Abstract
SARS-CoV-2 (COVID-19) spreads and develops quickly worldwide as a new global crisis which has left deep socio-economic damage and massive human mortality. This virus accounts for the ongoing outbreak and forces an urgent need to improve antiviral therapeutics and targeted diagnosing tools. Researchers have been working to find a new drug to combat the virus since the outbreak started in late 2019, but there are currently no successful drugs to control the SARS-CoV-2, which makes the situation riskier. Very recently, new variant of SARS-CoV-2 is identified in many countries which make the situation very critical. No successful treatment has yet been shown although enormous international commitment to combat this pandemic and the start of different clinical trials. Nanomedicine has outstanding potential to solve several specific health issues, like viruses, which are regarded a significant medical issue. In this review, we presented an up-to-date drug design strategy against SARS-CoV-2, including the development of novel drugs and repurposed product potentials were useful, and successful drugs discovery is a constant requirement. The use of nanomaterials in treatment against SARS-CoV-2 and their use as carriers for the transport of the most frequently used antiviral therapeutics are discussed systematically here. We also addressed the possibilities of practical applications of nanoparticles to give the status of COVID-19 antiviral systems.
Similar content being viewed by others
Introduction
A rising number of cases of novel coronaviruses recognized as severe acute respiratory coronavirus-2 syndrome known as SARS-CoV-2 have been causing serious human infections with viral pneumonia (COVID-19) since December 2019 (Huang et al. 2019; Zhou et al. 2020a). In fall 2019, many of the enigmatic pneumonia patients of unknown etiology have been recorded in Wuhan, Hubei, a province of China which has received worldwide attention (Zhu et al. 2019). Unlike, previous coronavirus outbreaks like the severe acute respiratory syndrome coronavirus (SARS-CoV) from China and the Middle East Respiratory Syndrome coronavirus (MERS CoV) from Saudi Arabia (Wit et al. 2016), SARS-CoV-2 has a high infectious rate. By the time of this writing, COVID-19 has infected almost all inhabited areas of the world and seriously impacted on more than 123 million of people and 2,727,837 who have died (WHO, report) (as on 25th March, 2021) with a worrisome feature of major global rises in the number of cases every day. Therefore, the World Health Organization has formally declared COVID-19 a pandemic. Although several nations and international research groups are working on COVID-19 vaccine development, no specific antiviral therapies, monoclonal antibodies (mAbs) or vaccines have been available to date. However, this is not a fast process; scientists estimate the period to be, some few more months. Treatments focus mainly on symptomatic and respiratory assistance according to protocols issued in each country by health authority, where several countries follow the World Health Organization (WHO) protocol (Guan et al. 2019; Guo et al. 2019).
Nanotechnology has received much interest and application over more than two decades to particles with dimension(s) falling within the range of the nanometer (10–9 or 1 billionth of a meter) due to their unique properties (Parboosing et al. 2012). Because of their physical and chemical characteristics and the adaptable nanotechnological accomplishments of the past, it is clear that nanoscience is gradually accelerating its worldwide perspective in the present and will be in the centuries ahead (Aiswarya et al. 2019; Kerry et al. 2019). Furthermore, nanomaterials may play protective feature, prevent the encapsulated drug or anti-infection agent from degradation due to the shielding properties of these nano-sized materials (Wang et al. 2018). These nanoparticles can be biomimetic in nature, and the adjustable surface charge makes them attractive tools for viral treatment, resulting in endogenous antiviral properties (Bowman et al. 2008; Sanvicens and Marco 2008; Caron et al. 2010; Petros and Desimone 2010; Gagliardi 2017) against various viruses including Herpes Simplex Virus (HSV), human immunodeficiency virus (HIV), hepatitis B virus (HBV), (Galdiero et al. 2011). Silver sulfadiazine is officially classified as an essential anti-infective topical medication by the World Health Organization (WHO 2015). The potential of metal nanoparticles for early detection, diagnosis, and treatment of disease was explored in medicine, but their biological properties remained mostly unexplored (Bhattacharya and Mukherjee 2008). Silver nanoparticles (SNPs) are one of the most successful symbols of noble nanoparticles but have also had strong antimicrobial activity, so particular attention has been paid to the production of various products to fight infectious diseases (Abou El-Nour et al. 2010; Marambio-Jones and Hoek 2010; Lara et al. 2011). Some silver nanoparticles applications have earned US Food and Drug Administration clearance (Dunn and Edwards-Jones 2004). Metallic NPs, such as gold NPs (AuNPs) capped by properly engineered antisense oligonucleotides, have recently been introduced to detect the SARS-CoV-2, such as in the naked-eye virus screening approach (Moitra et al. 2020). Novel nano-vaccine metastasis tools, as well as effective nano drugs for treating SARS-CoV-2 infections, are being developed as delivery vehicles for SARS-CoV-2 therapeutics. Dexamethasone, a nanotechnology based therapeutic drug against SARS-CoV-2 was developed using an anti-edema and anti-fibrotic mechanism as an effective drug delivery system (Lammers et al. 2020). Different nanoparticulate delivery systems against viral infections are discussed here, in addition to their use as carriers for delivering in the widely used antiviral therapies.
