Abstract
The application of microorganisms for the synthesis of nanoparticles as an eco-friendly and promising approach is welcome due to its non-toxicity and simplicity. The aim of this study was to synthesize silver nanoparticle using Streptomyces sp. (09 PBT 005). 09 PBT 005 was isolated from the soil sample of the agriculture field in Vengodu, Thiruvannamalai district, Tamil Nadu, India. 09 PBT 005 was subjected to molecular characterization by 16S rRNA sequence analysis. It was found that 09 PBT 005 belonged to Streptomyces sp. The isolate Streptomyces sp. 09 PBT 005 was inoculated in fermentation medium and incubated at 30 ºC for 12 days in different pH conditions. The 0.02 molar concentration showed good antibacterial activity against Gram-positive and Gram-negative bacteria at pH-7. The synthesis of silver nanoparticles was investigated by UV–Vis spectroscopy, scanning electron microscopy and Fourier Transform Infrared analysis. The synthesized AgNPs sizes were found to be in the dimensions ranging between 198 and 595 nm. The cytotoxicity of the synthesized nanoparticles was studied against A549 adenocarcinoma lung cancer cell line. It showed 83.23 % activity at 100 μl with IC 50 value of 50 μl. This method will be useful in the biosynthesis of nanoparticles.
Similar content being viewed by others
Introduction
Nanotechnology is an emerging field of science which involves synthesis and development of various nanomaterials (Basavaraj et al. 2012). At present, different types of metal nanomaterials are being produced using copper, zinc, titanium, magnesium, gold, alginate and silver. These nanomaterials are used in various fields such as optical devices (Anderson and Moskovits 2006), catalytic (Zhong-jie et al. 2005), bactericidal (Rai and Yadav et al. 2009), electronic (Rao and Kulkarni et al. 2000), sensor technology (Vaseashta et al. 2005), biological labelling (Nicewarner-Pena and Freeman et al. 2001) and treatment of some cancers (Sriram and Manikanth et al. 2010). Currently, there is a growing need to develop environmentally benign nanoparticles that do not use toxic chemicals in the synthesis protocol. As a result, researchers in the field of nanoparticles have turned to biological systems for inspiration. Biosynthetic methods have been investigated as alternatives to chemical and physical ones. This is not surprising given that many organisms, both unicellular and multicellular, are known to produce inorganic materials either intra- or extra-cellularly (Simkiss et al. 1989; Mann 1996).
The metal–microbe interactions have important role in several biotechnological applications including the fields of bioremediation, biomineralization, bioleaching and microbial corrosion. However, it is only recently that microorganisms have been explored as potential biofactory for synthesis of metallic nanoparticles such as cadmium sulphide, gold and silver (Sastry et al. 2003; Ahmad et al. 2003). An important area of research in nanotechnology is the biosynthesis of nanoparticles such as nanosilver of different chemical compositions, sizes and controlled monodispersity. Silver nanoparticles are undoubtedly the most widely used nanomaterials among all. Silver nanoparticles are used as antimicrobial agents, in textile industries, water treatment, sunscreen lotions, etc. (Rai et al. 2009; Sharma et al. 2009). Microorganisms such as bacteria, moulds, yeasts, and viruses in the living environment are often pathogenic and cause severe infections in human beings. There is a pressing need to search for new antimicrobial agents from natural substances (Kim et al. 1998; Cho et al. 2005). Therefore, biological and biomimetic approaches for the synthesis of nanomaterials are being explored. Cell mass or extracellular components from microorganisms such as Klebsiella pneumoniae, Bacillus licheniformis, Fusarium oxysporum, Aspergillus flavus, Cladosporium cladosporioides, Aspergillus clavatus, and Penicillium brevicompactum have been utilized for the reduction of silver ions to AgNPs (Ahmad et al. 2003; Shahverdi et al. 2007; Kalishwaralal et al. 2008; Balaji et al. 2009; Shaligram et al. 2009; Verma et al. 2010).
Actinomycetes are microorganisms that share important characteristics of fungi and prokaryotes such as bacteria (Okami et al. 1988). Even though they are classified as prokaryotes due to their close affinity with mycobacteria and the coryneforms (and thus amenable to genetic manipulation by modern recombinant DNA techniques), they were originally designated as ‘ray fungi’ (Strahlenpilze). Focus on actinomycetes has primarily centred on their phenomenal ability to produce secondary metabolites (Sasaki et al. 1988). The present study was aimed at using Streptomyces sp. (09 PBT 005) to synthesis silver nanoparticles (AgNPs) and to screen the antibacterial and cytotoxic activities.
