Abstract
Water quality of Tiruvallur Taluk of Tiruvallur district, Tamil Nadu, India has been analysed to assess its suitability in relation to domestic and agricultural uses. Thirty water samples, including 8 surface water (S), 22 groundwater samples [15 shallow ground waters (SW) and 7 deep ground waters (DW)], were collected to assess the various physico-chemical parameters such as Temperature, pH, Electrical conductivity (EC), Total dissolved solids (TDS), cations (Ca, Mg, Na, K), anions (CO3, HCO3, Cl, SO4, NO3, PO4) and trace elements (Fe, Mn, Zn). Various irrigation water quality diagrams and parameters such as United states salinity laboratory (USSL), Wilcox, sodium absorption ratio (SAR), sodium percentage (Na %), Residual sodium carbonate (RSC), Residual Sodium Bicarbonate (RSBC) and Kelley’s ratio revealed that most of the water samples are suitable for irrigation. Langelier Saturation Index (LSI) values suggest that the water is slightly corrosive and non-scale forming in nature. Gibbs plot suggests that the study area is dominated by evaporation and rock–water dominance process. Piper plot indicates the chemical composition of water, chiefly controlled by dissolution and mixing of irrigation return flow.
Similar content being viewed by others
Introduction
Water is the basic of life on this planet and the foundation of civilization. The quality must be considered in any assessment of water resources (Anon 1993). Detailed works on groundwater assessment, evaluation and management in relation to hydrology and water resources engineering were initiated by several authors (Todd 1980; Prince 1985; Karanth 1987). Hydrogeochemical processes are the major responsible for the alteration of groundwater in their chemical composition. The quality of ground water depends on the nature of the soil and the rock masses present along the pathway of groundwater saturation zone (Olayinka et al. 1999; Foster et al. 2000; Chidambaram et al. 2008). There are many processes involved during the movement of groundwater from recharge to discharge areas that include precipitation, mixing, ion exchange, redox condition, leaching and dissolution. The chemical and biological characteristics of water determine whether or not it can be used for domestic, industrial or agricultural supply. Nevertheless, groundwater is a complex chemical substance that owes its composition mainly to the solution in, and chemical reaction between, the water and the rock or soil masses through which it travels. Water also remains the most important factor of illness and infant mortality in many developing countries and even in industrialized countries, where the number of cases of infectious intestinal diseases continues to increase (Jones and Watkins 1985). Water contamination can be classified as having either natural or anthropogenic sources. In this situation, water quality and chemistry gain their importance for the management and assessment of groundwater. Hence, the study focuses on the assessment of the quality and geochemistry of groundwater in the Tiruvallur Taluk of Tamil Nadu.
The study area covers the Tiruvallur Taluk, Tamil Nadu, which is located at 40 km from the State Headquarter, with the geological location of 13°08′N and 79°54′E. In the Tiruvallur taluk, there are about 136 villages and the total population is around 3,725,697. The average rainfall of the area is about 1104 mm of which 52 % is during the Northeast monsoon and 41 % during Southwest monsoon. The district occupies an area of 3422 km2 (1321 sq. mi) and the Taluk covers an area of 10.75 km2 (4.15 sq. mi). The region is mainly occupied by sedimentary formation and also rocks of Archean and Proterozoic. Tiruvallur district is a part of the east flowing Arniar–Korataliar and Cooum sub-basins. The Monsoon Rivers like Araniyar, Korattalayar, Cooum, Nagari and Nandhi are the important rivers and the drainage pattern is generally dendritic. Ground water occurs under phreatic conditions in the weathered mantle and under semi-confined conditions in the fissured and fractured zones at deeper levels. The depth to water level in the district varied between 2.38 and 7.36 m bgl (CGWB 2007). The study area is mainly influenced by agriculture activities chiefly donated for Groundnut, Rice, Sugarcane, Ragi, Cumbu. Hydrogeomorphological units such as Flood plain, Low land, Alluvial plain, Buried channel, and Upland are observed in the regions. Among the fluvial geomorphic units, flood plain, buried channel, alluvial plain, and valley fill contribute to more ground water resources to the area. The aim of the study is to assess the water quality for irrigation and drinking water purposes in Tiruvallur District, Tamil Nadu, India.
