Abstract
Enhanced oil recovery (EOR) processes have a great potential to maximize oil recovery factor of the existing reservoirs, where a significant volume of the unrecovered oil after conventional methods is targeted. Application of chemical EOR techniques includes the process of injecting different types of chemicals into a reservoir to improve the overall sweep efficiency. Surfactant flooding is one of the chemical EOR used to reduce the oil–water interfacial tension and to mobilize residual oil toward producing wells. Throughout the process of surfactant flooding, selecting a suitable surfactant for the reservoir conditions is quite challenging. Surfactants tend to be the major factor associated with the cost of an EOR process, and losing surfactants leads to substantial economic losses. This process could encounter a significant loss of surfactant due to adsorption into the porous media. Surfactant concentration, salinity, temperature, and pH were found to be as the main factors that influence the surfactant adsorption on reservoir rocks. Most of the research has been conducted in low-temperature and low-salinity conditions. Only limited studies were conducted in high-temperature and high-salinity (HT/HS) conditions due to the challenging for implementation of surfactant flooding in these conditions. This paper, therefore, focuses on the reviews of the studies conducted on surfactant adsorption for different surfactant types on different reservoir rocks under different reservoir conditions, and the influence of surfactant concentration, salinity, temperature, and pH on surfactant adsorption.
Similar content being viewed by others
Introduction
Rising demand for oil has been noticed due to the fact that it remains the world’s most powerful source of energy. This was observed by the increases in exploration and production of oil reservoirs. There was a huge development on the fields of maximizing the oil recovery and production enhancement in progress by oil and service companies. In conventional resources, the recovered volumes from the original oil in place are around 30%. Therefore, the development of more advanced techniques to recover additional oil is required in order to meet energy demands. Also, conventional methods are not sufficient to increase the amount of recoverable volumes more than the existing reserves (Curbelo et al. 2007).
The applications of enhanced oil recovery (EOR) techniques include the process of injecting extra fluids represented in injecting chemicals or gases and/or thermal energy into a reservoir. The injected fluids will enhance the existing reservoir natural energy by the displacement of oil to a producing well. The mechanism of recovery enhancement involves the formed conditions caused by the interactions between injected fluids and oil resulting in lowering the interfacial tension, oil swelling, oil viscosity reduction, and wettability alteration. The selection of the suitable EOR method for implementation depends on the screening and the evaluation of reservoir properties and conditions as well as the economic feasibility (Green and Willhite 1998). Throughout the past 60 years, a major development has been made on chemical flooding that increased the potentiality of making it the most important EOR method (Demirbas et al. 2015). It was reported that chemical EOR has been successfully applied in many countries such as in the USA, China, Germany, France, Austria, and Canada. However, chemical flooding is an expensive recovery method because of the high cost of chemicals.
Chemical EOR application is divided into polymer flooding, surfactant flooding, and alkaline flooding and their combinations. The process of each chemical type is different, and the enhancement achieved by each type will influence the oil recovery by different mechanisms (Buchgraber et al. 2006; Dang et al. 2011). Surfactant flooding is known as the most promising methods among all chemical EOR processes. The mechanism of using surfactants during the surfactant flooding is mainly to reduce the interfacial tension, and for wettability alteration in order to increase the capillary number and to mobilize more oil toward the producing wells (Hirasaki and Zhang 2004). Several studies determined that most surfactants cannot be used in harsh reservoir conditions. Therefore, their poor performance at high-temperature and salinity conditions has led to developing new technologies, chemicals, and formulations in order to overcome these harsh conditions (Azam et al. 2013; Karnanda et al. 2013; Sheng 2015).
Surfactant flooding process encounters a significant loss of surfactant due to retention in the porous media (Amirianshoja et al. 2013). Surfactant retention is divided into precipitation, phase trapping, and adsorption. Surfactant retention due to precipitation and phase trapping can be avoided by choosing surfactants that are tolerant for temperature and salt. However, surfactant adsorption can be only minimized (Kamal et al. 2017; Liu et al. 2004). During the surfactant flooding process, the adsorption of surfactants from the injected slug may impact the effectiveness and the cost of the process (Amirianshoja et al. 2013). Usually, the cost of surfactants can reach half or more of the total project cost (Sheng 2011). Therefore, for an economic perspective, minimizing the amount of surfactant adsorption is a key point in designing surfactant flooding (Barati-Harooni et al. 2016). Surfactant adsorption can lead the surfactant flooding process to fail by affecting the performance of the surfactants which can influence the function of surfactants to lower the oil–water interfacial tension (Curbelo et al. 2007; Zargartalebi et al. 2015). Alkali is used in the alkaline–surfactant (AS) flooding to generate in situ surfactants which are formed from the chemical reaction between the alkali and acidic components in crude oil which can contribute in lowering the interfacial tension. In addition to that, alkali can increase the pH of the aqueous phase and minimize the surfactant used, and thus, it minimizes the surfactant adsorption which contributes to reducing the cost (Sheng 2011). This review will provide information on surfactant adsorption from recent studies to gain an in-depth understanding of all the factors affecting surfactant adsorption process as well as their mechanisms.