Coronavirus origin and its virology
Coronaviruses have developed several times over the past 1000 years (Forni et al. 2017). These viruses are enveloped with a positive-sense single-stranded RNA genome (26–32 kb) (Su et al. 2016). The initial discovery of diseases in animals proceeded by the diagnosis of infectious bronchitis virus (IBV) from chickens in 1937 (Beaudette and Hudson 1937) and murine hepatitis viruses (MHV) from mice in 1949 (Cheever et al. 1949). Later on, numerous other coronavirus strains were reported in several animals like cats, calves, sparrows, dogs, bats, turkeys and rabbits (Lai et al. 2007). Human coronaviruses first emerged from respiratory tract infections in the 1960s (Kahn and McIntosh 2005). Coronavirus virions have large peplomers (spike projections from the virus envelop) under the electron microscope which making it look like a crown, hence the title corona, meaning in Latin 'crown' or 'halo' and the diameter of the virus is variable from 60 to 140 nm (Wang et al. 2006; Ge et al. 2013; Chen et al. 2016).
Classification according to International Committee of Taxonomy of Viruses (ICTV) (2018), 4 coronavirus genera (α, β, γ, δ) have been described in the Coronaviridae family. Gammacoronaviruses and Deltacoronaviruses can infect mammals and birds but have never caused any disease in humans (Woo et al. 2012; Cui et al. 2019). In contrast to this, the genera Alphacoronaviruses and Betacoronaviruses are capable of causing gastrointestinal illness in animals and respiratory disease in humans especially Human coronavirus NL63, Human coronavirus 229E, Betacoronavirus 1, Human coronavirus HKU1, Severe Acute Respiratory Syndrome-related coronavirus(SARS-CoV), Middle East Respiratory Syndrome-related coronavirus (MERS-CoV)can able to infect humans (Helmy et al. 2020). Based on the genomic analysis the recently identified SARS-CoV-2 belongs to the Betacoronavirus lineage B, having the RNA genome of about 30 kb, which has 74–99% identity than that of pangolin coronavirus (Munisjavanica) and horseshoe bat (Rhinolophussinicus), respectively (Zhou et al. 2020a; Zhu et al. 2019; Woo et al. 2012; Cui et al. 2019).
Evolutionary study of COVID-19 showed that it is most similar to SARS-like coronaviruses and was called SARS-CoV-2 for the similarity (Alanagreh et al. 2020). Coronaviruses are zoonotic pathogens, and bats have been documented as the rich source (Chen et al. 2020a; Yang et al. 2011) and can be transmitted by direct contact to humans through intermediate host animals. The human race has seen three coronavirus outbreaks with high rates of morbidity in the last two decades. The world was stunned by the first pandemic of this century in 2003, with the emergence of SARS-CoV in Guangdong, China that resulted in 744 deaths in 29 countries and more than 8000 patients in 29 countries corresponding to a 9.6% fatality rate (Wang et al. 2006; Alanagreh et al. 2020). The outbreak was linked explicitly to market civet cats and was considered a SARS-CoV intermediate host, with the bats as the natural host (Hu et al. 2017; Guan et al. 2003).
Years later, on 23 September 2012, the WHO declared MERS-CoV, a new coronavirus, was discovered, which appears to have a higher mortality rate (> 35%) from the Middle East in with more than 80% of Saudi Arabia cases recorded. A detailed study of the evolutionary relationships showed that MERS-CoV was transmitted from dromedary camels as an intermediate host to humans that may originate from bats (Zaki et al. 2012; Wang et al. 2015; Dudas and Rambaut 2016). SARS-CoV-2 is believed to have originated on the seafood market in Wuhan, China (Huang et al. 2019). Recent researches have indicated that SARS-CoV-2 is a modified bat-derived coronavirus (Zhou et al. 2020a; Zhang et al. 2019) resulting from zoonotic transmission to humans (Lu et al. 2019; Xu et al. 2020). A coronavirus detected in the Malayan pangolin was found to be 99% similar to SARS-CoV-2 (Nadeem et al. 2020). Infected pangolins exhibit similar pathological signs to those suffering from COVID-19, and the antibodies circulating in their blood can react with the SARS-CoV-2 spike protein. Latest researches point out that SARS-CoV-2 may derive from Pangolin-CoV recombination with bat-CoV-RaTG13-like virus where pangolin is considered as one of the possible intermediate hosts between bat and human (Ge et al. 2013; Lu et al. 2019; Xu et al. 2020). Investigations are underway to identify possibilities of snakes, minks, and tortoises as intermediate hosts (Li et al. 2020a; Xiao et al. 2020). The majority of the scientific study believes that SARS-CoV-2 was transmitted to humans on the seafood market which originated from bats through the intermediate animal host (Lu et al. 2019). However, a conclusive response to which animals function as intermediate hosts is yet to be found.