Materials and methods
Chemicals
AgNO3 was obtained from Qualigen Mumbai, India. All other chemicals were purchased from Himedia, Mumbai, India. Freshly prepared doubly distilled water was used throughout the experimental work.
Isolation of actinomycetes from soil samples
Soil sample from sugarcane rhizosphere was collected from Vengodu, Thiruvannamalai district, Tamil Nadu, India (Latitude: 12º54′2383′′, North; Longitude: 79º69′9216′′, East elevation ft/m 227.5/65.4). The samples were collected from 5 to 25 cm depth in sterile plastic bags and transported aseptically to the laboratory. The soil samples were air-dried for 1 week at room temperature. Isolation and enumeration of actinomycetes were performed by serial dilution and spread plate technique (Elliah et al. 2004). One gram of soil was suspended in 9 ml of sterile double-distilled water. The dilution was carried out up to 10−5 dilutions. Aliquots (0.1 ml) of 10−2, 10−3, 10−4, and 10−5 were spread on the Actinomycetes isolation agar (Himedia, Mumbai). To minimize the fungal and bacterial growth, actidione 20 mg/l and nalidixic acid 100 mg/l were added. The plates were incubated at 30 °C for 10 days. Based on the colony morphology, the actinomycetes cultures were selected and purified on ISP2 (International Streptomyces Project 2) medium. In our pilot scale screening, a total of 27 actinomycetes were isolated and designated as 09 PBT 001 to 09 PBT 027. They were used for the screening of AgNPs synthesis; effective synthesizer (09 PBT 005) was further characterized by 16S rRNA sequencing technique.
Morphological, physiological and biochemical observations
Cultural and morphological features of 09 PBT 005 were characterized following the directions (Shirling and Gottlieb 1966). Cultural characteristics of pure isolates in various media (AIA—actinomycetes isolation agar, MHA—Mueller–Hinton agar, SCA—starch casein agar, SDA—Sabouraud dextrose agar, STP—Streptomyces agar, YPG—yeast peptone glucose agar, ZMA—Zobell marine agar, ISP—International Streptomyces Project) were recorded after incubation at 30 °C for 7–14 days. Morphology of spore bearing hyphae with entire spore chain was observed with a light microscope (Model SE; Nikon) using cover-slip method in ISP medium (ISP 3–6). The shape of cell, Gram-stain, colour determination, the presence of spores, and colony morphology were assessed on solid ISP agar medium. Biochemical reactions, different temperatures, NaCl concentration, pH level, pigment production, enzyme reaction and acid or gas production were done following standard methods (Balachandran et al. 2012a; Valanarasu et al. 2009).
Biological synthesis of silver nanoparticles
The Streptomyces sp. (09 PBT 005) strain was grown in 500-ml Erlenmeyer flasks containing 150 ml of fermentation medium which was composed of tryptone (7.0 g), peptone (3.0 g), sodium chloride (5.0 g) and potassium hydrogen phosphate (1.25 g); the pH of the medium was adjusted to pH-3.0, 5.0, 7.0, 9.0 and 11 using 1 M HCl and 1 M NaOH. The culture was grown with continuous shaking on a rotary shaker (150 rpm) at 30 °C for 12 days. After the fermentation of the culture, biomass was harvested by centrifugation (5,000 rpm) at 20 °C for 20 min, and then the mycelia were washed thrice with sterile distilled water under aseptic conditions. The biomass was brought into contact with 100 ml sterile double-distilled water for 24 h at 30 °C in an Erlenmeyer flask and agitated at 150 rpm. After incubation the cell filtrate was filtered by Whatman No. 1 filter paper. A carefully weighed quantity of silver nitrate was added to the Erlenmeyer flask containing 100 ml of cell filtrate to yield an overall Ag+ ion concentration of 0.01, 0.02, 0.03, 0.04, 0.05 M and the reaction was carried out under dark conditions. A control experiment containing only 0.01, 0.02, 0.03, 0,04 and 0.05 M of silver nitrate solution was also performed. Formation of AgNPs was characterized using UV–visible spectroscopy, Fourier Transform Infrared (FT-IR) Spectroscopy analysis and scanning electron microscopy (SEM).