Surfactants
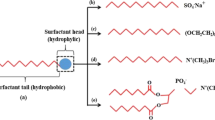
The term surfactant comes from surface-active agent, and surfactants are chemical compounds utilized to reduce the IFT between two different phases by adsorbing on a surface or a fluid–fluid interface. Surfactants are extensively used chemicals having various EOR applications due to their significance in IFTs reduction and their capability in changing wetting properties (Green and Willhite 1998). Surfactants are known as amphiphilic or amphipathic molecules which contain a polar (hydrophilic) portion and a nonpolar (hydrophobic or hydrocarbon loving) portion. The origin of the term amphiphilic comes from the Greek word “amphi,” meaning “both,” and this describes the fact that all surfactant molecules have at least two parts, the hydrophilic one which is soluble in a specific fluid, e.g., water, and the hydrophobic part which is insoluble in water (Tadros 2014).
According to the nature of the hydrophilic head group, surfactants are classified into different types, and this classification of surfactants is made based on the charges of the polar head group of the surfactant molecule. Surfactants are divided into the classes: anionics (negative charge), cationics (positive charge), nonionics (no charge), and zwitterionics (negative and positive charge) (Bera and Belhaj 2016; Tadros 2014).
Anionic surfactants
Anionic surfactants are known by having a negative charge on their head group when they are in aqueous solution. These surfactants are widely used in EOR processes, and this is due to (1) their relatively low cost of manufacture, (2) they exhibit relatively low adsorption on sandstone rocks whose surface charge is negative, (3) their efficiency to reduce IFT, (4) their stability at high temperatures (Tadros 2014). Anionic surfactants based on their head polar groups can be classified into carboxylate, sulfate, sulfonate, and phosphate (Kronberg et al. 2014).
Nonionic surfactants
Nonionic surfactants in aqueous solution do not have any charge on their head group, and they are mainly used as co-surfactant to improve the phase behavior of the surfactant. Nonionic surfactants are much more tolerant of high salinity. Nevertheless, their function of IFT reduction is less as compared to anionic surfactants which restrict them to be used as a primary surfactant in EOR applications. Therefore, a combination of anionic and nonionic is useful to increase the tolerance to salinity (Sheng 2011). The most widely used nonionic surfactants are those based on ethylene oxide (EO) known as ethoxylated surfactants (Holmberg et al. 2002).
Cationic surfactants
Cationic surfactants have positive charges on their head groups when they are in the aqueous phase, where they depend mainly on the atom of nitrogen to carry the charge (Kronberg et al. 2014). Cationic surfactants show high adsorption in sandstone reservoir and hence cannot be used for EOR application. However, these surfactants can be used for wettability alteration from oil wet to water wet in carbonate reservoir (Sheng 2011).
Zwitterionic surfactants
These surfactants consist of two opposite charge active groups. The zwitterionic surfactant can be anionic, nonionic, anionic–cationic, or nonionic–cationic. The positive charge group is always ammonium, and the most common negative charge group is carboxylate. They are also called amphoteric surfactants (Holmberg et al. 2002). These surfactants are tolerant of temperature and salinity. However, their high cost has quit a restriction (Bera and Belhaj 2016; Sheng 2011).
Surfactant flooding
Surfactants have a great potential in EOR applications, and they are used to enhance the recovery process efficiently by increasing the quantity of the residual oil extracted after secondary recovery process, which can possibly be around 60% of the original oil in place (Thomas and Ali 1999). Surfactant flooding is a chemical EOR method used for enhancing the oil recovery mechanism by recovering the capillary-trapped residual oil after waterflooding (Barati-Harooni et al. 2016). Surfactant flooding process depends on injecting surfactants to the reservoir along with injecting other chemicals. During surfactant flooding process, favorable phase behavior is targeted to achieve ultralow IFT between oil and water in order to mobilize the trapped oil (Sandersen 2012). The crude oil may contain organic acids, salts, alcohols, and other natural surface-active agents. Once crude oil is brought in contact with brine, these natural surfactants accumulate at the crude oil–brine interface and form an adsorbed film which lowers the interfacial tension of the crude oil–water interface (Olajire 2014). The main constraint influencing the surfactant flooding process is surfactant stability at reservoir conditions especially in high-temperature and high-salinity conditions. Other constraints include losses of the surfactants due to surfactant adsorption in the reservoir rock and trapping of the fluid in the pore structure (Sandersen 2012). These losses should be minimized where the successful implementation of surfactant flooding process depends mainly on the cost of surfactants (Hirasaki et al. 2008).
Surfactant flooding in EOR is divided into three types. The first type is micelle/polymer flooding where it can assist in achieving high displacement efficiency. The procedure involves injecting a slug containing surfactant, co-surfactant, alcohol, brine, and oil. The second type is microemulsion flooding, and it can be beneficial in high-temperature and high-salinity conditions. It is also useful for low-permeable zones where the polymer and/or alkali cannot operate. The main mechanism of this type is to reduce the IFT to an ultralow value by generating microemulsions in the reservoir. The injection slug in this process mainly consists of surfactants, co-surfactants, alcohol, and brine. The third type is the alkaline–surfactant–polymer (ASP) flooding. In this type, low IFT value is achieved by adding alkaline at low surfactant concentration, and this will contribute in cost reduction as lower surfactant concentration is used (Rosen et al. 2005; Sandersen 2012; Schramm 2000).