Andersen et al. (2020) analyzed the spike protein amino acids from SARS-CoV and SARS-CoV-2 revealed that they differ in the hypothesis that the theory of laboratory origin of SARS-CoV-2 by manipulating SARS-like viruses. According to the literature, urbanization, more and more frequent combining of different animals, may have facilitated the emergence and re-emergence of some of these viruses in densely populated regions. In contrast, coronaviruses are known to have high rates of mutation and recombination that may allow them to cross barriers to species and adapt to a new host (Liu et al. 2020). Further studies are therefore needed to confirm intermediate hosts of coronaviruses for zoonotic transmission control and to prevent future outbreaks of such viral infections (Lau and Chan 2015).
New variants of SARS-CoV-2
Viruses keep evolving by mutation and a new form emerges in itself, and is not a serious concern. Many new mutants would not selectively favor the virus. However, certain mutations or mutation blends may have a selective benefit for the virus, such as increased transmissibility resulting in an increase in receptor binding, or the capacity of the host immune response to modify antibodies-recognized surface structures. Prior studies into the D614G variant showed that while the 614G variant offered a select gain, there was no observable influence on the severity or result of the infection due to increased cellular infectivity (Li et al. 2020b).
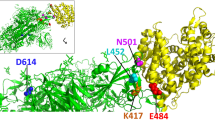
In mid-December 2020, the world watched with interest and growing concern as scientists in the United Kingdom characterized the newly discovered coronavirus variant (‘VUI – 202012/01’ (in December 2020 first variant to be investigated)) as being more communicable than existing circulating viruses. By 21 December 2020, 1108 cases with this variant were detected, mainly in the South and East of England and recently also in Australia, United States, Denmark, India, Iceland, Italy and the Netherlands (WHO). The mutations found in this new form have to do with the receptor binding site as well as other surface structures that may change the virus' antigenic properties. Initial report reveals that the variant can spread easier among individuals and this variant having mutation in ‘spike’ protein. Alterations of this spike protein will make the virus more contagious and also more easily spread through people. According to WHO report, the variant have mutations resulting in 14 amino acid modifications and 3 deletions (deletion 144, deletion 69–70, D614G, N501Y, A570D, P681H, S982A, T716I, D1118H). Any one of these mutations can impact human transmissibility of the virus. One mutation (N 501Y) found is that an amino acid varies within the receptor binding domain (RBD) of the six main residues. Another biologically significant mutation, P681H, was detected in the RBD. Finally, deletion at 69/70 was found that impact the efficiency of some diagnostic PCR tests that use an S gene target (https://www.who.int/csr/don/21-december-2020-sars-cov2-variant-united-kingdom/en/).
Pathogenesis of SARS-CoV-2
COVID-19 patients display multiple clinical signs and symptoms, such as fever, running nose, sore throat, nonproductive cough, myalgia, dyspnea, fatigue, loss of smell and taste, normal or decreased leukocyte counts, and viral conjunctivitis which are evidence of SARS-CoV and MERS-CoV infections (Huang et al. 2019; Lu et al. 2019; Volz et al. 2020; Chan et al. 2019). However, the complete and systematic clinical expression for COVID-19 has not been clear to date. The related SARS-CoV and MERS-CoV mechanisms can still provide us with a significant amount of knowledge on pathogenesis of infection with SARS-CoV-2 to promote our identification of COVID-19. Patients with moderate signs and symptoms have been reported to improve their health after one week, at the same time, the patients with significant respiratory symptoms leading to acute respiratory distress syndrome (ARDS) (Xia et al. 2020). Epidemiological studies indicate the time of incubation for COVID-19 was estimated to be between 1 and 14 days, while the serial period was estimated at 4–8 days. It requires around 3–7 days for the epidemic to double the infections (Adhikari et al. 2020).
COVID-19 showed higher rates of pandemic and transmission risk compared with SARS-CoV as it was found that the sufficient reproductive number (R) of COVID-19 (2.9) was more than the ones mentioned earlier sufficient reproductive number (R) of SARS-CoV (1.77) (Park et al. 2020). Four structural proteins in the coronavirus family include the large homogeneous 'spike surface glycoprotein' (S), matrix protein (M), a small envelope protein (E), and nucleocapsid protein (N). The Spike protein in coronaviruses has been reported as a significant determinant for receptor binding and interacting with the host cells and responsible for the infection (Wrapp et al. 2020). The life cycle of the virus with the host consists of five phases: attachment, penetration (reach the host cells by endocytosis or membrane fusion), biosynthesis (viral RNA enters the nucleus for replication and mRNA is used to generate viral proteins), maturation (making of new viral particles) and release. After entering the target cell's cytoplasm and being a positive-sense RNA genome, the viral genome translates into two polyproteins 1a, b (pp1a, pp1b) and form a replication-transcription complex (RTC) that pertains to genome transcription and replication (Lau and Chan 2015). The newly synthesized glycoproteins are then incorporated into the endoplasmic reticulum or Golgi membrane, and the nucleocapsid is developed by merging of genomic RNA with the nucleocapsid protein. Viral particles then germinate into the endoplasmic reticulum-Golgi intermediate compartment (ERGIC), and the vesicles containing the virus particles merge into the plasma membrane for virus release (Adhikari et al. 2020).
