/l, respectively. Further, to check the Na % and sodium absorption ratio at inlet and outlet which 27.89 %, 0.67 and 28.19 %, 0.68, respectively, for the suitability of the wastewater. Finally, the agglomerative hierarchical clustering techniques were used to study the similarity in the sewage treatment plants. The result suggests that there is considerable improvement in the wastewater quality after treatment except at the Pappankalan and Coronation Pillar, Timarpur.
Similar content being viewed by others
Introduction
Discharge of untreated sewage water in the water body is a common practice in many countries. This is the common cause for pollution of surface and groundwater because there is a large gap between generation and treatment of domestic wastewater in India. In general, the wastewater discharged from domestic premises like residences, institutions, and commercial establishments is termed as sewage or wastewater. Normally domestic and municipal wastewater are composed of 99.9 % water and remaining 0.1 % suspended, colloidal and dissolved solids, mainly organic in nature because it consists of maximum amount of carbon compounds, viz., human waste, paper, vegetable matter, etc., and it also contributes pathogens which consumes available oxygen from water bodies. Besides this, industrial wastewater gets mixed with municipal waste polluting the water bodies and land which is irrigated by the wastewater.
The wastewater is not used directly for drinking purposes because it contains ions, metals and microorganism which could be harmful to humans if their concentration or numbers exceeds from permissible limit. Therefore, the increasing demand for fresh drinking water and for others purposes such as water for gardening, washing, etc., is met by groundwater, continuously withdrawn from ground and it also poses many environmental and sociological problems in the region. The treated wastewater could act as an alternative to groundwater for some uses.
The treatment of sewage water requires physical, chemical and biological methods. Many previous studies have shown that after the treatment of sewage water, sewage sludge forms which still contains much higher amount of organic contaminants, which are present in higher concentrations, are applied to agricultural soils (Singh et al. 2004; Frank Laturnus et al. 2007). Similarly, another study (Foresti et al. 2006) had also reported that the utility of anaerobic processes as the core technology for sustainable domestic wastewater treatment. Anaerobic digesters have been responsible for the removal of large fraction of organic matter (mineralization of waste sludge) in conventional aerobic sewage treatment plants since the early years of domestic sewage treatment (DST). Orhon et al. 1997 have studied the domestic sewage water quality in terms of major polluting parameters. Biomethanation process is very common in domestic and industrial waste treatment in Indian scenario (Tare et al. 1997). Domestic and industrial sewage generated within the National Capital Territory (NCT) of Delhi is the main source of pollution of the river Yamuna during its passage through the NCT. Almost the entire treated and untreated sewage of Delhi which is discharged in river Yamuna contributes 80 % of the river Yamuna’s pollutant load.
Despite over 10 years of efforts and expenditure of Rs. 872 crore since 1994 on establishment of sewage treatment infrastructure for treating the domestic and industrial sewage before its release into the river, water quality of the river Yamuna is still very far away from normal river water (Govt. report 2005). Low polycyclic aromatic hydrocarbons are also present in the sewage sludge. These can also be successfully treated by aerobic bioreactors (Trably and Patureau 2006). Sato et al. 2005 have studied the prospect for a self-sustainable sewage treatment system. Monitoring of different sites requires money and manpower; this could be reduced by clustering techniques. The application of hierarchical classification approach is well known for the interpretation of data and provides a valuable tool for reliable and effective monitoring and management (Singh et al. 2009).
Methods of wastewater treatment were first developed in response to the adverse conditions caused by the discharge of wastewater to the environment and the concern for public health. Further, as cities became larger and larger, limited land was available for wastewater treatment and disposal, principally by irrigation and intermittent filtration. Also, as populations grew, the quantity of wastewater generated rose rapidly and the deteriorating quality of this huge amount of wastewater exceeded the self-purification capacity of the streams and river bodies.
Objectives of study: The main objective was to collect wastewater samples from various sewage treatment plant (STP) operating within Delhi region to understand the physicochemical property of wastewater generated as domestic waste. In addition, specific objective was to compare the physicochemical property of wastewater at inlet and outlets of STP to analyze the efficiency of treatment plants for those analyzed parameter and identification of similar sites.