Surfactant losses
The success of surfactant flooding is subjected to the reduction in surfactant loss in the reservoir. The injected slug may witness a reduction in the surfactant concentration as it transports through the reservoir. Surfactant losses take place in the reservoir due to different mechanisms, i.e., surfactant adsorption, surfactant precipitation, surfactant degradation, surfactant polymer mixing, and surfactant partitioning in the residual oil phase (Donaldson et al. 1989). When surfactant slug comes in contact with the reservoir rock, adsorption of surfactant takes place on the rock surface. Due to adsorption, the surfactant concentration in the injected slug decreases and the amount remaining behind is insufficient to achieve ultralow IFT and to mobilize the trapped residual oil (Trushenski et al. 1974).
Surfactant adsorption
Surfactants adsorb onto solid surfaces as monomers rather than as micelles. Surface-active molecules can be adsorbed onto reservoir rocks from aqueous solutions by a number of mechanisms, i.e., ion exchange, ion association, hydrophobic bonding, adsorption by the polarization of π electrons, and adsorption by dispersion forces (Dang et al. 2011; Paria and Khilar 2004; Somasundaran and Huang 2000; Zhang and Somasundaran 2006). Surfactant adsorption during the surfactant flooding process is the most critical problem that can influence the success or failure of this process (Azam et al. 2014). Surfactant adsorption may occur on the rock surface due to the electrostatic interaction and van der Waals interactions that arise between the surfactant and solid surface (Kamal et al. 2017).
Generally, surfactant adsorption depends on many factors such as surfactant type, surfactant concentration, surfactant equivalent weight, ionic strength, pH, salinity, and temperature (Azam et al. 2014; Baviere et al. 1988; Paria and Khilar 2004; Siracusa and Somasundaran 1987). These factors can also influence the dissolution behavior of minerals, and therefore, it will cause significant changes in the adsorption of surfactants into the rock surface (Siracusa and Somasundaran 1987). In this review, we will discuss the effect of the main factors affecting surfactant adsorption which are: surfactant concentration, surfactant, salinity, temperature, and pH. Practically, surfactant adsorption can only be reduced to a certain limit due to the fact that it cannot be fully eliminated. The performance of the surfactant flooding process will be improved, and good recovery efficiency can be achieved only if the process is economically optimized by reducing surfactant adsorption (Park et al. 2015).
Surfactant concentration
Surfactant adsorption is a major factor that strongly affects the surfactant flooding process. Therefore, any reduction in surfactant concentration from the injected slug may decrease the surfactant efficiency to reduce oil–water IFT. This may lead the whole process to economic failure. Several studies discussed the effect of surfactant concentration on the adsorption of ionic and nonionic surfactants onto reservoir rocks. Based on the rock type, the rock surface charge is either negatively charged such as sandstone or positively charged such as carbonates. At low surfactant concentrations, surfactant adsorption is determined according to the charge on the electrical double layer of the solid surface. The adsorption of surfactant molecules at low concentrations on the rock surface occurs as a single monomer. When surfactant concentration increases, these monomers start to aggregate and associate among themselves to form micelles. Micelles are accumulated molecules where they usually contain 50 or more surfactant molecules (Bera et al. 2013a; Liu 2008; Li et al. 2011; Miura et al. 2013; Torn et al. 2003; Xu et al. 2008).
Anionic surfactants adsorption increases with increasing surfactant concentration. At low surfactant concentration below critical micelle concentration (CMC), the charge in the electrical double layer controls the extent of adsorption. This is described by the electrostatic interactions that arise between the surfactant head group and the net charge present on the solid surface. As surfactant concentration increases, lateral interactions will appear between the adsorbed surfactant molecules; it drives surfactant to aggregate the rock surface showing an increase in the adsorption density. When reaching CMC, any addition of surfactant will not have any effect on adsorption and it displays a plateau behavior. In this case, adsorption remains constant (Adak et al. 2005; Budhathoki et al. 2016; Kamal et al. 2017).
Boomgaard et al. (1987) explained the adsorption of nonionic surfactants. At low surfactant concentration, hydrogen bonding between the nonionic surfactant chain and the hydroxyl groups on the rock surface is the main mechanism of adsorption. Nonionic surfactants through hydrogen bonding adsorb as monomers. As surfactant concentration increases, micelles are formed due to the hydrophobic interactions which occur between the adsorbed monomers gathering at the liquid–rock interface (Curbelo et al. 2007).
Salinity
Salinity is one of the factors that influence surfactant adsorption. The most commonly used surfactants in chemical EOR are anionic surfactants. Usually, these surfactants are strongly influenced by adsorption on rock surfaces due to the presence of salt and divalent cations. Thus, it is a challenge to design surfactant formulations that are salinity and hardness resistant (Tabary 2013).
High-salinity water is not desirable for anionic surfactants due to the fact that it can precipitate resulting from the interaction between salt ions and the surfactant. On the other hand, increasing the salinity will reduce the repulsive forces arising between the anionic surfactant molecules and the rock surface (Azam et al. 2013; Kamal et al. 2017). This agrees with the experimental investigation done by Baviere et al. (1988) and Mannhardt et al. (1993).