Immune reaction to SARS-CoV-2
As reported earlier by several scientist groups from China, angiotensin-converting enzyme 2 (ACE2) is the primary receptor for both SARS-CoV2 and SARS-CoV (Zhou et al. 2020a; Li et al. 2003). Since ACE2 expresses itself strongly in the alveolar space of epithelial cells on the apical side of the lung, this virus will probably enter and kill them (Hamming et al. 2004; Jia et al. 2005). A recent report indicated that the binding efficiency between SARS-CoV-2 and ACE2 glycoprotein is 10–20 folds higher than that of SARS-CoV, which could clarify SARS-CoV-2's highly infectious capability (Guo et al. 2019; Letko et al. 2020). ACE2 is widely expressed in the nasal mucosa, lung, bronchus, heart, esophagus, stomach, kidney, ileum, and bladder, all of which are vulnerable for SARS-CoV-2 (Zou et al. 2020). Clinicians have also recently suggested that SARS-CoV-2 be potentially pathogenic to testicular tissues, which implies fertility concerns in young patients (Letko et al. 2020).
Once the virus got entry into the cell, the SARS-CoV antigen presentation depends primarily on the MHC I molecules, to the antigen presentation cells (APC), which is a central part of antiviral immunity of the body but also contributes to its presentation by MHC II (Liu et al. 2010). Afterwards, antigen presentation stimulates body's humoral and cellular immunity, mediated by virus-specific B and T cells.
Acute respiratory distress syndrome (ARDS) and cytokine storm
ARDS is a cytokine storm, which threatens life, a joint immunopathological event for SARS-CoV, MERS-CoV, and SARS-CoV-2 infections. The main mechanism of ARDS is to prevent adequate oxygen from entering the lungs and circulation, leading to death by most respiratory disorders and acute lung injury (Thompson et al. 2017). Individuals experience extreme respiratory failure involving mechanical ventilation in fatal human infections with SARS-CoV, MERS-CoV and SARS-CoV-2, and the histopathology findings also support ARDS (Xu et al. 2020; Ng et al. 2014). Rapid viral replication can cause massive death and vascular leakage of the epithelial and endothelial cells, triggering the release of pro-inflammatory cytokines (TNF-α, TGF-β, IFN-α, IFN-γ, IL-1β, IL-12, IL-18 etc.) and chemokines (CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10, etc.) in large amount by immune effector cells in SARS-CoV and MERS-CoV infections (Huang et al. 2019; Cameron et al. 2008; Williams and Chambers 2014; Min et al. 2016; Channappanavar and Perlman 2017). SARS-CoV-2 commandeers the same entry receptor, ACE2, indicates the probability of the targeted and infected cell population as SARS-CoV for infection (Gu et al. 2005; Jin et al. 2020). For SARS-CoV and SARS-CoV-2, few patients have chronic inflammation, ARDS, and even sudden death, especially those who produce early neutralizing antibodies, though most patients overcome inflammatory response and clear the virus (Jin et al. 2020; Fu et al. 2020).
Diagnostic tests for COVID-19
Laboratory diagnosis is a priority for the management and control of COVID-19 outbreaks. Respiratory samples have the highest yield, but other specimens, such as blood and stools, may also be used (Wang et al. 2020; WHO 2020). Molecular techniques are more appropriate for accurate diagnosis as they can target particular pathogens and classify them. Nucleic acid detection technology used to detect COVID-19 is a real-time quantitative polymerase chain reaction (RT-qPCR) and high-throughput sequencing. RT-qPCR is still widely used because of high-throughput sequencing is equipment dependency and high cost. Researchers have added more than 1000 sequences on the Global Initiative on Sharing All Influenza Data (GISAID) and GeneBank, which helps scientists to establish a RT-qPCR diagnostic assay (Chudhary et al. 2019). Primers are designed with a two-target system, where the first detects various coronaviruses, like SARS-CoV-2, and the second one is specific for the SARS-CoV-2. Though RT-qPCR is very specific, and its false-negative rate leads clinicians to propose computed tomography (CT scan) as a necessary auxiliary diagnostic method. Both RT-PCR and CT scans have their drawbacks. The significant problems in RT-PCR are a shortage of kits and lack in the identification of the early stage of infection or asymptomatic patient. In contrast, CT scans are expensive and require technical expertise. The problems, as mentioned earlier, should be rectified by adopting more tests that are sensitive. Moreover apart from rtPCR and CT scan diagnosis, antibodies such as IgM and IgG were also detected where it will take at least 10 to 15 days after onset of symptoms to develop these antibodies by the patient (Zhao et al. 2020). Another study indicated that, it may take up to 22 days to produce antibodies after onset of symptoms (Guo et al. 2020). However, further research is necessary to investigate the pathogenesis and design an efficient diagnoses method to overcome the false-negative results.
Possible nano-based method for diagnosing viruses
Colorimetric bioassays based on nanotechnology are comfortable and desirable for their usability; visual performance and no need for sophisticated Biosensor instruments design (Pan et al. 2010,2014). Over the last two decades, gold nanoparticles (AuNPs) have gained tremendous interest in colorimetric-based biosensing applications. They have been used for detecting a wide range of biological and chemical targets, such as small molecules, metal ions, proteins and nucleic acids, where particles change color in response to nanosized particle reactivity to external conditions (Saha et al. 2012; Curry et al. 2014; Misra et al. 2018; Peng et al. 2018; Li et al. 2018).