Microbial organisms
The following Gram-positive and Gram-negative bacteria were used for the experiment. Gram positive: Micrococcus luteus MTCC 106, Bacillus subtilis MTCC 441, Staphylococcus epidermidis MTTC 3615 and Methicillin resistance Staphylococcus aureus (MRSA). Gram negative: K. pneumoniae MTCC 109, Enterobacter aerogenes MTCC 111, Salmonella typhimurium MTCC 1251, Shigella flexneri MTCC 1457, Proteus vulgaris MTCC 1771 and Salmonella typhi-B. The reference cultures were obtained from the Institute of Microbial Technology, Chandigarh, India-160 036. Bacterial inoculums were prepared by growing cells in Mueller–Hinton broth (Himedia) for 24 h at 37 °C.
Antibacterial assay
The antibacterial activity of the silver nanoparticles was assayed using the standard Kirby–Bauer disc diffusion method (Bauer and Kirby et al. 1966). Petri plates were prepared with 20 ml of sterile MHA (Himedia, Mumbai). The test cultures were swabbed on the top of the solidified media and allowed to dry for 10 min. One hundred micro litres of the synthesized AgNPs was filled into the well and kept for incubation overnight at 37 ºC and left for 30 min at room temperature for AgNPs diffusion. Streptomycin (10 μg/well) and culture supernatant (100 μl/well) were used as a positive and negative control, respectively. The plates were incubated for 24 h at 37 °C. Diameters of the zones of inhibition were measured using a zone scale from Himedia and expressed in millimetres.
Cell line maintenance and growth conditions
A549 adenocarcinoma lung cancer cell line was obtained from National Institute of Cell Sciences, Pune. A549 adenocarcinoma lung cancer cell line was maintained in complete tissue culture medium (Dulbecco’s modified eagle’s medium) with 10 % foetal bovine serum and 2 mM l-Glutamine, along with antibiotics (about 100 IU/ml of penicillin, 100 μg/ml of streptomycin) with the pH adjusted to 7.2. The cell lines were maintained at 37 ºC at 5 % CO2 in CO2 incubator (Hsu et al. 2011). Cultures were viewed using an inverted microscope to assess the degree of confluency and the absence of bacterial and fungal contaminants was confirmed.
Cytotoxic properties
The cytotoxicity was determined according to an available method with some changes (Balachandran et al. 2012b). Cells (5000 cells/well) were seeded in 96 well plates containing medium with different concentrations such as 100, 75, 50, 25, 12.5 and 6.25 μl. The cells were cultivated at 37 °C with 5 % CO2 and 95 % air in 100 % relative humidity. After various durations of cultivation, the solution in the medium was removed. An aliquot of 100 μl of medium containing 1 mg/ml of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) was loaded to the plate. The cells were cultured for 4 h and then the solution in the medium was removed. An aliquot of 100 μl of DMSO was added to the plate, which was shaken until the crystals were dissolved. The cytotoxicity against cancer cells was determined by measuring the absorbance of the converted dye at 570 nm in an ELISA reader. Cytotoxicity of each sample was expressed as IC50 value. The IC50 value is the concentration of test sample that causes 50 % inhibition of cell growth, averaged from three replicate experiments.
Characterization
UV–vis spectroscopy
The reduction of silver ions was confirmed by measuring the UV–visible spectrum of the reaction medium. Three millilitres of supernatant was withdrawn after 72 h and absorbance was measured using UV–visible spectrophotometer Thermo Fisher, UV–vis double beam, Serial No.3628/0509 and software version 6.89 in the wavelength range from 200 to 600 nm. The absorption in the visible range directly reflects the perceived colour of the chemical involved.
Fourier Transform Infrared (FT-IR) Spectroscopy analysis
The sample was subjected to FT-IR Spectroscopy analysis on a PerkinElmer grating spectrophotometric instrument in Kbr disc. Two milligrams of the sample was mixed with 200 mg KBr (FT-IR grade) and pressed into a pellet. The sample pellet was placed into the sample holder and FT-IR spectra were recorded in the range 4,000–400 cm–1 in FT-IR spectroscopy at a resolution of 1 cm−1.
Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) was used to observe the size, shape and morphology of the resultant nanoparticles. A specimen for SEM sample was made by casting a drop of suspension on a carbon-coated copper grid and the excess solution was removed by tissue paper and allowed to air dry at room temperature. Scanning electron microscopy study was observed on S-3400 2010 (Japan) at an accelerating voltage of 10,000 V and fitted with a CCD camera.