Methodology
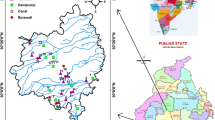
Eight surface water and twenty-two ground water samples were collected along the study area (Fig. 1). The surface water was sampled in pond, reservoirs and tank. The ground water samples were collected from shallow (depth <70 feet) and deep (depth >70 feet) wells. Sampling and analysis was carried out using standard procedures (Ramesh and Anbu 1996; APHA 1992). Five hundred millilitres of water samples was collected in polyethylene bottle. Bore water was collected by pumping out the stagnant water for 20 min with hand pump, to get representative samples. Then, it was sealed and brought to the laboratory for analysis, stored properly (4 °C), and filtered with 0.45-µm filter paper before analysis. The temperature, Electrical conductivity (EC), and pH were measured using a handheld portable kit in situ. The carbonate and bicarbonate were determined by titration with 0.2 N sulphuric acid using phenolphthalein and methyl orange indicators. The analytical precision of the measurements of ions was determined by calculating the ionic balance error, which was observed to be within the standard limit of ±10 %. The trace elements were analysed by Inductively Coupled Atomic Absorption Spectrophotometer (ICPAES-Model No. IRIS INTREPID II XSP-Thermo Electron Corporation). The geochemical results are calculated and plotted on piper trilinear plot, LSI calculation, USSL and Wilcox plot in Aqua Chem 4.0 software. Gibbs diagram is plotted to assess the quality controlling mechanism and dominated hydrogeochemical facies of the study area. Statistical analysis was performed using an IBM SPSS software package (SPSS 2001). The physico-chemical parameters of the analytical results of groundwater were compared with standard guideline values recommended by the WHO and BIS. Sodium absorption ratio (SAR), sodium percentage (Na %), Residual sodium carbonate (RSC), Residual Sodium Bicarbonate (RSBC) and Kelley’s ratio will be used to evaluate the suitability of water for irrigation purposes.
Results and discussion
The statistical parameters like minimum, maximum and mean concentration of physico-chemical parameters, major ion concentrations are tabulated in Table 1.
Physico-chemical characteristics
Surface water showed a minimum temperature of 25 °C and maximum of 31 °C, the average temperature is 31 °C; in the shallow well, ranging from 22 to 31 °C, average temperature was 31 °C. In the deep well category, temperature ranges from 26 to 35 °C with a mean value of 29.5 °C. pH is the term used universally to express the intensity of the acid or alkaline condition of a solution. The pH of groundwater is a very important indicator of its quality and is controlled by the amount of dissolved CO2, carbonate and bicarbonate (Ghandour et al. 1985). The combination of CO2 with water forms carbonic acid, which affects the pH of the water. For the three types of water samples, the pH ranged from 5.4 to 8.4. It clearly indicated that all three types fall within the permissible limit except few (the permissible limit of pH is 6.5–8.5).
The measurement of EC is directly related to the concentration of ionized substances in water and may also be related to the problems of excessive hardness and other mineral contamination. Dissolved solids present in natural water consist mainly of inorganic salts such as carbonates, bicarbonates, chlorides, sulphates, phosphates and nitrates of calcium, magnesium, sodium, potassium, iron, and small amount of organic matter and dissolved gases. The assessment of geochemical characteristics of water is essential to classify the ground water and surface water depending upon their hydrochemical properties based on their TDS values (Davis and De Wiest 1966; Freeze and Cherry 1979). The most desirable limit of EC in drinking water is prescribed as 1500 µs/cm (WHO 2004). The electrical conductivity can be classified as type I, if the enrichments of salts are low (EC: 1500 µs/cm); type II, if the enrichment of salts are medium (EC: 1500 and 3000 µs/cm); type III, if the enrichments of salts are high (EC 3000 µs/cm; Sarath Prasanth et al. 2012). According to the classification of EC, all 8 surface water samples (Sample no 5, 9, 10, 18, 19, 20, 28 and 30–100 % of samples) come under the category I. From the 15 shallow well water samples, 13 samples (Sample no 6, 7, 8, 12, 13, 14, 15, 17, 21, 22, 23, 24, 27–87 % of the samples) come under the category I and 2 samples (25 and 29–13 % of the samples) come under the category II. In the deep well samples, nearly 71.4 % of the samples are listed under category I and 28.5 % of samples fall under the category of II.