In 2017, Teengam et al. (2017) developed a multiplex colorimetric paper-based analytical tool using AgNPs as a colorimetric reagent for viral infection-related DNA detection such as MERS-CoV. They reached a detection limit of 1.53 nM under optimum conditions. In 2019, Layqah and Eissa (2019) identified an electrochemical immunosensor using a range of carbon electrodes modified with AuNPs that allowed human coronavirus (HCoV) and MERS-CoV proteins to be detected in spiked nasal samples. They observed a strong linear relationship between the sensor response and the concentration of viruses where linear ranges of 0.01–10.000 ngml-1and 0.001–100 ng/mL were obtained for HCoV, and MERS-CoV, respectively.
Point-of-care testing (PoCT)
Transferring diagnostic tests for COVID-19 from laboratory environments to the point of treatment is theoretically revolutionary in the pace and quantity of tests that may be done. PoC testing is described as testing conducted at home or at a location where the patient is treated using a kit or strip. The biosensor, which is used to conduct a biochemical assay to identify the pathogen, is the most important part of PoC research (Vashist 2020). Colorimetric biosensors are desirable POCTs because they allow the analyte to be detected through simple colour changes that are noticeable to the eye without assistance. One such PoC method that is under development for the diagnosis of COVID-19 is the lateral flow assay (LFA) for SARS-CoV-2 detection (Xiang et al. 2020). The nanoparticles-connected LFA makes the device more stable and sensitive. An LFA has demonstrated 81, 100, and 86% clinical sensitivity, specificity, and accuracy for IgG and 57, 100, and 69% for immunoglobulin M (IgM), respectively. The test identifies IgM and IgG with 82% sensitivity (Teengam et al. 2017). Anti-SARS-CoV-2 antibodies could be detected using these LFA in healthy blood donors and health-care staff. From COVID-19 positive patients, Villarreal et al. (2021) recorded an 87% positivity rate for both IgM and IgG antibodies.
In another study, Huang et al. (2020a) achieved a rapid diagnosis and on-site detection of the IgM antibody against the SARS-CoV-2 virus using colloidal gold nanoparticle-based lateral-flow (AuNP-LF) assay. The SARS-CoV-2 nucleoprotein (SARS-CoV-2 NP) was coated to a sample capture analytical membrane to prepare AuNP-LF strips, and the antihuman IgM was combined with AuNPs to form the detector reporter. AuNP-LF test output was evaluated by analyzing serum samples of COVID-19 patients and healthy humans. AuNP-LF's sensitivity and specificity proved 100 and 93.3%, respectively, and the results were obtained within 15 min, which requires only 10–20 μL serum for each test.
Colorimetric assay
The simple, quick and precise "naked-eye" colorimetric SARS-CoV-2 identification test is a matter of urgency at this time. Moitra et al. (2020) developed a gold nanoparticulated (AuNPs) colorimetric assay, capped with appropriately designed thiol-modified antisense oligonucleotides (ASOs) specific to SARS-CoV-2's N-gene (nucleocapsid phosphoprotein), used for the detection of positive COVID-19 cases from isolated RNA samples within 10 min. The thiol-modified ASO-capped AuNPs agglomerate targets the SARS-CoV-2 RNA sequence and demonstrate a change in its plasmon resonance on the surface. Furthermore, the virus RNA material leads to a visually detectable precipitate from the solution mediated by the new agglomeration between the AuNPs. The unique designs of the diagnostic test SARS-CoV-2 can selectively detect SARS-CoV-2 presence in a biospecimen by the naked eye without requiring any complex instrument (Fig. 1) (Zhu et al. 2020).
Nanoparticle-based virus colorimetric detection. This figure shows the process by which the virus allows nanoparticles to be aggregated, leading to a change in color from red to purple (Zhu et al. 2020)
Separation based on magnetic nanoparticle
The very first step in the molecular diagnosis of SARS-CoV-2 is nuclear acid extraction from a clinical specimen which takes a lot of work and time. Magnetic carboxyl polymer-coated nanoparticles (pcMNPs) have been developed to simplify extraction to detect SARS-CoV-2 RNA sensitively (Zhao et al. 2020) which may also incorporated in RT-PCR protocol. The advantages of pcMNPs over a traditional column based extraction process include strong viral RNA binding efficiency that results tenfold increase in sensitivity. This approach can also be used for the design of PoC devices.
Biomolecules-based detection
A biosensor includes components of biorecognition, are referred as biomolecules that are used to target based on the assay type. Antibodies are the commonly used biomolecules in ELISA and the other biomolecules are nucleic acids and enzymes. For specific diagnosis of SARS-CoV-2 nucleic acid, a double functional plasmonic biosensor was designed. This biosensor is a two-way platform that combines the plasmonic photothermal (PPT) and with localized surface plasmon resonance sensing transduction. The system consists of a complex two-dimensional gold nano-islands (AuNIs) chip where a RdRp, ORF1ab, or E gene cDNA receptor has been immobilised by gold-thiol attachment linkage. The output of this biosensor has yet to be tested in clinical samples (Qiu et al. 2020). Teengam et al. suggested an alternative paper-based colorimetric assay technique based on pyrrolidinyl peptide nucleic acid-induced NP aggregation for the detection of SARS-CoV-2 DNA (Teengam et al. 2017). These nucleic acid probes showed significant selectivity for the DNA targets. Seo et al. reported a novel antibody-based biosensor for detecting SARS-CoV-2 spike proteins. The antibody of SARS-CoV-2 was coated on field-effect transistor (FET) graphene sheets employing nasopharyngeal swab specimens from clinical patients as antigens. The virus was also observed in the growth media with an identification value of 1.6 × 101 pfu/mL using this sensor. The COVID-19 FET detector can distinguish among infected and safe individuals with a detection limit of 2.42 × 102 copies/mL (Seo et al. 2020).