16S rRNA region-based characterization
Genomic DNA Isolation
The freshly cultured cells were pelleted by centrifuging for 2 min at 12,000 rpm to obtain 10–15 mg (wet weight). The cells were resuspended thoroughly in 300 μl of Lysis solution; 20 μl of RNase. A solution was added, mixed and incubated for 2 min at room temperature. About 20 μl of the Proteinase K solution (20 mg/ml) was added to the sample and mixed; the resuspended cells were transferred to Hibead Tube and incubated for 30 min at 55o. The mixture was vortexed for 5–7 min and incubated for 10 min at 95 °C followed by pulse vortexing. Supernatant was collected by centrifuging the tube at 10,000 rpm for 1 min at room temperature. About 200 μl of lysis solution was added, mixed thoroughly by vortexing and incubated at 55 °C for 10 min. To the lysate, 200 μl of ethanol (96–100 %) was added and mixed thoroughly by vortexing for 15 s. The lysate was transferred to new spin column and 500 μl of prewash solution was added to the spin column and centrifuged at 10,000 rpm for 1 min and the supernatant was discarded. The lysate was then washed in 500 μl of wash solution and centrifuged at 10,000 rpm for 3 min. Two hundred micro litres of the elution buffer was pipetted out and added directly into the column without spilling and incubated for 1 min at room temperature. Finally, the DNA was eluted by centrifuging the column at 10,000 rpm for 1 min. (Hipura Streptomyces DNA spin kit-MB 527-20pr from Himedia).
Preparation and analysis of 16S rRNA
The primer 27F (51 AGT TTG ATC CTG GCT CAG 31) and 1492R (51 ACG GCT ACC TTG TTA CGA CTT 31) were used to amplify 16S ribosomal sequence from genomic DNA in Thermal Cycler (ep gradient Eppendorf). The cyclic conditions were as follows: initial denaturation at 94 °C for 3 min, 35 cycles of 94 °C for 1 min, 54 °C for 1 min, and 72 °C for 2 min, and final extension of 10 min at 10 min and hold at 4 °C. The PCR products were confirmed by 1 % agarose gel electrophoresis (Farris et al. 2007).
DNA sequence determination
Automated sequencing was carried out according to the dideoxy chain-termination method using Applied Biosystems automated sequencer by synergy Scientific Services (Sanger et al. 1977).
Database searching and nucleotide sequence accession number
The sequence was compared for similarity with the reference species of bacteria contained in genomic database banks, using the NCBI BLAST (Blast‘n’) tool (http://www.ncbi.nlm.nih.gov/BLAST). The partial 16S rRNA gene sequences of isolate 09 PBT 005 have been deposited in the GenBank. A phylogenetic tree was constructed using the neighbour-joining DNA distance algorithm using software MEGA (version 4.0) (Tamura et al. 2007).
Results and discussion
Isolation and culture characterization
Nanomaterials are the leading substances in the field of nanomedicine and nanobiotechnology. Silver nanoparticles have recently been shown to be promising antibacterial and anticancer material. Streptomyces cultures were isolated from the soil sample collected from agricultural field in Vengodu, Thiruvannamalai district, Tamil Nadu, India. This strain 09 PBT 005 was Gram-positive filamentous bacterium. The colour of the substrate mycelia was dark green. The spore’s chains were green. These characteristic morphological properties strongly suggested that the isolate belonged to Streptomyces genus (Table 1). It showed good growth on medium amended with sodium chloride up to 11 %; no growth was seen at 15 %. The temperature for growth ranged from 25 to 37 °C with optimum of 30 °C and the pH range was 6–10 with normal pH of 7 (Table 2). 09 PBT 005 showed resistance towards ampicillin, ceftazidime, ceftazidime/clavulanic acid, co-trimoxazole, oxacillin, penicillin-G, piperacillin and ticarcillin/clavulanic acid (Table 3). The Streptomyces isolate was characterized on the basis of colony characteristics and microscopic appearance (Cappuccino et al. 2006) (Fig. 1). The 16S rRNA sequencing showed it to be Streptomyces sp. 09 PBT 005 (bases 1–802 linear DNA). The isolate 09 PBT 005 showed 98 % homology to Streptomyces ghanaensis 16S ribosomal RNA gene, partial sequence (HE797851) NCBI BLAST available at http://www.ncbi-nlm-nih.gov/. The DNA sequences were aligned and phylogenetic tree was constructed using mega4 software (bootstrap method) (Fig. 2). The sequences were deposited in GenBank (NCBI) with accession number JF710843. Streptomyces sp. 09 PBT 005 was further selected for the biosynthesis of silver nanoparticles because there are only very few reports on the synthesis of silver nanoparticles using Streptomyces sp. Earlier Faghri and Salouti (2011) (Sathishkumar et al. 2009; Faghri Zonooz. 2011) have reported the biosynthesis of silver nanoparticles using aqueous extract of Streptomyces sp.