Total dissolved solid (TDS) denotes the various types of minerals present in water in dissolved form. In natural waters, dissolved solids are composed of mainly carbonates, bicarbonates, chlorides, sulphate, phosphate, silica, calcium, magnesium, sodium and potassium. TDS affects water supply system (scaling), excessive soap consumption, calcification of arteries, may cause urinary concretions, diseases of kidney or bladder and stomach disorder. According to WHO specification, TDS up to 500 mg/l is highly desirable and up to 1500 mg/l is the maximum permissible limit. Degree of groundwater quality can be classified as fresh, if the TDS is less than 1000 mg/l; brackish, if the TDS is between 1000 and 10,000 mg/l; saline, if the TDS varied from 10,000 to 1,000,000 mg/l; brine, if the TDS is more than 1,000,000 mg/l (Todd 1980). Total dissolved salts in the surface water range from 409 to 960 mg/l. According to Todd (1980), all surface water samples 100 % (8 samples) in the study area are classified as fresh water, in the case of shallow well TDS value ranges from 294.4 to 1324 mg/l, but 87 % of water samples (13 samples) come under fresh water and 13 % of water samples (2 samples) are categorized as brackish water. Deep well type water samples range from 288 to 1600 mg/l and 71 % of water samples (5 samples) fall under the category of fresh and 29 % of the samples (2 samples) are brackish type. This indicates the influence of rock–water interaction in relation to recharge water. The observed TDS values range from 288 to 1600 mg/l in the deep well water sample. The occurrence of high TDS is due to the influence of anthropogenic sources, such as domestic sewage, septic tanks and agricultural activities.
The concentration of carbonates in natural waters is a function of dissolved carbon dioxide, temperature, pH, cations and other dissolved salts. Bicarbonate concentration of natural waters is generally held within a moderate range by the effects of the carbonate equilibrium. Most surface streams contained less than 200 mg/l of carbonate and bicarbonate, but in ground water somewhat higher. The concentration of bicarbonate in the surface water samples ranges from 61.0 to 506.3 mg/l, it shows that seven (87 %) samples are within the permissible limit (500 mg/l) of WHO and one (13 %) sample exceeds the limit (506.3 mg/l). The shallow well type samples range from 67 to 628.0 mg/l. 11 samples (73 %) are within permissible limit and 4 samples (27 %) come beyond the permissible limit. Notably, sample no 12, 15 and 25 showed higher concentrations beyond the limit of WHO standards, i.e. 506.3, 512.4 and 628 mg/l, respectively. The deep well sample ranges from 73.2 to 408.7 mg/l.
Chloride is the most predominant natural form of the element chlorine and is extremely stable in the water. Chloride in groundwater may be from diverse sources such as weathering, leaching of sedimentary rocks and soil, and domestic and municipal effluents (Sarath Prasanth et al. 2012; Krishna Kumar et al. 2014). As irrigation is concerned, chloride is not adsorbed or held back by soils; therefore, it moves readily with the soil–water, gets adsorbed by crops, moves in the transpiration stream, and accumulates in the leaves (Ayers and Westcot 1994). Higher Cl− intake beyond the crop tolerance limit in plants develops symptoms such as leaf burn and drying of leaf tissues. Excessive necrosis (dead tissue) is often accompanied by an early leaf drop or defoliation (SubbaRao 2006). Chlorides play an important role in balancing the level of electrolytes in blood, but higher concentration of Cl can give hypertension and renal stones (McCarthy 2004). As per World Health Organization (WHO 2011) and Bureau of Indian standards (BIS 1991), the desirable limit and permissible limit for chloride are 250 and 1000 mg/l, respectively. Chloride found in the surface water varies between 159.5 and 425.4 mg/l. Of the eight surface water samples, 6 samples (75 %) fall within the desirable limit (within 250 mg/l) and two samples (25 %) fall within the permissible (between 250 and 1000 mg/l) limit. A notable point is that samples are not found beyond the permissible limit of chloride concentration in the surface water type. In the case of shallow well, it ranges from 150.6 to 726.7 mg/l. Of the fifteen shallow well water samples, seven samples (47 %, Sample no 6, 8, 12, 13, 22, 23, 29) fall within the desirable limit, eight samples (53 %) fall between the desirable and permissible limit. This may be due to the lack of underground drainage systems and bad maintenance of the environment around the sources. In the deep well water samples, it ranges from 62.03 to 248.15 mg/l. All deep water samples fall within the desirable limit.