Current potential treatment
No drug synthesized or in use for SARS-CoV-2 infection (unfortunately), up to now; the latter leads to a lethal and acute inflammatory response and pulmonary injury. To establish an effective COVID-19 treatment using nanoparticles, one must understand the nanoparticles' versatile mode of viral inhibition mechanism. SARS-CoV-2 also uses a 'lock and key' mechanism similar to SARS-CoV and MERS-CoV, in which the angiotensin converting enzyme II (ACE2) acts as a 'key' for entering specialized cells keeping its “lock”, which is mostly present in lungs, heart, intestine cells, arteries, and kidneys (Yang et al. 2019). After the virus has reached the cell, they replicate and invade other cells of the host cell and organelles, on that basis, the treatment that stops the virus from entering the cell may be beneficial (Itani et al. 2020). SARS-CoV-2 Mpro protease is one of the most promising candidates for antivirals in the design and production of SARS products. Ton et al. (2020) created a groundbreaking forum for profound learning called deep docking (DD) and performed docking tests of 1.3 billion compounds in the library of ZINC15, and listed the top 1000 ligand capacity for SARS-CoV-2 Mpro protein. These systems are made available for subsequent studies in cell culture and animal pattern experiments by the scientific community. This research is very important as it is very easy to obtain docking results from in-silico experiments and allows a structural simulated virtual screening of billions of compounds to be purchased in a limited period of time. But in the other side, contradictory evidence suggests that SARS-CoV-2 Mpro is not appropriate since the protein target has already been mentioned (Bzowka et al. 2020). Qamar et al. (2020) performed a successful SARS-CoV2 multi-epitope vaccine (MEV) by the use of seven proteins (B cell, IFN-β and T-cell). Docking experiments have shown a solid and clear affinity between MEV and TLR8 and TLR3, while optimization of codon and in-silico cloning has enhanced the output of the E. coli K-12 system. For the production of vaccines, potential experimental validations in this direction will yield useful outcomes.
Usage of supportive drugs
As there is no scientifically proven active antiviral agent against SARS-CoV-2, a variety of drugs are licensed for use in clinical trials such as Chloroquine phosphate, Darunavir, Favipiravir, etc., (most commonly used antiviral drugs are listed in Table 1). Moreover, these drugs are not specific against SARS-CoV-2 but have general antiviral activity, which can interfere with viral entry or block receptors of the virus. Coronaviruses are usually not responsive to existing antiviral drugs, and in the case of coronavirus infections, combinations of various treatments were also used for treatment (Zylka-Menhorn 2020). Such successful combinations for the treatment of COVID-19 are lopinavir/ritonavir plus arbidol (Huang et al. 2015) and lopinavir with ritonavir (Han et al. 2020; Lim et al. 2020). Another study suggests that ribavirin could be a potent drug inhibiting coronaviruses replication if combined with interferon-β (Al-Tawfiq et al. 2014; Arabi et al. 2020). Very recently, a combination of remdesivir and chloroquine gained more attention because of its effectiveness in halting SARS-CoV-2 replication process (Alanagreh et al. 2020). Some of the therapies mentioned above are not unique to COVID-19 and are supportive treatments, including cardiovascular/hemodynamic or respiratory therapies that assist patients with the virus. However, these drugs can reduce symptoms and risks but should not kill the virus effectively.
Antibody and plasma therapy
Convalescent plasma therapy contains the acellular components of blood from the COVID 19 recovered patients that contains SARS-CoV-2 antibodies. This passive immunization (PI) has also been reported for short-term fortification against infectious agents in which the coronavirus-specific human monoclonal antibody (mAb) (CR3022) can bind powerfully to the SARS-CoV-2 receptor-binding domain (RBD) (Tian et al. 2019). When the human body is infected with viruses, it produces antibodies to fend off the virus. Such antibodies can be obtained as convalescent plasma in a healed patient's blood and transferred to a newly infected patient's blood where they can neutralize the pathogen, and improve the patient's immunity. Patients with pandemic influenza A(H1N1) 2009 virus infection who received convalescent plasma had lower respiratory tract viral load, serum cytokine response, and mortality in previous studies (Hung et al. 2011). In another report, SARS patients who received convalescent plasma had a higher percentage of hospital discharge at day 22 from the initial infection than those who did not receive convalescent plasma (Cheng et al. 2005). These results suggest that the use of convalescent plasma transfusion can be candidate therapy for the prevention and treatment of COVID-19 patients, particularly in life-threatening situations.
