Abstract
In this study, deposition of a Ni3P nano-scale layer on B4C nanoparticles via simple electroless plating in an acidic bath was investigated. B4C nanoparticles were produced by mechanical milling with the average size of about 95 nm. Electroless nickel plating was carried out at temperature and pH of 85°C and 5.5, respectively. The uncoated and composite powders were characterized by transition electron microscope and the phases present were revealed by X-ray diffraction. Also, nickel and phosphorous contents of the coating were measured by inductively coupled plasma analysis. The results confirmed deposition of a Ni3P layer with the average thickness of about 25 nm on B4C nanoparticles.
Similar content being viewed by others
Introduction
Ceramic particles like B4C, Al2O3, SiC and MgO in nanometric scale have absorbed attractions to be used as reinforcements since they reduce interparticle spacing resulting in increased mechanical properties (Ansary Yar et al. 2009).
Various mechanical methods like high energy processes such as planetary, attrition and jet milling are available to prepare ultra fine particles (Aparecida et al. 2006). Attrition mill has been widely used for fine grinding of different materials. The main advantages of attrition mills are relatively high energy utilization, fast and efficient fine grinding and simple operation (Shinohara et al. 1999).
Boron carbide has a low density, high hardness and offers distinct advantages such as neutron absorption, wear resistance and impact resistance (Lee et al. 2001). These unique properties make B4C a proper candidate to fabricate MMCs especially Al matrix composites. One of the major problems to do this is poor wettability of ceramic particles like B4C with the matrix (Kerti and Toptan 2008). To overcome this problem, there are several ways including: (i) adding surface-active elements into the matrix; (ii) increasing metal liquid temperature; (iii) oxidizing or coating the ceramic particles; (iv) using inert or reactive gases during production; and (v) changing the chemical composition of the matrix alloy (Hashim et al. 2001; Tekmen and Cocen 2006; Tekmen et al. 2007). Therefore, one of the most important procedures to enhance wettability is deposition of a metallic or alloy coating on ceramic particles. One way to do this is through electroless plating of metals on ceramic particles. Electroless plating has been used in preparation of low dimensional nanostructured materials. Some results have been reported to deposit metals or metallic compounds on the surface of carbon nanotubes, nano and micron particles of SiC, and Al2O3 powders, etc. (Kong et al. 2002; Chen et al. 2003; Chang and Lin 1996; Zhang et al. 2003).
The reduction of nickel during electroless plating is a simple reaction. It is clear that electroless nickel plating consists of autocatalytic deposition of Ni–P alloy from an aqueous solution with no usage of external current (Abrantes and Correia 1994; Ashassi-Sorkhabi and Rafizadeh 2004). The characteristics of the alloy coating expand the physical properties beyond those of pure nickel coating. These unique properties include corrosion and wear resistance (Sankara Narayanan and Seshadri 2004), hardness, lubricity, uniformity of deposit regardless of geometries and nonmagnetic properties (Ramalho and Miranda 2005).
The major novelty of the present study is that there are few reports on deposition of a metallic or compound coating on the nano-scale ceramic particles and no reports on formation of such a coating on micron or nano-scale B4C particles. Due to agglomeration of nanoparticles, deposition of a coating layer on these particles is very difficult. On the other hand, the product of this study can be considered as a nanocomposite powder which can be used as modern nano-scale reinforcement in fabrication of MMCs especially Al matrix composites with better wettability of the ceramic reinforcement with the metallic matrix. In this study, deposition of a Ni3P nano-scale layer on B4C nanoparticles via simple electroless in an acidic bath was carried out.
Experimental
B4C nanoparticles were synthesized by milling of original B4C powders with the mean size of 0.8 μm in an attrition mill (union process, model 1-S) using a hardened stainless steel vial and hardened stainless steel balls with 6 mm in diameter. The ball to powder ratio and rotational speed were 15:1 and 400 rpm, respectively. Isopropyl alcohol was used as milling media and the grinding media occupied 80% of the chamber volume. The final mean size of the attrition-milled particles was about 95 nm after 140 h milling.
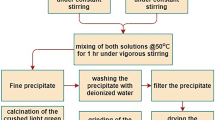
The process of electroless plating consists of two stages: pretreatment of B4C nanopowder and electroless plating.
Pretreatment of boron carbide nanopowder
The pretreatment of B4C particles was carried out according to the following procedure:
-
1.
Cleaning by immersing in 150 ml acetone with ultrasonication for 15 min.
-
2.
Cleaning and etching in 150 ml HNO3 with ultrasonication for further 15 min.
-
3.
Sensitization in a solution containing 10 g/l of SnCl2 and 40 ml/l HCl for 20 min.
-
4.
Activation in a solution of 0.25 g/l of PdCl2 and 2.5 ml/l of HCl for 20 min.
-
5.
Drying at 100°C.