Sulphates occur naturally in various minerals, including Barite (BaSO4), Epsomite (MgSO4·7H2O) and Gypsum (CaSO4·2H2O) (Greenwood and Earnshaw 1984). The primary mineral sources of sulphate ions include evaporate minerals such as Anhydrite, Gypsum and sulphates of Magnesium and Sodium and the leaching of fertilizers (Thivya et al. 2013a) may also increase the sulphate concentration in groundwater. These dissolved minerals contribute to the mineral content of drinking water (WHO 2004). There have been a number of studies conducted to determine the toxicity of sulphate in humans. Case reports of diarrhoea in three infants exposed to water containing sulphate at concentrations ranging from 630 to 1150 mg/l have been presented (Chien et al. 1968). However, diarrhoea could not be explained as being solely due to exposure to high sulphate levels and other factors may have played a role (e.g. consumption of infant formula with high osmolarity or the presence of microbial pathogens). Sodium, potassium and magnesium sulphates are all highly soluble in water, whereas calcium and barium sulphates are less soluble. WHO and BIS suggests the maximum permissible limit of SO4 in drinking water is 400 mg/l; all the surface water, shallow water and deep well water samples are within the permissible limit (Table 1).
The concentration of nitrogen in groundwater is derived from the biosphere (Saleh et al. 1999). High concentration of nitrate in drinking water is toxic and causes blue baby disease/methaemoglobinaemia in children and gastric carcinomas (Alam et al. 2012; Gilly et al. 1984). Nitrogen is a plant nutrient that stimulates crop growth, when applied in excess affects the crop by over stimulation of growth, delayed maturity and poor quality of crop yield. The concentration of nitrate in surface and groundwater samples is less than certified limit (45 mg/l). The average concentration of phosphate in surface and groundwater samples is less. The phosphate and nitrate in water samples were probably derived from application of phosphate and nitrogen fertilizers in the agricultural fields.
Soil containing large proportions of sodium with carbonate as the predominant anion is termed as alkali soil, whereas with chloride or sulphate as the predominant cations is termed as saline soil (Brindha et al. 2014). The mean concentration of Ca and Mg for surface water and groundwater is slightly higher than the BSI- and WHO-suggested standard limit (70 mg/l). The mean concentration of Na and K is 171.2 and 4.75 mg/l for surface water, 177.2 and 30.1 for shallow wells, 64.4 and 14.2 for deep well samples (Table 1), in a general case, which is the dominant ion among the cations, contributing approximately 52 to 55 % of the total cations. The maximum concentration of sodium and potassium noticed in a few samples (Sample no 14, 15, 17 and 25) may be due to dissolution of Na- and K-bearing minerals from soil column, and also by weathering of plagioclase-bearing rocks (Chidambaram 2000). In general, consuming of groundwater with high sodium content is harmful and causes heart diseases and kidney problems. The lower concentration of potassium is due to the more resistant nature of potash feldspar against chemical weathering process (Hem 1985a; Thivya et al. 2013b), because the major part of the aquifer chiefly consists of hornblende biotite gneiss.
Water quality characteristics for irrigation and drinking purposes
The Gibbs diagram was proposed to understand the relationship between the chemical components of water (Gibbs 1970, Eqs. 1, 2). The quality characteristics of water are identified from three distinct fields of Gibbs diagram, namely precipitation dominance, evaporation dominance and rock–water interaction dominance where all ions are expressed in meq/l.
The Gibbs ratio of the water samples is plotted against their respective total dissolved solid concentration (TDS) and calculated Gibbs equation values. According to Gibbs Eq. 1 for anions, nearly 24 % of the samples fall under evaporation dominance field and 76 % of the samples follow the rock–water dominance field. Likewise, according to Gibbs equation II for cations, 50 % of the samples fall under rock–water dominance and evaporation dominance fields (Fig. 2).