Microbial synthesis of silver nanoparticles
Synthesis of silver nanoparticles was observed by the addition of selected culture supernatant of Streptomyces sp. (09 PBT 005) to 0.01, 0.02, 0.03, 0.04 and 0.05 aqueous AgNO3 at room temperature. The Streptomyces sp. (09 PBT 005) aqueous filtrate incubated with deionized water (positive control) and the silver nitrate solution (negative control) was observed to retain its original colour, and the silver nitrate-treated supernatant turned dark brown after 72 h due to the deposition of silver nanoparticles (Fig. 3). Generally, the formation of silver can be primarily identified through visible observation in the change of colour solution during the reaction from colourless to pale yellow or dark brown (Sathishkumar et al. 2009). The colour formation is dependent on the excitation of surface plasmon vibrations of silver nanoparticles (Kannan et al. 2011).
Biosynthesis of silver nanoparticles—colour change reaction: conical flasks containing the extracellular filtrate of the Streptomyces sp. (09 PBT 005) biomass (a) and conical flasks are containing the extracellular filtrate of the Streptomyces sp. (09 PBT 005) biomass after exposure to AgNO3 solution (b)
Antibacterial activity of synthesized AgNPs
The biologically synthesized AgNPs showed good antimicrobial activity against Gram-positive and Gram-negative bacteria by well diffusion method (Fig. 4a–e). At the 0.02 molar concentrations at pH-7, the Streptomyces sp. (09 PBT 005)-based nanosilver particles showed good activity against Gram-negative bacteria such as S. flexneri and E. aerogenes with maximum zones of inhibition (16 mm). Klebsiella pneumoniae, S. typhimurium and S. typhi-B and P. vulgaris showed 13 mm zones. A moderate activity was seen in Gram-positive bacteria such as S. epidermidis (15 mm), MRSA (13 mm) and B. subtilis (12 mm). Nanoparticles synthesized using 0.02 M AgNO3 was the most efficient to inhibit the tested pathogens when compared to higher concentrations. There was a decrease in antibacterial activity as the concentration of AgNO3 increased. This shows that a minute quantity can itself act against many pathogenic microorganisms. The antimicrobial activity of colloidal silver particles is influenced by the particle dimensions (Kaviya et al. 2011). Similar results have been reported previously by Panácˇek et al. (2006) and Augustine et al. (2013). A number of possible mechanisms are proposed for the antibacterial activity of AgNPs. The synthesized AgNPs with smaller size can act drastically on cell membrane and further interact with DNA and cause damage (Morones et al. 2005). In addition, the pitting of the cell membranes by silver nanoparticles causes an increase in permeability and results finally in cell death (Shahverdi et al. 2007). Silver ions have been known to bind with the negatively charged bacterial cell wall resulting in the rupture and consequent denaturation of proteins which leads to cell death (Lin and Vidic et al. 1998). There was not much activity against Gram-positive and Gram-negative bacteria at pH-3, 5, 9 and 11. Based on the bactericidal activity the nanoparticles were taken to assess cytotoxic activity and further characterization.
Cytotoxic properties of synthesized AgNPs
AgNPs showed cytotoxic activity in vitro against A549 adenocarcinoma lung cancer cell line. It showed 83.23 % activity at 100 μl with IC50 value of 50 μl. All concentrations used in the experiment decreased the cell viability significantly (P < 0.05) in a concentration-dependent manner (Fig. 5).