The effect of salinity on the anionic surfactant adsorption at the solid–liquid interface was discussed by many researchers (Behrends and Herrmann 1998; Koopal et al. 1996; Nevskaia et al. 1998; Paria and Khilar 2004). The presence of salt improves the adsorption of anionic surfactants on a negatively charged solid surface. Koopal et al. (1996) explained the influence of ionic strength on the adsorption of anionic and cationic surfactants onto an oppositely charged solid surface. At low surfactant concentration, the initial adsorption occurs at low-salinity conditions. Attractions between the head group and the surface arise due to an increase in the ionic strength that causes adsorption to be reduced. As surfactant concentration increases, ionic strength rises which shows a decrease in mutual head group repulsion, and thus, adsorption is increased.
Salinity has also an impact on nonionic surfactants which it can change its solubility, surface activity, and adsorption at the solid–liquid interface (Paria and Khilar 2004; Rosen and Kunjappu 2012). Table 1 summarizes several studies that highlighted the effect of salinity on surfactant adsorption.
Temperature
Researchers initially explained the effect of temperature on surfactant adsorption where adsorption is generally an exothermic process. They indicated that the increase in temperature leads to a considerable decrease in the adsorption of surfactants due to an increase in the kinetic energy of the species (Fava and Eyrin 1955; Hartman et al. 1946; Somasundaran and Fuerstenau 1972; Ziegler and Handy 1981). Kulkarni and Somasundaran (1976) addressed the effect of ionic strength and temperature on the adsorption of surfactants. Adsorption increases with the increase in temperature at low ionic strength while it decreases at high ionic strength with the temperature decrease (Ziegler and Handy 1981). The effect of temperature on surfactant adsorption depends on the adsorption density. The process of surfactant adsorption can be either enthalpy driven or entropy driven (Hirasaki and Zhang 2003). For surfactants with low adsorption density (enthalpy-driven adsorption), when the temperature increases, it causes the adsorption density to increase. Meanwhile, adsorption density is reduced with temperature increase for surfactants with high adsorption density (entropy-driven adsorption) (Kamal et al. 2017).
Surfactant flooding is commonly operated under low-temperature and low-salinity conditions. Anionic and nonionic surfactants are the most favorable surfactants to be used in these conditions. On the other hand, at high-temperature and high-salinity conditions, anionic surfactants show low slat resistance (Kamal et al. 2018).
For nonionic surfactants generally, adsorption increases with increasing temperature. This was proposed by Corkill et al. (1966), and they found that adsorption of surfactant molecules at different temperatures increases as temperature increases. The increase in temperature affects the surfactant’s head group making it compact and less hydrophilic, therefore increasing the surface activity and adsorption values. Nonionic surfactants behavior was deeply investigated at various temperatures where it was also suggested that at low surfactant concentrations, adsorption of nonionic surfactants is reduced as temperature increases. Meanwhile, at high surfactant concentrations, the opposite is correct (Ziegler and Handy 1981).
Puerto et al. (2010) explained that reservoirs with temperatures varying from 70 to 120 °C are suitable candidates for surfactant flooding. However, high-temperature reservoir conditions can affect the stability of surfactants to operate for the period of the project which could be for years. Sheng (2015) discussed that most researchers consider 93.3 °C as reservoir temperature limit even though specific surfactants can be applied at high-temperature reservoirs up to 150 °C. These surfactants could be stable under such conditions. However, they must be also applicable corresponding to other conditions as well such as low adsorption which can contribute to minimizing the cost. Table 2 summarizes several studies that highlighted the effect of temperature on surfactant adsorption.
pH
The pH has a major influence on surfactant adsorption where the charge of solid surfaces varies with the change of pH. Surfactant adsorption magnitude differs at different pHs depending on the surfactant charge which interacts with the charges available at the surface. As the pH of the surfactant solution increases, it reduces the number of hydroxyl groups in the surface affecting the formation of hydrogen bonding. This makes hydrated mineral oxides on the solid surface to be negatively charged. At lower pH, surfactant solution drives the mineral hydroxyl to acquire a positive charge which increases adsorption of surfactants by attracting the negatively charged surfactant molecules to the rock surface. Berea sandstone, e.g., contains mainly silica oxides, and by increasing pH from a low value (acid condition) to the medium pH (5–7) or higher pH (base condition), it imposes an increase in the negative charges of the rock surface (Azam et al. 2013; Hanamertani et al. 2017; Lv et al. 2011).
Surface charges that exist on surfactants as well as the rock surface have a direct effect on the surfactant adsorption. Anionic surfactants carry negative charge where cationic surfactants carry a positive charge and they are by default attracted to positively charged surfaces and negatively charged surfaces, respectively. Surface charges are intensely affected by salinity and pH of the formation brine. Generally, if brine chemistry effect is neglected and water is at neutral pH, anionic surfactants have a tendency for adsorption on carbonates due to the existence of positive charges (basic state) on the rock surface, while cationic surfactants have a tendency for adsorption on sandstone due to the existence of negative charges (acidic state) on the rock surface (Bera et al. 2013b; Harkot and Jańczuk 2009; Wei et al. 2012).
Another important factor that affects the surface charge of the rock is the solution pH. The rock surface charge density depends on the pH variation while in contact with the surfactant solution. The pH value at zero surface charge density on the surface is named the point of zero charges (PZC) (Grigg et al. 2004; Mushtaq et al. 2014).