Study area
Delhi lies in the latitude/longitude 28°38′/77°13′. At present, the total quantity of sewage generated is 2,871 million liters per day (mld), whereas the total capacity of the sewage treatment plants in Delhi is 1,478 mld (see Tables 1, 2 for detailed capacity of different plants and common treatment method used). The remaining 48 % untreated sewage (1,393 mld) finds its way into the Yamuna River through the 19 major drains which carry sewage and industrial effluents from the city. As a result, the quality of river water has been deteriorating and the water in the river is at present unfit for drinking (even for animals) and for use in agriculture. The sewerage facilities cover only about 75 % of the population. The sewage system is nonexistent in large parts of the trans-Yamuna area, all the resettlement colonies, and illegal settlements. The conventional method for estimation of generated wastewater is derived at 80 % of the water supplied. However, this may not be realistic in the areas like Delhi (see Fig. 1) where large quantity of ground water is simultaneously extracted and utilized. Efforts have been made to find the cumulative pollution load addition to river Yamuna (23 km stretch from Wazirabad Barrage to Okhla Barrage) at various points by the major drains carrying wastewater from NCT of Delhi; making these open sewers in turn causing foul smell, bad quality and groundwater contamination all along the drain and ultimately polluting disposal sink to river Yamuna.
Materials and methods
A total of 14 samples were collected for the study in the year 2007–2008. These 14 samples were representing seven inlets and seven outlets of the same STP. The pH and conductivity were measured from unfiltered water samples. The pH was measured by Rachho (model no. 123) pH Meter. Systronics conductivity meter was used for the measurement of conductivity and TDS. The bicarbonate content was determined following the potentiometric titration method (American Public Health Association (APHA) 1995). Nitrate was estimated by brucine method. Nitrate and brucine react to produce a yellow color, the intensity of which can be measured at 410 nm. The method is suitable for the samples with a very wide range of salinity. Sulphate was analyzed by turbidimetric method. Suspended matter and original color of the sample may interfere with the sulphate determination. Suspended matter can be removed by filtration. Presence of silica in excess of 500 mg/l and large quantity of organic matter may affect the satisfactory precipitation of barium sulphate. The ascorbic acid method (APHA 1995) determined phosphate. Optical density was measured at 650 nm using Cecil Spectrophotometer (model no. 594). Chloride was determined by spectrophotometer. Cations such as Na, K, Ca and Mg were analyzed using atomic absorption spectrophotometer, Shimadzu-AA-6800. Excessive sodium content in water renders it unsuitable for soils containing exchangeable calcium and magnesium ions. The exchange capacity of water is expressed in sodium absorption ratio (SAR) by Eq. 1 and sodium percentage was calculated by using Eq. 2
Results and discussions
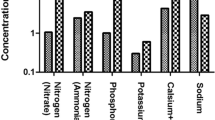
Wastewater samples were collected at inlet and outlet and analyzed for various chemical parameters and results are given in (Table 3). The pH of the wastewater was slightly acidic to alkaline and it ranges from 6.4 to 7.3 before treatment and after treatment its values varies from 7.0 to 7.9 indicates its alkaline nature. The EC value indicate amount of dissolve constituents present in water and used in determining the suitability of water for irrigation (Paliwal and Yadav 1976). The EC of wastewater samples ranges from 1,470 to 4,210 μS/cm at inlet while the average of 2,169 μS/cm and at outlet their value varies from 1,420 to 3,820 μS/cm with an average 2,161 μS/cm. The high EC value is attributed to the high salinity and high mineral content. It also corresponds to the highest concentrations of dominant ions which are the result of ion exchange and solubilisation in the water. It shows strong positive correlation with TDS, HCO3−, and Ca2+ in inlet as well as outlet (Tables 4, 5). Bicarbonate is the major anion present in the fresh water body. The source of bicarbonate is atmospheric CO2, which dissolves in water to form carbonic acid. Reaction of carbonic acid with limestone containing mineral like albite (Garrels and Christ 1965), from partial or complete decomposition of organic matter (Berner 1971) give rise to bicarbonate ion concentration in water body. The bicarbonate concentration of wastewater ranges from 199.26 to 696.33 mg/l with an average of 515.88 mg/l in before treatment and after treatment bicarbonate value varies from 316.22 to 550.13 mg/l with an average 436.52 mg/l. Bicarbonate is having strong positive correlation with Ca2+ and K+ in inlet and with Ca2+ and SO42− at outlet (Tables 4, 5). The source of nitrate in wastewater is domestic sewage, runoff from the agriculture field, leachates from the landfill sites. The concentration of nitrate in inlet and outlet wastewater samples varies from 2.28 to 5.08 mg/l with an average of 4.28 mg/l, 0.09 to 5.67 mg/l (average 1.25 mg/l), respectively. The source of sulphate in water is the solvent action of gypsum, pyrites, galena, chalcopyrite, sphalerite, anhydrite and it is also present as final oxidation products of disulphides, sulphite and thiosulphates. The value of sulphate in inlet value ranges from 51.24 to 107.06 mg/l with an average of 82.85 mg/l and after treatment its concentration varies from 69.47 to 150.61 mg/l with an average of 99.22 mg/l. The source of Na+ in water is weathering of plagioclase, pyroxene and hornblende; evaporate minerals and atmospheric precipitation (2 mg/l) (Davis and De Wiest 1967). The concentration of Na+ in water samples varies from 21.99 to 25.42 mg/l with an average of 23.08 mg/l in before treatment and after treatment its concentration varies from 22.79 to 24.6 mg/l (average 23.18 mg/l). Na+ is showing a strong correlation with Mg2+ at inlet. Sodium is showing higher values because it behaves like conservative element, i.e., it is not used up in biological process or clay mineral formation (Subramanian and Saxena 1983).