Characterization
UV–visible spectral analysis
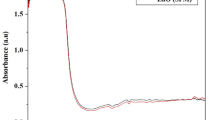
In this study, AgNPs were successfully synthesized by Streptomyces sp. (09 PBT 005), when the cell-free extract was subjected to AgNO3. The biosynthesis reaction started within few minutes and the colour reaction was observed in which clear AgNO3 solution changed into yellowish-brown coloured solution which indicated the formation of silver nanoparticles (Fig. 6a). The appearance of a yellowish-brown colour in the silver nitrate-treated flask indicated the formation of silver nanoparticles, whereas no colour change was observed in either the culture supernatant without silver nitrate or the silver nitrate control experiments. The UV–visible spectra for the aqueous AgNO3-culture supernatant of synthesized AgNPs by Streptomyces sp (09 PBT 005) alone were recorded. In the UV–visible spectrum, a strong and broad peak was observed at 440 nm, indicating the presence of AgNPs. In agreement with previous reports, the absorption peak at 441 nm is probably due to the excitation of longitudinal plasmon vibrations and formation of quasi-linear superstructures of nanoparticles (Shankar et al. 2003). The optimum time required for the completion of reaction in our study was 72 h; as the duration of reaction and concentration of silver nitrate increase, more silver nanoparticles are formed resulting in large size particle. Different concentrations of silver nitrate solution were used to get maximum antibacterial activity of AgNPs. As the size increases, the peak of plasmon resonance shifts to longer wavelengths and broadens. The nanoparticles in this study are big and have an average diameter from 198 to 595 nm. Similar result was reported by Veerasamy et al. (2011).
Characterizations of AgNps. a UV–vis absorption spectra of silver nanoparticles synthesized by Streptomyces sp. (09 PBT 005). b FT-IR analysis of silver nanoparticles biosynthesis using Streptomyces sp. (09 PBT 005). c Scanning electron microscopy image shows formation of AgNPs by Streptomyces sp. (09 PBT 005), c (i) agglomeration of AgNPs at 100 μm, c (ii) agglomeration of AgNPs at 20 μm and c (iii). Size of the AgNPs at 20 μm (198–595 nm)
FT-IR analysis
Fourier Transform Infrared spectrum showed that the active biomolecules of culture supernatant were responsible for the reduction of Ag+ ions into metallic AgNPS, which revealed distinct peak in the range of 3,586–666 cm−1 (Fig. 6b). The broad peak at 3,586 cm−1 is due to strong stretching vibration of phenolic OH (Gopinath and Mubarkali et al. 2012). The band at 3,547 cm−1 is due to NH stretching of amide group. The band at 3,394 is assigned to the N–H group from peptide linkage present in the cell-free extract of Streptomyces sp. (Mubarkali and Thajuddin et al. 2011). The peak at 3,367 cm−1 may be ascribed to NH stretching of primary amine (NH2). The presence of characteristic peak of amide carbonyl is shown by the peak at 1634 (NHCO). IR spectroscopic study has confirmed that the carbonyl group from amino acid residues and peptides of proteins has stronger ability to bind metal, so that the proteins could most possibly form a coat covering the metal nanoparticles (i.e. capping of AgNPs) to prevent agglomeration of the particles and stabilizing in the medium. This evidence suggests that the biological molecules could possibly perform the function in the formation and stabilization of the AgNP in aqueous medium. It is well known that proteins can bind to AgNPs through free amine groups in the proteins and, therefore, stabilization of the AgNPs by surface-bound proteins is a possibility (Gole and Dash et al. 2001). The peak at 1,634 cm−1 is due to the carbonyl stretch vibrations in the amide linkages of proteins (Basavaraja and Balaji et al. 2008). The carbonyl groups of amino acid residues and peptides have strong ability to bind to silver (Balaji et al. 2009). It is also reported that proteins can bind to nanoparticles either through free amine or cysteine groups in proteins (Mandal et al. 2005).
SEM
The morphology of the silver nanoparticles was observed using scanning electron microscope. The nanoparticles are polydispersed with a roughly spherical shape Fig. 6c (i, ii), although the exact shape of the nanoparticles was not clearly predicted. Higher magnification showed the average diameter of these spherical nanoparticles to be about 198–595 nm 6c (iii). The SEM analysis of Ag nanoparticles is in agreement with the results of Faghri Zonooz and Salouti (2011) and Sastry et al. 2003. The above results suggested that the silver nanoparticles were synthesized due to the action of Streptomyces cell-free extract, which act as good bioreductant for biosynthesis.