Generally, the use of alkali in surfactant flooding is to generate in situ soap from its reaction with crude oil components so that the amount of the injected surfactant can be reduced. Therefore, it reduces the surfactant concentration to be used and that will decrease operating costs and significantly improves the profit of chemical flooding projects. Alkali is also used as a chemical agent to reduce surfactant adsorption on the rock surface by increasing the pH of the medium to enhance surfactant stability. In the case of anionic surfactants which their head group structure is negatively charged, when alkali is used it increases the pH of the environment and that will generate a strong electrostatic repulsive force between the surfactant and the reservoir rock surface, and thereby surfactant adsorption is significantly reduced (Dang et al. 2011). However, the influence of alkali in lowering adsorption of anionic surfactants is limited to reservoirs with low salinity/hardness, due to the fact that alkali is sensitive to divalent cations Ca2+ and Mg2+, which reduces its effectiveness and drives it to precipitate. On the other hand, cationic surfactants which their head group is positively charged are usually used for positively charged carbonate reservoirs (ShamsiJazeyi et al. 2013).
The most common used alkali is sodium carbonate where it consumes the multivalent cations that cause surfactant to precipitate. Sodium metaborate is another alkali which is used in high-salinity conditions due to its ability to sustain under high-salinity conditions (Flaaten et al. 2010). Sodium polyacrylate is introduced as a sacrificial agent as it can be used to reduced surfactant adsorption on dolomite (ShamsiJazeyi et al. 2013). Table 3 summarizes several studies that highlighted the effect of pH on surfactant adsorption.
Conclusion
Adsorption of surfactants on the rock surfaces may result in a reduction in their concentrations that may possibly reduce their efficiency and affect their performance in practical EOR applications. Reduction in IFT of the oil–water–rock system to an ultralow value is the main function of surfactants. However, surfactant loss due to adsorption impairs their effectiveness and may turn the process to be economically unfeasible. Reducing the amount of surfactant adsorption is necessary to avoid the failure of the whole process. This review highlights the influence of surfactant concentration, salinity, temperature, and pH on surfactant adsorption. Adsorption increases with increasing surfactant concentration. When a surfactant is at a concentration below the CMC, surface aggregation occurs and a large increase in the adsorption is noticed. However, at concentrations above the CMC, an increase in the concentration has no effect on the adsorption behavior. It was also found that salinity has an influence on surfactant adsorption due to the interactions that arise between salt ions and the surfactant molecules. Increasing reservoir brine salinity increases the adsorption of surfactants on rock surfaces due to the decrease in the repulsive forces between adsorbed molecules. The increase in temperature generally leads to a considerable decrease in the adsorption of surfactants due to an increase in the kinetic energy of the species. The pH effect on surfactant adsorption is significant where surfactant adsorption magnitude varies at different pHs depending on the surfactant charge which interacts with the charges available at the surface. Most of the research has been conducted in low-temperature and low-salinity conditions. Only limited studies were conducted in high-temperature and high-salinity (HT/HS) conditions due to the implementation challenges of surfactant flooding in these conditions.
References
Adak A, Bandyopadhyay M, Pal A (2005) Adsorption of anionic surfactant on alumina and reuse of the surfactant-modified alumina for the removal of crystal violet from aquatic environment. J Environ Sci Health 40(1):167–182
Amirianshoja T, Junin R, Idris AK, Rahmani O (2013) A comparative study of surfactant adsorption by clay minerals. J Pet Sci Eng 101:21–27
Azam MR, Tan IM, Ismail L, Mushtaq M, Nadeem M, Sagir M (2013) Static adsorption of anionic surfactant onto crushed Berea Sandstone. J Pet Explor Prod Technol 3(3):195–201
Azam MR, Tan IM, Ismail L, Mushtaq M, Nadeem M, Sagir M (2014) Kinetics and equilibria of synthesized anionic surfactant onto Berea Sandstone. J Dispers Sci Technol 35(2):223–230
Barati-Harooni A, Najafi-Marghmaleki A, Tatar A, Mohammadi AH (2016) Experimental and modeling studies on adsorption of a nonionic surfactant on sandstone minerals in enhanced oil recovery process with surfactant flooding. J Mol Liq 220:1022–1032
Bataweel MA, Nasr-El-Din HA (2012) ASP vs. SP flooding in high salinity/hardness and temprature in sandstone cores. SPE 15567 79