Controlling mechanism of groundwater chemistry (Gibbs 1970)
Piper diagram was made in such a way that the milli-equivalent percentages of the major cations and anions are plotted in a separate triangle. These plotted points in the triangular fields are projected further into the central diamond field, which provides the overall character of the water. The triangular fields are plotted separately with epm values of cations, (Ca, Mg) alkali earth, (Na+K) alkali, (HCO3) weak acid and (SO4 and Cl) strong acid. Piper (1944) diagram is useful for identification of similarities and differences in groundwater because it separates the similar qualities as groups (Todd 1980). Samples of shallow water fall under the mixed region of Ca–Mg–Cl type indicating the predominance of anthropogenic impact. Most of the surface water and deep water samples fall under Na–Cl type indicating the dissolution and anthropogenic processes. Some of the samples of surface water fall under the Ca–Cl type. Most of the samples reflect the mixing types of cations and anions (Fig. 3).
Langelier saturation index (LSI)
Groundwater chemistry is a tool to probe into mechanisms of chemical interaction of water with ions from different sources. The LSI calculation was calculated as suggested by Langelier (1946). The LSI value explains about the dissolution, precipitation and neutral state of the water samples (Eq. 3). The corrosive action of water is mainly due to an availability of excess of free CO2 and its interaction with carbonates. The corresponding concentration of calcium, magnesium, and carbon dioxide in the groundwater prevents conversion of bicarbonate to carbonate. Low pH values of water are due to the presence of low alkalinity and high free carbon dioxide.
where PH = −log[H+]
PHs = (9.3 + a + b) − (c + d)
a = log10 (TDS) – 1/10
b = −13.12 × log10(°C + 273) + 34.55
c = log10(Ca2+ as CaCO3 mg/l) − 0.4
d = log10(alkalinity as CaCO3 mg/l)
The calculated LSI values indicate that nearly 50 % of the samples have a tendency to precipitate the carbonates from the water and approximately 54 % of the samples are willing to dissolve the carbonate minerals from the aquifer due to free CO2 and slightly corrosive nature of water. The overall results of the LSI calculation indicate that the majority of the samples are slightly corrosive with scale forming (Table 2).
United States salinity laboratory (USSL) diagram
Sodium toxicity is recorded as a result of high sodium in water as Na % and SAR ratios. Typical toxicity symptoms in plants and trees are leaf burn and dead tissue along the outside edges of leaves. The source of Na+ into the groundwater is due to the weathering of feldspar and due to over exploitation of groundwater (Hem 1985b). The measured value of SAR ranges from 0.28 to 8.49. SAR is the combination of Na+ and K+ ion concentrations with Ca2+, Mg2+, Na+ and K+ ion concentrations, which is multiplied by 100. Here, all the ions are represented in meq/l. The geochemical parameters of the water samples are plotted in USSL diagram (Salinity hazards versus Sodium Hazards, USSL 1954). The sodium and salinity hazards are classified into four zones in the USSL diagram. Approximately, 27 % of the samples are fall under C1S1 (low salinity and low sodium) category, which can be used for irrigation purposes in most of the soil types. In the zone of high salinity and low sodium hazard category (C3S1), approximately 44 % of the samples are noticed. This water can be used for irrigation applications with cautions of the exchangeable character of sodium. 27 % of the samples fall under the C3S2 (high salinity with moderate sodium hazards) category, followed by 4 % of the samples categorized under C4S2 (very high salinity and moderate sodium hazards) category, which can be suitable for plants after special consideration of soil management and maintaining the good irrigation system (Fig. 4).
Residual sodium carbonate (RSC) and residual sodium bicarbonate (RSBC)
Residual sodium carbonate (RSC) is calculated to determine the hazardous effects of carbonate and bicarbonate on the quality of water used for irrigation activities (Raju 2007). The residual sodium carbonate (RSC) is changing their quality of water through the precipitation of alkali earth elements (Ca2+, Mg2+) thereby increase the percentage of sodium (Eaton 1950; Eq. 4). The water-soluble excess carbonate is combined with alkaline earth ions to form the NaHCO3. The relationship between carbonates and alkali earth’s concentration can be explained by the RSC for irrigation quality of water. Here, all the concentrations are expressed in meq/l. The suitability of RSC value for irrigation is <1.25 meq/l. The higher concentration of RSC may lead to the poor quality of soil for irrigation. The value of RSC ranges from −12.29 to 2.51. Based on the value of the RSC, all the samples are suitable for irrigation purposes, except few (Sample no 9, 20, 23, 25 and 30).