The source of K+ is weathering of orthoclase, microcline, biotite, K-feldspar, etc., and rainwater (0.1 mg/l) (Davis and De Wiest 1967). The concentration of K+ in water samples varies from 24.60 to 39.30 mg/l with an average value of 29.34 mg/l in inlet and outlet its concentration varies from 25.00 to 35.71 mg/l with an average 29.07 mg/l. The main sources of Ca2+ and Mg2+ in water are calcite, dolomite, magnesite, anhydrite, gypsum, feldspar, pyroxene, amphiboles, etc. The concentration of Ca2+ in inlet water sample varies from 70.46 to 99.78 mg/l with an average value of 84.31 mg/l and outlet its values varies from 69.98 to 99.78 mg/l (average 82.65 mg/l). The concentration of Mg2+ in water samples is varies from 3.11 to 5.39 mg/l with an average value of 4.14 mg/l in inlet and after treatment concentration of Mg2+ varies from 3.77 to 4.96 mg/l (average 4.40 mg/l) (Figs. 2, 3, 4, 5, 6, 7)
Suitability for irrigation uses
The treated wastewater is not used for the irrigation purpose because it contains high sodium content, which may be harmful for most soils and requires special water and soil management practices. Water with high amount of bicarbonates and relatively low in calcium is also known to be hazardous for irrigation (Richards 1954) and higher EC in water also creates an adverse effect on saline soil; whereas salt content in irrigation water causes an increase in soil solution osmotic pressure (Thorne and Peterson 1954). EC and sodium concentration are used in classifying irrigation water. The total concentration of soluble salts in irrigation water can be expressed for the purpose of classification of irrigation water as low (EC = <250 μS/cm), medium (250–750 μS/cm), high (750–2,250 μS/cm) and very high (2,250–5,000 μS/cm) salinity zone (Richards 1954).
The calculated value of SAR in the wastewater after treatment ranges from 0.61 to 0.77 with average value 0.68 while before treatment 0.60–0.71 with average 0.67. The plot of data was performed on the US salinity diagram (Fig. 8). In this diagram the EC is taken as salinity hazard and SAR as alkalinity hazard, which shows that most of the wastewater samples fall in the category C3S1 and one in C4S1. Indicating low to medium salinity and low sodium water, this can be used for irrigation in most soil and crops with little danger of development of exchangeable sodium, and salinity.
USSL salinity diagram of wastewater after treatment for irrigation purposes (Richards 1954)
Sodium percentage (Na %)
Sodium concentration is important in classifying irrigation water because it reacts with soil to reduce its permeability. Excess sodium in water produces undesirable effects of changing soil properties and reducing soil permeability (Kelley 1951). The Na % is calculated using the formula given below, where all the concentrations in meq/l. The Wilcox (1955) diagram is relating sodium percentage and electrical conductivity (Fig. 9). The sodium percentage in the inlet wastewater ranges from 24.31 to 29.99 % with average 27.89 % and after treatment 24.58 to 31.75 % with average 28.19 %. As per the Bureau of Indian Standard (BIS), maximum sodium of 60 % is recommended for irrigation water. After treatment most of the samples on the Wilcox diagram fall in the categories of good to permissible region, but in Pappankalan region sample fall in the unsuitable category.
Na % versus EC in wastewater after treatment (Wilcox 1955). One sample falls in the doubtful category
Cluster analysis
Cluster analysis approach is subjective in nature. To investigate the results, further CA was performed to check the similarity between the different STP with the help of outlet parameter. The cluster-rendered dendrogram map was drawn using single linkage Ward’s method. Following clusters (see Fig. 10 for details) were formed on the basis of physicochemical parameters which link similar STP site, viz., cluster 1 (3, 1, 2, 7 and 6) cluster 2 (2 and 4) and cluster 3 (2, 4 and 5). This clustering will help in identifying the similar sites, which will further help in long-term monitoring.