Baviere M, Bazin B, Noik C (1988) Surfactants for EOR: olefin sulfonate behavior at high temperature and hardness. SPE Reserv Eng 3(2):597–603
Behrends T, Herrmann R (1998) Partitioning studies of anthracene on silica in the presence of a cationic surfactant: dependency on pH and ionic strength. Phys Chem Earth 23(2):229–235
Bera A, Belhaj H (2016) Ionic Liquids as alternatives of surfactants in enhanced oil recovery—a State-of-the-Art review. J Mol Liq 224:177–188
Bera A, Mandal A (2015) Microemulsions: a novel approach to enhanced oil recovery: a review. J Pet Explor Prod Technol 5(3):255–268
Bera A, Kumar T, Ojha K, Mandal A (2013a) Adsorption of surfactants on sand surface in enhanced oil recovery: isotherms, kinetics and thermodynamic studies. Appl Surf Sci 284:87–99
Bera A, Kumar T, Ojha K, Mandal A (2013b) Adsorption of surfactants on sand surface in enhanced oil recovery: isotherms, kinetics and thermodynamic studies. Appl Surf Sci 284(March):87–99
Boomgaard TVD, Tadros T, Lyklema J (1987) Adsorption of nonionic surfactants on latices and silica in combination with stability studies. J Colloid Interface Sci 116(1):8–16
Buchgraber M, Clemens T, Omv E, Castanier LM, Kovscek AR (2006) The displacement of viscous oil by associative polymer solutions. SPE 122400
Budhathoki M, Barnee SHR, Shiau BJ, Harwell JH (2016) Improved oil recovery by reducing surfactant adsorption with polyelectrolyte in high saline brine. Colloids Surf A 498:66–73
Corkill JM, Goodman JF, Tate JR (1966) Adsorption of non-ionic surface-active agents at the graphon/solution interface. Trans Faraday Soc 62:979–986
Curbelo Fabíola D S, Santanna VC, Neto ELB, Dutra TV, Dantas TNC, Neto Afonso A Danta, Garnica Alfredo I C (2007) Adsorption of nonionic surfactants in sandstones. Colloids Surf A 293(1–3):1–4
Dang CTQ, Chen Z, Nguyen NTB, Bae W, Phung TH (2011) Development of isotherm polymer/surfactant adsorption models in chemical flooding. SPE Asia pacific oil and gas conference and exhibition Held in Jakarta, Indonesia (SPE 147872)
Demirbas A, Alsulami HE, Hassanein WS (2015) Utilization of surfactant flooding processes for enhanced oil recovery (EOR). Pet Sci Technol 33(12):1331–1339
Denoyel R, Rouquerol J (1991) Thermodynamic (including microcalorimetry) study of the adsorption of nonionic and anionic surfactants onto silica, kaolin, and alumina. J Colloid Interface Sci 143(2):555–572
Donaldson EC, Chilingarian GV, Yen TF (1989) Enhanced oil recovery, II: Processes and operations. Elsevier
Elraies KA (2012) An experimental study on ASP process using a new polymeric surfactant. J Pet Explor Prod Technol 2(4):223–227
Fava A, Eyrin H (1955) And kinetics of detergent adsorption- a generalized equilibration theory. 627(1951):890–898
Flaaten A, Nguyen Q, Zhang J, Mohammadi H, Pope G (2010) Alkaline/surfactant/polymer chemical flooding without the need for soft water. SPE J 15(1):184–196
Green DW, Willhite GP (1998) Enhanced oil recovery, vol 6. Henry L. Doherty Memorial Fund of AIME, Society of Petroleum Engineers Richardson, Richardson
Grigg RB, Baojun B, Yi L (2004) Competitive adsorption of a hybrid surfactant system onto five minerals, Berea Sandstone, and limestone. In: SPE annual technical conference and exhibition (1)
Han M, Alsofi A, Fuseni A, Zhou X, Hassan S, Aramco S (2013) Development of chemical EOR formulations for a high temperature and high salinity carbonate reservoir. Iptc 1–13
Hanamertani AS, Pilus RM, Idris AK, Irawan S, Tan IM (2017) Ionic liquids as a potential additive for reducing surfactant adsorption onto crushed Berea Sandstone. J Pet Sci Eng 162:480
Harkot J, Jańczuk B (2009) The role of adsorption of dodecylethyldimethylammonium bromide and benzyldimethyldodecylammonium bromide surfactants in wetting of polytetrafluoroethylene and poly(methyl methacrylate) surfaces. Appl Surf Sci 255(6):3623–3628
Hartman RJ, Kernt RA, Bobalek EG (1946) Adsorption isotherms of some substituted benzoic acids. Colloid Laboratory, Indiana University Bloomington, Bloomington
Hirasaki GJ, Zhang DL (2003) Surface chemistry of oil recovery from fractured, oil-wet, carbonate formation. SPE international symposium on oilfield chemistry held in Houston, Texas, USA (SPE 80988)
Hirasaki G, Zhang DL (2004) Surface chemistry of oil recovery from fractured, oil-wet, carbonate formations. SPE J 9(02):151–162
Hirasaki GJ, Miller CA, Puerto M (2008) SPE 115386 recent advances in surfactant EOR. (September):21–24
Holmberg K, Bo J, Kronberg B (2002) Surfactants and polymers in aqueous solution. Wiley
Ingrid H (2014) Investigation of adsorption of surfactants onto illite and relations to enhanced oil recovery methods. Norwegian University of Science and Technology (NTNU), Trondheim
Kamal MS, Hussein IA, Sultan AS (2017) Review on surfactant flooding: phase behavior, retention, ift, and field applications. Energy Fuels 31(8):7701–7720