The residual sodium bicarbonate (RSBC) calculation was proposed by Gupta and Gupta (1987) to identify the suitability of water (Eq. 5). The RSBC values range from 6.39 to 6.40 meq/l (Table 2). The satisfactory level of RSBC is <5 meq/l. All the samples followed the satisfactory level of irrigation water except one (Sample no 30)
Water quality indexes
Permeability index is a vital parameter to evaluate the quality of irrigation water in relation to soil for improvement in agriculture (Thilagavathi et al. 2012; Thivya et al. 2013a). The PI in a groundwater sample measures the total concentration of Na and HCO3 to the total cation content based on Doneen (1964). Based on the PI values, the irrigated water can be classified as Class I (>75 %), Class II (25–75 %) and Class III (<25 %). The concentrations of the ions are in meq/l. The permeability index of the study area ranges from 28.88 to 96.11 %. Nearly 30 % of the samples are fall under Class II category and another 70 % of the samples belong in the Class I category indicating that the water is moderate to good for irrigation purposes with minor indication of poor irrigation quality (Table 2).
Magnesium hazards (MH) were developed by Paliwal (1972) to an index for calculating the magnesium hazard (magnesium ratio (MR). Ca2+ and Mg2+ maintain a state of equilibrium in most groundwater (Hem 1985a; Eq. 7). During equilibrium, more Mg2+ in groundwater will adversely affect the soil quality (Kumar et al. 2007). The maximum permissible limit MH value is 50, the maximum value of magnesium hazards is considered to be harmful and unsuitable for irrigation. The magnesium ratio varied from 10.44 to 97.96 (Table 2). The majority of the samples fall under suitable for irrigation except two (Sample no 27 and 30).
Kelly’s index (KI) is used for the classification of water for irrigation purposes. Sodium measured against calcium and magnesium is considered for calculating this parameter (Kelly 1940; Eq. 8). The suggested limit of the Kelly’s ratio is <1 for irrigation waters. The Kelly’s ratio for studying samples ranges from 0.09 to 1.94 (Table 2). The majority of the samples fall under suitable for irrigation category except nine samples (Sample no 2, 9, 14, 15, 17, 19, 20, 28, and 25).
The calculated value of sodium percentage (Na %) for surface water, deep water and shallow water ranges from 1.15 to 6.77 in the studied water (Eq. 9). The sodium percentage value should not be exceeding 60 for irrigation water. All the samples are falling under the suitable category based on the Na % (Table 3).
The electrical conductivity and sodium percentage value of the studied samples are plotted in the Wilcox Plot. As per the water quality classification based on total salt concentration and Na %, 27 % of the samples are very good to good for irrigation, 40 % of the samples fall under good to poor field, 20 % of the samples are grouped under poor to doubtful category, remaining 10 % of the samples form the zone of unsuitability for irrigation (Fig. 5).
Specific conductance and sodium percentage (Na %) relationship for rating of irrigation water quality (Wilcox 1955)
Trace elements
Groundwater gets polluted by various trace metals from natural and anthropogenic sources such as chemical weathering of rocks and soils, decomposing vegetation and animal matter, fall out of atmospheric particulate matter anthropogenic activities including the discharge of various domestic and industrial effluents. The water samples were analysed for trace elements such as Pb, Zn, Cu, Cr, Fe and Mn. Analytical results show that all the elements are below the detection limit except Fe, Mn and Zn. The concentration of Fe and Mn in surface and groundwater samples is below the WHO- and BIS-suggested standards except few (Table 1). Zinc is considered as an essential element for life and acts as a micronutrient when present in trace amounts. The problem of Zn toxicity includes irritability, muscular stiffness, loss of appetite and nausea. The mean concentration of Zn in the shallow and deep well samples is 0.9 and 3.1 ppm. The slightly increasing trends of Fe and Mn in few samples are probably due to chemical weathering dissolution and the process of ferromagnetism.
Correlation matrix
The correlation coefficient expresses numerically the extent to which two variables are statistically associated. A correlation coefficient of <0.5 exhibits poor correlation, 0.5 indicates good correlation and >0.5 represents excellent correlation. Good correlation existing between pH and PO4 may also be due to the presence of Apatite (Brindha et al. 2011) which is normally present in acid charnockites and granites (Chidambaram 2000). Good correlation of EC with HCO3, Cl, Ca, Mg, Na and K indicates that these ions dominate in the region (Table 4). HCO3 exhibits a good correlation with SO4 and Na indicating the process of weathering. Good correlation existing between Cl–Ca, Mg, Na and K indicates a complex factor of pollution and reverse ion exchange (Thilagavathi et al. 2012). SO4 shows good correlation with Na whereas Ca exhibits a good correlation with Mg and Na. Mg exhibits a good correlation with Na.