Current vaccines
Scientific research on vaccine development strategies against SARS-CoV-2is growing and research-tested in animals and humans, such as inactivated virus, live attenuated virus, recombinant protein, subunit vaccines and nucleic acid vaccines (Chen et al. 2020b) are in their preliminary stage. Out of more than 200 candidate vaccines, few of them completed clinical trials and are administered to humans as successful SARS-CoV-2 vaccines to prevent viral shedding and transmission, thereby helping to control coronavirus outbreaks. There are numerous studies underway, especially in the US, India, China, Russia and the UK. Pfizer and BioNTech have developed mRNA-based vaccine (BNT162b2), where it got authorization in several countries for public use. By coding a mutated version of full-spike protein of the virus, BNT162b2 produces an immune response to SARS-CoV-2. Another important mRNA-based vaccine (mRNA-1273) was developed by Moderna, is also ready for human. Russian company ‘Gamaleya Research Institute’ developed Sputnik V, a non-replicating viral vector vaccine in partnership with Health Ministry of the Russian Federation. Vaccine developed by Oxford University and AstraZeneca named ChAdOx1 nCoV-19 (chimpanzee adenovirus-vectored vaccine expressing the SARS-CoV-2 spike protein) (NCT04324606) has demonstrated an appropriate safety profile and a homologous improvement in antibody responses (Folegatti et al. 2020). A randomized, double-blind, placebo-controlled, was designed to determine the immunogenicity and safety of Ad5-CoV, which codes for a full-length SARS-CoV-2 spike protein (NCT04341389).
Possible effects and efficacy of vaccine on VUI –202012/01 new variant
The new variant has no phenotypic information and no data are available about the ability of antibodies developed by the emerging vaccines to neutralize this variant. As already described, the latest virus strain reveals multiple mutations on the spike protein, as well as in the receptor binding site. The latest vaccines candidates are mostly based on the spike protein sequence. Therefore the modifications in spike protein of the circulating SARS-CoV-2 strains must be observed for assessing potential antigenic changes. The antigenic description of the new variant is underway and the findings are expected in the upcoming days. Monitoring of field efficacy of COVID-19 vaccines in use would be significant, if possible having variant-virus-specific estimates. Monitoring of primary vaccine problems using variant-virus-specific effects can also help to understand whether there is an influence on vaccine efficacy. It must be noted that T-cell immunity plays an important role in the defense against and evacuation of COVID-19 viral diseases. While T-cell immunity is evaluated both after infections with SARS-CoV-2 and after vaccination, it is still unclear what function it could play in defense correlations.
Traditional herbal medicines
Researchers are focusing on complementary and alternative medicinal systems (CAM’s) due to the lack of specific treatments or vaccines against SARS-CoV-2. Several compounds of medicinal plants have gained significant attention for their efficacy in various therapies for viral diseases where the bioactive compounds have no or limited side effects (Tian et al. 2019). CAM’s are currently part of the medicinal system in various countries such as India, China, South Korea, Singapore etc., (Qamar et al. 2019). Indian traditional medicine method includes the convergence of Ayurveda, Yoga, Unani, Siddha, and Homeopathy under one umbrella called AYUSH. Ayurveda and Homeopathy employ natural drugs of plant, animal and mineral origin for treatment and are accepted worldwide (Prajapati 2020). Rastogi et al. (2020) proposed Ayurvedic intervention with graded response depending on the stage of infection and disease proximity among individuals within a population. Though there is no proven effective medicine against the SARS-CoV-2 pathogen, their proposal may give remedies and subsequent treatment choices. Girija and Sivan (2020) tested Ayurvedic medicine such as Sudarsana Churna, Talisadi Churna and Dhanwantara Gutika against a positive COVID-19 patient. They found the medication to halt the progression of the disease to a more severe state.
Traditional Chinese and South Korean medicine also comprises of herbal medicines close to Ayurveda, which appear to have some effect in the promotion of COVID-19 prevention and treatment therapies (Ang et al. 2020) and also used in the past during SARS-CoV and H1N1 influenza epidemic outbreaks (Chen et al. 2011; Xiaoyan et al. 2018). A total of 28 traditional medicine guidelines are provided out of this 26 were Chinese government-issued, and two were by Korean medicine-professional associations (Yang et al. 2020). In China, more than 85% of COVID-19 patients had received the Traditional Chinese Medicine form of treatments (Ma et al. 2020). These traditional medicines may be capable of targeting ACE2, and these demonstrate some promises to avoid SARS-CoV-2 infection (Girija and Sivan 2020; Zhou et al. 2020b) and also manifest possible immunosuppressive effects by reducing TNF-α, IL-1β, IL-6, IL-8, IL-10 and other cytokines levels (Rastogi et al. 2020; Wang et al. 2008; Chang et al. 2015; Liu et al. 2019).