Between the above mentioned stages, complete rinsing was required to prevent contamination of solutions.
Electroless plating
Electroless plating of nickel was performed by introducing about 0.5 g of the pretreated B4C nanopowder into an electroless plating bath using composition given in Table 1. To expose all of the particles to the electroless nickel solution, continuous stirring was done at 200 rpm. The bath operation started under acidic conditions; the particles were plated at 85°C. Since this temperature has been used as bath temperature in some previous studies (Palaniappa et al. 2007; Kretz et al. 2004; Pai and Rohatgi 1975; Tekmen and Cocen 2008; Zhang et al. 2007a, b) we selected 85°C as the plating temperature. It has been reported that the higher the plating temperature, the more the rate of plating and deposited layer but the temperatures higher than 90°C may lead to bath instability and fail to deposit a coating layer on the particles (Krishnan et al. 2006).
The bath pH measured to be 5.5 and the plating time was 25 min.
The as-received and Ni3P-deposited B4C powders were characterized by X-ray diffraction (Siemens X-ray diffractometer, 30 kV and 25 A), scanning electron microscope (SEM-Philips XL 30) and transition electron microscope (TEM- Hitachi H 800). Also, to determine the composition of the coating layer, inductively coupled plasma analysis (Liberty-RL ICP Varian) was carried out. In order to obtain a more trustable data, the analysis was repeated two more times and the average value was presented in the article.
Results and discussion
Morphology and particle size distribution of B4C nanoparticles
As-received B4C powder with the mean particle size of about 0.8 micron was used as the starting material. SEM micrographs of as-received powder reveal that the powder has a wide size distribution and irregular shape Fig. 1a.
Figure 1b shows the variation of particle size distribution and median size (D50) as a function of milling time measured by particle size analyzer. By increasing the milling times, decrease in particle size and a narrow sized distribution can be seen.
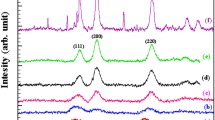
During milling, a combination of fracture mechanisms occurs which include abrasion, compression and impact mechanism. The particle size distribution of a material after grinding will be determined by the combination of all mechanisms. The predominant mechanism is determined according to material properties and operating conditions. Since hard materials are difficult to be abraded on their surfaces, the impact mechanism plays the dominant role, thus, the distribution curve shows a monomodal distribution curve. This result is consistent with that reported regarding grinding of diamond by a researcher (Shinohara et al. 1999). Figure 1c presents the TEM image of the nanoparticles. The nanoparticles are spherical and have a uniform distribution. The XRD pattern, shown in Fig. 2 a, reveals that the attrition-milled nanopowder consists B4C phase with no major impurities.
Ni3P-deposited powder
The mechanism of reduction of nickel ion by hypophosphite has been under constant revision in recent years. The following partial equations occur during electroless nickel plating process in a hypophosphite bath according to the reports of a study (Krishnan et al. 2006):
Therefore, nickel and phosphorous are reduced in the bath simultaneously. The reduced nickel reacts with the reduced phosphorous in the following reaction:
According to reaction (4), an alloy layer of nickel and phosphorous is formed on the particles. This is confirmed by the XRD pattern of the electroless-coated powder in Fig. 2b. The XRD patterns of attrition-milled B4C powder and coated powder are shown in Fig. 2. In Fig. 2a, only diffraction peaks of B4C can be observed but in Fig 2b, peaks of Ni3P can be seen in addition to B4C peaks.
Although in some studies (Krishnan et al. 2006, Ebrahimian-Hosseinabadi et al. 2006), it has been reported that the coating deposited through electroless plating is amorphous and the associated XRD peaks turn up only after heat treatment of the product at 400°C for 1 h, but it should be mentioned that according to results reported by a research (Vafaei-Makhsoos et al. 1978), electroless coatings containing up to 5 wt. % phosphorous are crystalline. Thus, phosphorous content of the coating determines the structure of the deposited coating. Table 2 shows the results of ICP analysis which confirms that the phosphorous content of the deposited layer in the present study is below 5 wt. %.
The transition electron microscope images of the as-received and Ni3P-deposited powders prove deposition of a coating on B4C nanoparticles. It is clear that the particles are sphere-like. Figure 3 reveals the Ni3P-deposited nanoparticles by electroless nickel plating with two different magnifications. From Fig. 3b, the average thickness of the nickel phosphide coating layer can be estimated as 25 nm. However, because of high chemical activity of nanoparticles (Zhang et al. 2007a, b), agglomerates of the powder have been formed.
Conclusion
Deposition of a Ni3P nano-scale layer on B4C nanoparticles via simple electroless plating was performed at 85°C, pH 5.5, plating time of 25 min and sodium hypophosphite content of 15 g/l. B4C nanoparticles were produced by mechanical milling. ICP analysis of Ni3P-deposited particles showed the existence of nickel and phosphorus and the XRD pattern of the product confirmed the presence of these two elements in the coating in the form of Ni3P. TEM images of the product confirmed this conclusion by showing a coating layer of about 25 nm in thickness.