Conclusion
The study classifies the waters according to the utility and chemistry of surface water, deep and shallow groundwater in the study area. The major ion concentration suggests that predominant samples belong to the suitable category for drinking and irrigation purposes based on WHO and ISI standards. Gibbs diagram showing the hydrogeochemical characteristics is chiefly controlled by rock–water interaction process followed by evaporation dominance. Piper diagram showing the majority of the samples is falling under Ca–Mg–Cl, Na–Cl and Ca–Cl fields and the study area water quality is chiefly controlled by anthropogenic activities, dissolution processes and ion exchange processes. The calculated results of the LSI calculation indicate that the majority of the samples are slightly corrosive with scale formation. The irrigation water quality parameters indicated that the majority of the water samples are suitable for irrigation purposes, except less than 5 % of the samples. The trace element concentration indicates that the slightly increasing trends of Fe and Mn in few samples are probably due to chemical weathering dissolution and the process of ferromagnetism. The statistical results explain the association of ions and they are dominated by the anthropogenic activities, weathering, and dissolution processes. Hence, the study concludes that all these above processes control the quality and geochemistry of these regions.
References
Anon (1993) Ventura seeks desalted independence. US water news 9, 8
Alam M, Rais S, Aslam M (2012) Hydrochemical investigation and quality assessment of ground water in rural areas of Delhi, India. Environ Earth Sci 66(1):97–110
APHA (1992) Standard Methods for the Examination of Water and Wastewater. 19th Edn. Washington, DC, USASS: APHA
Ayers RS, Westcot DW (1994) Water quality for agriculture: FAO Irrigation and Drainage Paper 29. Revision 1:1–130
BIS (1991) (Bureau of Indian Standards) 10500 Indian Standard drinking water specification, 1st rev, 1–8
Brindha K, Rajesh R, Murugan R, Elango L (2011) Fluoride contamination in groundwater in parts of Nalgonda district Andhra Pradesh India. Environ Monit Assess 172:481–492
Brindha K, NeenaVaman KV, Srinivasan K, Sathis Babu M, Elango L (2014) Identification of surface water-groundwater interaction by hydrogeochemical indicators and assessing its suitability for drinking and irrigational purposes in Chennai, Southern India. Appl Water Sci 4:159–174
Chidambaram S (2000) Hydrogeochemical studies of groundwater in Periyar district, Tamil Nadu, India, unpublished Ph.D thesis, Department of Geology, Annamalai University
Chidambaram S, Ramanathan AL, Anandhan P, Srinivasamoorthy K, Prasanna MV, Vasudevan S (2008) A statistical approach to identify the hydrogeochemically active regimes in ground waters of Erode district, Tamilnadu. Asian J Water Environ Pollut 5(3):123–135
Chien L (1968) Infantile gastroenteritis due to water with high sulfate content. Can Med Assoc J 99:102–104
Davis SN, DeWiest RJ (1966) Hydrogeology. Wiley, NewYork
Doneen LD (1964) Notes on water quality in agriculture. Published in Water Science and Engineering. Univ. California, Davis
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 69(2):123–134
Foster S, Chilton J, Moench M, Cardy F, Schiffler M (2000) Groundwater in rural development: facing the challenges of supply and resource sustainability. World Bank Technical Paper 463. Washington, DC, USA
Freeze RA, Cherry JA (1979) Groundwater. Printice-Hall, New Jersey
Ghandour EIM, Khalil JB, Atta SA (1985) Distribution of carbonates, bicarbonates and pH values in groundwater of the Nile delta region, Egypt. Groundwater 23:35–41
Gibbs RJ (1970) Mechanism controlling world water chemistry. Sciences 170:795–840
Gilly G, Carrao G, Favilli S (1984) Concentration of nitrates in drinking water and incidence of carcinomas. First descriptive study of the Piemonate Region, Italy. Sci Total Environ 34:35–37
Greenwood NN, Earnshaw A (1984) Chemistry of the elements. Pergamon Press, Oxford