Conclusion
A critical need in the field of nanotechnology is the development of a reliable and eco-friendly process for synthesis of nanoparticles. Streptomyces sp. (09 PBT 005) has been effectively used for the synthesis of silver nanoparticles. We have demonstrated the use of a natural, renewable and low-cost bioreducing agent. Biosynthesized silver nanoparticles were confirmed by spectroscopic characterization of UV–visible, FT-IR and SEM. The biosynthesized silver nanoparticles using Streptomyces sp. (09 PBT 005) showed good antibacterial and cytotoxic activities.
References
Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, Sastry M (2003) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B 28:313–318
Anderson DJ, Moskovits M (2006) A SERS-active system based on silver nanoparticles tethered to a deposited silver film. J Phys Chem B 110:13722–13727
Augustine R, Kalarikkal N, Thomas S (2013) A facile and rapid method for the black pepper leaf mediated green synthesis of silver nanoparticles and the antimicrobial study. Appl Nanosci. doi:10.1007/s13204-013-0260-7
Balachandran C, Duraipandiyan V, Balakrishna K, Ignacimuthu S (2012a) Petroleum and polycyclic aromatic hydrocarbons (PAHs) degradation and naphthalene metabolism in Streptomyces sp. (ERI-CPDA-1) isolated from oil contaminated soil. Bioresour Technol 112:83–90
Balachandran C, Duraipandiyan V, Ignacimuthu S (2012b) Cytotoxic (A549) and antimicrobial effects of Methylobacterium sp. isolate (ERI-135) from Nilgiris forest soil, India. Asian Pac J Trop Biomed 2(9):712–716
Balaji DS, Basavaraja S, Deshpande R, Bedre MD, Prabhakara BK, Venkataraman A (2009) Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf B 68:88–92
Basavaraj U, Praveenkumar N, Sabiha TS, Rupali S, Samprita B (2012) Synthesis and characterization of silver nanoparticles. Int J Pharm Bio Sci 2(3):10–14
Basavaraja SS, Balaji SD, Lagashetty AK, Rajasab AH, Venkataraman A (2008) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater Res Bull 43:1164–1170
Bauer AW, Kirby WMM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496
Cappuccino JG, Sherman N (2006) Microbiology: a laboratory manual, Dorling Kindersley (India) Pvt. Ltd., India, 6:237
Cho KH, Park JE, Osaka T, Park SG (2005) The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim Acta 51:956–960
Elliah P, Ramana T, Bapi Raju KVVS, Sujatha P, Uma Sankar AM (2004) Investigation on marine actinomycetes from Bay of Bengal near Karnataka coast of AndhraPradesh. Asian J Microbiol Biotechnol Environ Sci. 6(1):53–56
Faghri Zonooz N, Salouti M (2011) Extracellular biosynthesis of silver nanoparticles using cell filtrate of Streptomyces sp. ERI-3. Scientia Iranica 18(6):1631–1635
Farris MH, Oslon JB (2007) Detection of actinobacteria cultivated from environmental samples reveals bias in universal primers. Lett Appl Microbiol 45:376–381
Gole A, Dash C, Ramachandran V, Sainkar SR, Mandale AB, Rao M, Sastry M (2001) Pepsin-gold colloid conjugates: preparation, characterization, and enzymatic activity. Langmuir 17:1674–1679
Gopinath V, MubarakAli D, Priyadarshini S, Meera PN, Thajuddin N, Velusamy P (2012) Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach. Colloids Surf B Biointerfaces 96:69
Hsu H, Huang K, Lu K, Chiou S, Yen J, Chang C, Houng J (2011) Typhonium blumei extract inhibits proliferation of human lung adenocarcinoma A549 cells via induction of cell cycle arrest and apoptosis. J Ethnopharmacol 135:492–500
Kalishwaralal K, Deepak V, Ramkumarpandian S, Nellaiah H, Sangiliyandi G (2008) Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater Lett 62:4411–4413
Kannan N, Mukunthan KS, Balaji S (2011) A comparative study of morphology, reactivity and stability of synthesized silver nanoparticles using Bacillus subtilis and Catharanthus roseus (L.) G. Don. Colloids Surf B Biointerfaces 86:378–383
Kaviya S, Santhanalakshmi J, Viswanathan B (2011) Green synthesis of silver nanoparticles using Polyalthia longifolia leaf extract along with d-sorbitol: study of antibacterial activity. J Nanotech 2011. doi:10.1155/2011/152970