Kamal MS, Hussain SMS, Fogang LT (2018) A Zwitterionic surfactant bearing unsaturated tail for enhanced oil recovery in high-temperature high-salinity reservoirs. J Surfactants Deterg 21(1):165–174
Karnanda W, Benzagouta MS, AlQuraishi A, Amro MM (2013) Effect of temperature, pressure, salinity, and surfactant concentration on IFT for surfactant flooding optimization. Arab J Geosci 6(9):3535–3544
Koopal LK, Lee EM, Bohmer MR (1996) Adsorption of cationic and anionic surfactants on metal oxide surfaces. J Colloid Interface Sci 177(2):478–489
Kronberg B, Holmberg K, Lindman B (2014) Surface chemistry of surfactants and polymers. Wiley, Hoboken
Krumrine PH, Falcone JS, Campbell TC (1982) Surfactant flooding 1: the effect of alkaline additives on ift, surfactant adsorption, and recovery efficiency. Soc Petrol Eng J 22(06):983–992
Kulkarni RD, Somasundaran P (1976) Mineralogical heterogeneity of ore particles tid its effects on their interfacial characteristics. 14:279–285
Li P, Ishiguro M (2016) Adsorption of anionic surfactant (sodium dodecyl sulfate) on silica. Soil Sci Plant Nutr 62(3):223–229
Li N, Zhang G, Ge J, Luchao J, Jianqiang Z, Baodong D, Pei H (2011) Adsorption behavior of betaine-type surfactant on quartz sand. Energy Fuels 25(10):4430–4437
Li Y, Zhang W, Kong B, Puerto M, Xinning Bao O, Sha ZS, Yang Y, Liu Y, Songyuan G, Miller C, Hirasaki GJ (2016) Mixtures of anionic/cationic surfactants: a new approach for enhanced oil recovery in low-salinity, high-temperature sandstone reservoir. SPE J 21(04):1164–1177
Lin W, Li X, Yang Z, Wang J, You Q, Zhang Y, He Y (2017) A laboratory study of surfactant flooding system for tertiary recovery in high-temperature and high-salinity reservoirs. J Residuals Sci Technol 14(Supplement 1):S125–S131
Liu S (2008) Alkaline surfactant polymer enhanced oil recovery process. Ph.D. Thesis, Rice University, p 242
Liu Q, Dong M, Zhou W, Ayub M, Zhang YP, Huang S (2004) Improved oil recovery by adsorption–desorption in chemical flooding. J Petrol Sci Eng 43(1–2):75–86
Lu J, Liyanage PJ, Solairaj S, Adkins S, Arachchilage GP, Kim DH, Britton C, Weerasooriya U, Pope GA (2014) Journal of petroleum science and engineering new surfactant developments for chemical enhanced oil recovery. J Petrol Sci Eng 120:94–101
Lv W, Bazin B, Ma D, Liu Q, Han D, Kangyun W (2011) Static and dynamic adsorption of anionic and amphoteric surfactants with and without the presence of alkali. J Petrol Sci Eng 77(2):209–218
Mannhardt K, Schramm LL, Novosad JJ (1993) Effect of rock type and brine composition on adsorption of two foam-forming surfactants. SPE Adv Technol Ser 1(01):212–218
Miura K, Li B, Tomita A, Ahmadi MA, Shadizadeh SR (2013) Experimental investigation of adsorption of a new nonionic surfactant on carbonate minerals. Fuel 104:462–467
Mushtaq M, Tan IM, Ismail L, Nadeem M, Sagir M, Azam R, Hashmet R (2014) Influence of PZC (point of zero charge) on the static adsorption of anionic surfactants on a Malaysian Sandstone. J Dispers Sci Technol 35(3):343–349
Mushtaq M, Tan IM, Rashid U, Sagir M, Mumtaz M (2015) Effect of PH on the static adsorption of foaming surfactants on Malaysian Sandstone. Arab J Geosci 8(10):8539–8548
Nevskaia DM, Guerrero-Ruíz A, López-González JDD (1998) Adsorption of polyoxyethylenic nonionic and anionic surfactants from aqueous solution: effects induced by the addition of NaCl and CaCl2. J Colloid Interface Sci 205(1):97–105
Olajire AA (2014) Review of ASP EOR (alkaline surfactant polymer enhanced oil recovery) technology in the petroleum industry: prospects and challenges, p 77
Paria S, Khilar KC (2004) A review on experimental studies of surfactant adsorption at the hydrophilic solid–water interface. Adv Colloid Interface Sci 110(3):75–95
Park S, Lee ES, Sulaiman WRW (2015) Adsorption behaviors of surfactants for chemical flooding in enhanced oil recovery. J Ind Eng Chem 21:1239–1245
Partyka S, Lindheimer M, Faucompre B (1993) Aggregate formation at the solid–liquid interface: the calorimetric evidence. Colloids Surf A 76(C):267–281
Puerto M, Hirasaki GJ, Miller CA, Barnes JR (2010) Surfactant systems for EOR in high-temperature, high-salinity environments. SPE J 17(01):11–19
Puerto MC, Lopez-Salinas JL, Jian G, Hirasaki GJ, Miller CA (2018) Laboratory studies of ternary surfactant formulation for EOR in oil-wet, high-temperature carbonate formations. IN: SPE improved oil recovery conference
Rosen MJ, Kunjappu JT (2012) Surfactants and interfacial phenomena, 4th edn. Wiley
Rosen MJ, Wang H, Shen P, Zhu Y (2005) Ultralow interfacial tension for enhanced oil recovery at very low surfactant concentrations. Langmuir 21(9):3749–3756
Sandersen SB (2012) Enhanced oil recovery with surfactant flooding. Technical University of Denmark “Danmarks Tekniske Universitet”, Center for Energy Resources Engineering, Ph.D. Thesis, pp 1–162