Gupta SK, Gupta IC (1987) Management of saline soils and water. Oxford and IBH Publication Co., New Delhi, p 399
Hem JD (1985a) Study and interpretation of the chemical characteristics of natural water. USGS Water Supp Paper 2254:117–120
Hem JD (1985b) Study and interpretation of the chemical characteristics of natural water: US Geological Survey Water-Supply Paper 2254, 3rd edn, p 263
Jones F, Watkins J (1985) The water cycle as a source of pathogens. J Appl Bacteriol 14:26–36
Karanth KR (1987) Role of hydrogeology in integrated water resource development and management. Environmental Centre, Groundwater News, pp 23–30
Kelly WP (1940) Permissible composition and concentration of irrigated waters. In: Proceedings of the ASCF 66. p 607
Krishna Kumar S, Bharani R, Magesh NS, Prince Godson S, Chandrasekar N (2014) Hydrogeochemistry and groundwater quality appraisal of part of south Chennai coastal aquifers, Tamil Nadu, India using WQI and fuzzy logic method. Appl Water Sci 4(4):341–350
Kumar M, Kumari K, Ramanathan AL, Saxena R (2007) A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab, India. Environ Geol 53:553–574
Langelier WF (1946) Chemical equilibria in water treatment. J Am Water Works Assoc 38:169
McCarthy MF (2004) Medical hypothesis 63, 138
Olayinka AI, Abimbola AF, Isibor RA, Rafiu AB (1999) A geoelectric hydrochemical investigation of shallow groundwater occurrence in Ibadan, South-Western Nigeria. Environ Geol 37:31–37
Paliwal KV (1972) Irrigation with saline water, Monogram no. 2 (Newseries). New Delhi, IARI, p 198
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analysis. Trans AGU 25:914–923
Prince M (1985) Introducing groundwater. George Alien and Unwin, London, Proceedings of an advisory group meeting, IAEA, Vienna. p 195
Raju NJ (2007) Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh, South India. Environ Geol l52:1067–1074
Ramesh R, Anbu M (1996) Chemical methods for environmental analysis—water and sediments, p 161
Saleh A, Al-Ruwih F, Shehata M (1999) Hydrogeochemical process operating within the main aquifers of Kuwait. J Arid Environ 42:195–209
Sarath Prasanth SV, Magesh NS, Jitheshlal KV, Chandrasekar N (2012) Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha District, Kerala, India. Appl Water Sci 2(3):165–175
SPSS (2001) SPSS for Windows, Rel. 11.0.1. 2001. Chicago: SPSS Inc
SubbaRao N (2006) Seasonal variation of groundwater quality in a part of Guntur district, Andhra Pradesh, India. Environ Geol 49:413–429
Thilagavathi R, Chidambaram S, Prasanna MV, Thivya C, Singaraja C (2012) A study on groundwater geochemistry and water quality in layered aquifers system of Pondicherry region, southeast India. Appl Water Sci 2:253–269
Thivya C, Chidambaram S, Singaraja C, Thilagavathi R, Prasanna MV, Jainab I (2013a) A study on the significance of lithology in groundwater quality of Madurai district, Tamil Nadu (India). Environ Dev Sustain 15:1365–1387
Thivya C, Chidambaram S, Thilagavathi R, Prasanna MV, Singaraja C, Nepolian M, Sundarrajan M (2013b) Identification of the geochemical processes in groundwater by factor analysis in hard rock aquifers of Madurai district, South India. Arab J Geosci 7:3767–3777
Todd DK (1980) Ground water hydrology. John Wiley and Sons New York, p 535
USSL (1954) Diagnosis and improvement of salinity and alkaline soil. USDA Hand Book no. 60, Washington
WHO (2011) WHO Guidelines for Drinking-water Quality, fourth ed. World Health Organization
WHO (World Health Organization) (2004) Guidelines for drinking water quality recommendations, vol 1. WHO, Geneva, p 515
Wilcox LV (1955) Classification and use of irrigation waters. USDA Circular No. 969, pp 19
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Krishna Kumar, S., Hari Babu, S., Eswar Rao, P. et al. Evaluation of water quality and hydrogeochemistry of surface and groundwater, Tiruvallur District, Tamil Nadu, India. Appl Water Sci 7, 2533–2544 (2017). https://doi.org/10.1007/s13201-016-0447-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-016-0447-7