References
Abrantes LM, Correia JP (1994) On the mechanism of Ni-P electroless plating. J Electrochem Soc 141:2356–2360
Ansary Yar A, Montazerianb M, Abdizadeh H, Baharvandi HR (2009) Microstructure and mechanical properties of aluminum alloy matrix composite reinforced with nano-particle MgO. J Alloy Compd 484:400–404
Aparecida M, Dos Santos P, Costa CA (2006) Comminution of silicon carbide powder in a planetary mill. Powder Technol 169:84–88
Ashassi-Sorkhabi H, Rafizadeh SH (2004) Effect of coating time and heat treatment on structures and corrosion characteristics of electroless Ni–P alloy deposits. Surf Coat Technol 176:318–326
Chang SY, Lin SJ (1996) Fabrication of SiCw reinforced copper matrix composite by electroless copper plating. Scr Mater 35(2):225–231
Chen Y, Cao M, Xu Q, Zhu J (2003) Electroless nickel plating on silicon carbide nanoparticles. Surf Coat Technol 172(1):90–94
Ebrahimian-Hosseinabadi M, Azari-Dorcheh K, Moonir Vaghefi SM (2006) Wear behavior of electroless Ni–P–B4C composite coatings. Wear 260:123–127
Hashim J, Looney L, Hashmi MSJ (2001) The wettability of SiC particles by molten aluminum alloy. J Mater Process Tech 119:324–328
Kerti I, Toptan F (2008) Microstructural variations in cast B4C-reinforced aluminium matrix composites (AMCs). Mater Lett 62:1215–1218
Kong FZ, Zhang XB, Xiong WQ, Liu F, Huang WZ, Sun YL, Tu JP, Chen XW (2002) Continuous Ni-layer on multiwall carbon nanotubes by an electroless plating method. Surf Coat Technol 155(1):33–36
Kretz F, Gacsi Z, Pieczonka T (2004) The electroless deposition of nickel on SiC particles for aluminum matrix composites. Surf Coat Tech 180–181:575–579
Krishnan KH, John S, Srinivasan KN, Praveen J, Ganesan M, Kavimani PM (2006) An overall aspect of electroless Ni-P depositions—a review article. Metall Mater Trans A 37A:1917–1926
Lee KB, Sim HS, Cho SY, Kwon H (2001) Tensile properties of 5052 Al Matrix composites reinforced with B4C particles. Metall Mater Trans A 32A:2142–2147
Pai BC, Rohatgi PK (1975) Copper coating on graphite particles. Mater Sci Eng 21:161–167
Palaniappa M, Veera Babu G, Balasubramanian K (2007) Electroless nickel–phosphorus plating on graphite powder. Mater Sci Eng A 471:165–168
Ramalho A, Miranda JC (2005) Friction and wear of electroless NiP and NiP+PTFE coatings. Wear 259:828–834
Sankara Narayanan TSN, Seshadri SK (2004) Formation and characterization of borohydride reduced electroless nickel deposits. J Alloy Compd 365:197–205
Shinohara K, Golman B, Uchiyama T, Otani M (1999) Fine-grinding characteristics of hard materials by attrition mill. Powder Technol 103:292–296
Tekmen C, Cocen U (2006) The effect of ceramic coatings on the wettability of Al–SiC composites. J Turk Ceram Fed 17:118–125
Tekmen C, Cocen U (2008) Squeeze casting of Ni coated SiC particle reinforced Al based composite. J Compos Mater 42:1271–1279
Tekmen C, Ervardar O, Cocen U (2007) Production of Al based composite reinforced with SnO2 coated SiC particles. J Compos Mater 41:3027–3033
Vafaei-Makhsoos E, Thomas EL, Toth LE (1978) Electron microscopy of crystalline and amorphous Ni-P electrodeposited films: insitu crystallization of an amorphous solid. Metall Trans 9A:1449–1460
Zhang C, Ling GP, He JH (2003) Co–Al2O3 nanocomposites powder prepared by electroless plating. Mater Lett 58:200–204
Zhang H, Liu Y, Jia Q, Jia X (2007a) Preparation of Ni/C core-shell composite powders by electroless plating method. Trans Nonferr Met Soc China 17:1144–1147
Zhang H, Wu X, Jia Q, Jia X (2007b) Preparation and microwave properties of Ni–SiC ultrafine powder by electroless plating. Mater Design 28:1369–1373
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hajizamani, M., Alizadeh, A. & Ehsani, N. Deposition of a Ni3P nano-scale layer on B4C nanoparticles by simple electroless plating in an acidic bath. Appl Nanosci 2, 417–421 (2012). https://doi.org/10.1007/s13204-011-0055-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-011-0055-7