Kim TN, Feng QL, Kim JO, Wu J, Wang H, Chen GC et al (1998) Antimicrobial effects of metal ions (Ag+, Cu2+, Zn2+) in hydroxyapatite. J Mater Sci Mater Med 9:129–134
Lin YE, Vidic RD, Stout JE, McCartney CA, Yu VL (1998) Inactivation of Mycobacterium avium by copper and silver ions. Water Res 32:997–2000
Mandal S, Phadtare S, Sastry M (2005) Interfacing biology with nanoparticle. Curr Appl Phys 5:118–127
Mann S (ed) (1996) Biomimetic materials chemistry. VCH Publishers, New York
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346–2353
MubaraAli D, Thajuddin N, Jeganathan K, Gunasekaran M (2011) Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf B Biointerfaces 85:360–365
Nicewarner-Pena SR, Freeman RG, Reiss BD, He L, Pena DJ, Walton ID, Cromer R, Keating CD, Natan MJ (2001) Submicrometer metallic barcodes. Science 294:137–141
Okami Y, Beppu T, Ogawara H (1988) Biology of Actinomycetes. Japan Scientific Societies Press, Tokyo, pp 88–508
Panàcˇek A, Kvitek L, Prucek R, Kolar M, Vecerova R, Pizurova N, Sharma VK, Nevecˇna´ T, Zboril R (2006) Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B 110(33):16248–16253
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Rao CNR, Kulkarni GU, Thomas PJ, Edwards PP (2000) Metal nanoparticles and their assemblies. Chem Soc Rev 29:27–35
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci 74(12):5463–5467
Sasaki T, Yoshida J, Itoh M, Gomi S, Shomura T, Sezaki M (1988) New antibiotics SF2315A and B produced by an Excellospora sp. I. Taxonomy of the strain, isolation and characterization of antibiotics. J Antibiot 41:835–842
Sastry M, Ahmad A, Khan MI, Kumar R (2003) Biosynthesis of metal nanoparticles using fungi and actinomyces. Curr Sci 5:162–170
Sathishkumar M, Sneha K, Won SW, Cho CW, Kim S, Yun YS (2009) Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf B Biointerfaces 73:332–338
Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian SA (2007) Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed Nanotechnol Biol Med 3:168–171
Shaligram NS, Bule M, Bhambure R, Singhal RS, Singh SK, Szakacs G, Pandey A (2009) Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochem 44:939–943
Shankar SS, Ahmad A, Sastry M (2003) Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Progr 19:1627–1631
Sharma VK, Ria AY, Lin Y (2009) Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci 145:83–96
Shirling JL, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Sys Evol Microbiol 16:313–340
Simkiss K, Wilbur KM (1989) Biomineralization. Cell Biology and Mineral Deposition Academic Press, New York, p 337
Sriram MI, ManiKanth SB, Kalishwaralal K, Gurunathan S (2010) Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int J Nanomed 5:753–762
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Valanarasu M, Duraipandiyan V, Agastian P, Ignacimuthu S (2009) In vitro antimicrobial activity of Streptomyces spp. ERI-3 isolated from Western Ghats rock soil (India). J Mycol Med 19:22–28
Vaseashta A, Dimova-Malinovska D (2005) Nanostructured and nanoscale devices, sensors and detectors. Sci Technol Adv Mater 6:312–318
Veerasamy R, Xin TZ, Gunasagaran S, Xiang TFW, Yang EFC, Jeyakumar N, Dhanaraj SA (2011) Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc 15:113–120
Verma VC, Kharwar RN, Gange AC (2010) Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomed 5:33–40
Zhong-jie J, Chun-yan L, Lu-wi S (2005) Catalytic properties of silver nanoparticles supported on silica spheres. J Phys Chem B 109:1730–1735
Acknowledgments
The authors are grateful to Entomology Research Institute, Loyola College, Chennai, for financial assistance. We thank the visiting Professorship Program, Deanship of Scientific Research at King Saudi University, Riyadh.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Saravana Kumar, P., Balachandran, C., Duraipandiyan, V. et al. Extracellular biosynthesis of silver nanoparticle using Streptomyces sp. 09 PBT 005 and its antibacterial and cytotoxic properties. Appl Nanosci 5, 169–180 (2015). https://doi.org/10.1007/s13204-014-0304-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-014-0304-7