Schramm LL (2000) Surfactants: fundamentals and applications in the petroleum industry. Press Syndicate of the University of Cambridge, Cambridge University Press, Cambridge
ShamsiJazeyi H, Verduzco R, Hirasaki GJ (2013) Reducing adsorption of anionic surfactant for enhanced oil recovery: part I. Competitive adsorption mechanism. Colloids Surf A 453:162
ShamsiJazeyi H, Verduzco R, Hirasaki GJ (2014) Reducing adsorption of anionic surfactant for enhanced oil recovery: part II. Applied aspects. Colloids Surf A 453:162
Sheng J (2011) Modern chemical enhanced oil recovery: theory and practice. Gulf Professional Publishing, Houston
Sheng JJ (2015) Status of surfactant EOR technology. Petroleum 1(2):97–105
Siracusa PA, Somasundaran P (1987) The role of mineral dissolution in the adsorption of dodecylbenzenesulfonate on kaolinite and alumina. Colloids Surfaces 26(C):55–77
Somasundaran P, Fuerstenau DW (1972) Heat and entropy of adsorption and association of long-chain surfactants at the alumina-aqueous solution interface. Trans SME AIME 252:275–279
Somasundaran P, Huang L (2000) Adsorption/aggregation of surfactants and their mixtures at solid–liquid interfaces. Adv Coll Interface Sci 88(1–2):179–208
Tabary R, et al (2013) Surfactant flooding in challenging conditions: towards hard brines and high temperatures. In: SPE middle east oil and gas show and conference. Society of Petroleum Engineers
Tadros TF (2014) An Introduction to surfactants. Walter de Gruyter GmbH, Berlin/Boston
Tagavifar M, Jang SH, Sharma H, Wang D, Chang LY, Mohanty K, Pope GA (2018) Effect of PH on adsorption of anionic surfactants on limestone: experimental study and surface complexation modeling. Colloids Surf A 538:549–558
Thomas S, Ali SMF (1999) Micellar flooding and ASP – chemical methods for enhanced oil recovery. CSPG and petroleum society joint convention, Calgary, Alberta, Canada
Torn LH, De Keizer A, Koopal LK, Lyklema J (2003) Mixed adsorption of poly(vinylpyrrolidone) and sodium dodecylbenzenesulfonate on kaolinite. J Colloid Interface Sci 260(1):1–8
Trushenski SP, Dauben DL, Parrish (1974) Micellar flooding-fluid propagation, interaction, and mobility. Amoco Production Co, Tulsa
Wei XL, Wang XH, Liu J, Sun DZ, Yin BL, Wang XJ (2012) Adsorption kinetics of 3-alkoxy-2-hydroxypropyl trimethyl ammonium chloride at oil–water interface. Appl Surf Sci 261:237–241
Xu Z, Yang X, Yang Z (2008) On the mechanism of surfactant adsorption on solid surfaces: free-energy investigations. J Phys Chem B 112(44):13802–13811
Yekeen N, Manan MA, Idris AK, Samin AM (2016) Influence of surfactant and electrolyte concentrations on surfactant adsorption and foaming characteristics. J Petrol Sci Eng 149(November):612–622
Yuan CD, Wan Fen P, Wang XC, Lin Sun Yu, Zhang C, Cheng S (2015) Effects of interfacial tension, emulsification, and surfactant concentration on oil recovery in surfactant flooding process for high temperature and high salinity reservoirs. Energy Fuels 29(10):6165–6176
Yuan T, Liu Z, Gao R, Guangfa H, Zhang G, Zhao J (2018) Enhanced oil recovery from high-salinity reservoirs by cationic Gemini surfactants. J Appl Polym Sci 135(14):1–7
Zaitoun A, Fonseca C, Berger P (2003) “Zaitoun - New Surfactant for Chemical Flood in High-Salinity Reservoir - SPE-80237-MS”
Zargartalebi M, Kharrat R, Barati N (2015) Enhancement of surfactant flooding performance by the use of silica nanoparticles. Fuel 143(November):21–27
Zhang R, Somasundaran P (2006) Advances in adsorption of surfactants and their mixtures at solid/solution interfaces. Adv Colloid Interface Sci 123–126(SPEC. ISS.):213–229
Zhang F, Ma D, Wang Q, Zhu Y, Luo W, Society of Petroleum Engineers (US) (2013) IPTC 17022 a novel hydroxylpropyl sulfobetaine surfactant for high temperature and high salinity reservoirs. In: International petroleum technology conference, vol 6. Curran Associates, inc., Red Hook, pp 4554–4561
Zhou M, Zhao J, Wang X, Yang Y (2013) Research on surfactant flooding in high-temperature and high-salinity reservoir for enhanced oil recovery. Tenside, Surfactants, Deterg 50(3):175–181
Ziegler VM, Handy LL (1981) Effect of temperature on surfactant adsorption in porous media. Society of Pet Eng J 21:218–228
Acknowledgements
The authors gratefully acknowledge Universiti Teknologi PETRONAS (UTP) for providing financial support through the Graduate Research Assistantship (GRA) Scheme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Belhaj, A.F., Elraies, K.A., Mahmood, S.M. et al. The effect of surfactant concentration, salinity, temperature, and pH on surfactant adsorption for chemical enhanced oil recovery: a review. J Petrol Explor Prod Technol 10, 125–137 (2020). https://doi.org/10.1007/s13202-019-0685-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-019-0685-y