Conclusion
The increasing demand for water supply can be met with the treated sewage water. In this paper, the sample’s pH value after treatment lies in alkaline range. EC is showing higher values (max 4,210 μS/cm) in Pappankalan inlet. The dominant anion in the water sample is HCO3− followed by SO42−, PO43−, Cl−, and NO3− and dominant cation is Ca2+ is followed by K+, Na+, and Mg2+. In Rithala, SPT outlet concentration of HCO3− is greater than the inlet concentration because of use of some chemicals during the treatment processes. Na+ concentration is higher at outlet as compared to inlet at Rithala and Keshopur STP. Similarly, concentration of K+ at Rithala is also more at outlet as compared to the inlet. One of the major problems with these wastewater treatment methods is that none of the available technologies has a direct economic return. The available technologies are unaffordable due to high capital and maintenance costs. The efficiency of STP is not good. In order to improve the efficiencies of the STPs, the treatment systems must be properly operated and maintained, sources of raw sewage identified, and existing facilities upgraded. Long-term monitoring strategy is required to study effectiveness of the STP in depth.
References
American Public Health Association (APHA) (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington DC
Berner RA (1971) Chemical Sedimentology. McGraw Hill Publication, New York
Davis SN, De Wiest RJM (1967) Hydrogeology. Wiley, Inc., New York
Foresti E, Zaiat M, Vallero M (2006) Anaerobic processes as the core technology for sustainable domestic wastewater treatment: consolidated applications, new trends, perspectives, and challenges. Rev Env Sci Bio Technol 5:3–19
Garrels RM, Christ CL (1965) Solutions, minerals and equilibria. Harper and Row, New York
Kelley KR (1951) Alkali soils- their formation properties and reclamation. Reinhold Publ. Corp, New York
Laturnus F, Arnold KV, Gron C (2007) Organic contaminants from sewage sludge applied to agricultural soils. Env Sci Pollut Res 14(1):53–60
Orhon D, Ate E, Sozen S, Cokgor EU (1997) Characterization and COD fractionation of domestic wastewaters. Environ Pollut 95(2):191–204
Paliwal KV, Yadav BR (1976) Irrigation water quality and crop management in Delhi. Geol Survey INd Misc Publ 14:130–139
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. US Department of Agriculture Handbook 60
Sato N, Okubo T, Onodera T, Ohashi A, Harada H (2005) Prospects for a self-sustainable sewage treatment system: A case study on full-scale UASB system in India’s Yamuna River Basin. Environ Mange. doi:10.1016/j.jenvman.2005.08.025
Singh KP, Mohan D, Sinha S, Dalwani R (2004) Impact assessment of treated/untreated wastewater toxicants discharged by sewage treatment plants on health, agricultural, and environmental quality in wastewater disposal area. Chemosphere 55:227–255
Singh SK, Singh CK, Kumar KS, Gupta R, Mukherjee S (2009) Spatial-temporal monitoring of groundwater using multivariate statistical techniques in Bareilly district of Uttar Pradesh, India. J Hydrol Hydromech 57(1):45–54. doi:10.2478/V10098-009-0005-1
Subramanian V, Saxena K (1983) Hydrogeology of ground water in Delhi region of India, Relation of water quality and quantity. In: Proceedings of the Hamberg symposium IAHS publication no. 146
Tare V, Ahammed M, Jawed M (1997) Biomethanation in domestic and industrial waste treatment-an Indian scenario. In: Proceedings of the eighth international conference on Anaerobic Digestion, vol 2. Japan, pp 255–262
Thorne DK, Peterson HB (1954) Irrigated soils. Constable and Company, London
Trably E, Patureau D (2006) Successful treatment of low PAH-contaminated sewage sludge in aerobic bioreactors. Environ Sci Pollut Res 13(3):170–176
Wilcox LV (1955) Classification and use of irrigation waters, USDA Circular No. 969, 19
Acknowledgments
The author (Sandeep Kumar Gautam) thanks CSIR for providing financial support to carry out the research work. The author also acknowledges the technical and administrative staff of his school for being always attentive and helpful during analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Gautam, S.K., Sharma, D., Tripathi, J.K. et al. A study of the effectiveness of sewage treatment plants in Delhi region. Appl Water Sci 3, 57–65 (2013). https://doi.org/10.1007/s13201-012-0059-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-012-0059-9