Nanotechnology against viral infections
Viruses have high mutation rates that make vaccination a difficult job because they can quickly develop resistance to existing drugs, and new viruses often emerge. There is a growing need for new medicines to be found, as well as enhancing the current drug formulations. In view of the lack of effective specific vaccine or treatment strategies against SARS-CoV-2, the development of a new method of treatment is required. Nanoparticles provide specific physical properties associated with delivery of the drugs and their usage as carriers for the transportation of most widely used antiviral therapies. Nanoparticle’s antiviral properties are mainly due to particle size that facilitating the delivery of drugs, large surface area by volume ratio to accommodate large drug payloads, and the particle’s adjustable surface charge with the possibility of encapsulation (Parboosing et al. 2012; Caron et al. 2010; Petros and Desimone 2010; Kumar et al. 2012). As a result of the chemical interactions on the virus surface, between the molecules-functionalities and the molecules-receptors, the particular nanoparticle exhibit the antiviral properties. Several nanomedicines are under investigation to establish possible nanotherapeutics for their antiviral activities. In the past, MERS or SARS have substantially lower infection rates than COVID-19, but treatment and vaccine candidates have not been thoroughly researched and developed. The initial research results on these viruses, on the other hand, provided a foundation for overcoming the current pandemic situation. Kato et al. (2019) developed nano-sized virus-like particles (VLPs) using recombinant S, membrane, and envelope proteins that found in MERS-CoV and SARS-CoV, and confirmed that the immunogenicity of the tested animal model was increased. In animal studies, these nano-sized VLPs have been shown to effectively defeat viruses by increasing the immune response.
Specific antiviral nanoparticles (Nanovaccinology)
Staroverov et al. (2011) analyzed the protective immune response in immunized mice and rabbits by administrating gold nanoparticles linked to a type of coronavirus known as transmissible swine gastroenteritis virus (TGEV). The authors stated that immunization with the antigen-colloidal gold complex increased T cell propagation by tenfold compared to free antigen response, resulting in enhanced expression of respiratory macrophage performance and increased protective immunity to TGEV. The research revealed that the gold nanoparticles conjugated to a virus could be suggested as a possible vaccine candidate for viruses. The antiviral effects of silver nanoparticles against herpes simplex virus type 1 (Baram-Pinto et al. 2009), HIV-1 (Sun et al. 2005), respiratory syncytial virus (Sun et al. 2008), hepatitis B virus (Lu et al. 2008), and monkeypox virus (Rogers et al. 2008) have been reported.
Schmitt et al. (2020) reported that the respiratory syncytial virus (RSV) was removed with RSV fusion protein-like nanoparticles (FVLP) in mice animal model. Natural killer cells activated IFN-γ(+), and T-cells are induced by the use of FLVP against infection stage, RSV in the lung and bronchiolar airways. Still, they do not form harmful lung plasmacytoid dendritic cells (DCs) and effector T-cells. Gold nanorods also successfully inhibit RSV by 56% in BALB/c mice through upgraded antiviral genes due to the NOD-like signaling pathways and RIG-I-like receptor. In another study by Gaikwad et al. (2013), the siRNA loaded lipid-based nanocarrier, ALN-RSV01, battles the “N” nucleocapsid gene, a core RSV viral protein. This was the first RNAi-based therapy approved for clinical trials and has now entered Phase II, showing very safe and effective antiviral effects.
The earlier report of Hendricks et al. (2013) has interestingly noted that the 'Nanotraps' is a heat-stimulating hydrogel that successfully traps live viral cells, RNA, and proteins, and it can also be used to treat infectious disorders including influenza virus. Hendricks et al. (2013) also successfully used liposomes to transport a synthetically derived receptor (glycan sialylneolacto-N-tetraose c (LSTc) sialoside) by dose-dependent fashion to bind and capture the influenza A virus. The antiviral ability of copper iodide nanoparticles has been extensively studied against the influenza A strain because of its impact on viral proteins such as hemagglutinin and neuraminidase, resulting in virus degradation and inactivation via the formation of reactive oxygen species (ROS) (Fujimori et al. 2009). Liu and Chen (2016) presented an insightful viewpoint on the use of nanotechnology in synthesizing HIV/AIDS vaccines. Their review highlights the potential of various nanomaterials and nano-architectures for use as vector or adjuvant HIV vaccines due to excellent potential to enhance conventional HIV vaccine delivery, permeability, stability, solubility and pharmacokinetics. In 2019, Cao and Woodrow (2019) analyzed the nanotechnology strategies used to remove HIV reservoirs and the nanocapsules of gene delivery and therapies used in cancer with the potential for HIV treatment.
Yadavalli et al. (2019) studied the ability of particles with highly porous activated carbon (HPAC) as a model for limiting the entry of HSV-1 and HSV-2 into target cells. The surface-active charcoal which could have antiviral effects via a virion sequestration method, and the HPAC compound showed a reduction of 40 to 60% HSV-1 and HSV-2 entry for concentrations as low as 1 mg/mL. The adsorbed or encapsulated acyclovir (ACV) molecules inside the HPAC pores showed a sustained drug release that works synergistically to achieve an enhanced therapeutic effect. In vivo studies of ocular (HSV-1) and genital (HSV-2) infection using a murine model, ACV loaded HPAC works by trapping the virus and releasing the encapsulated drug, preventing inflammation and penetration of the immune cells into the targeted tissue. The potent antiviral activity was allocated to the charged surface of its pores, which can interact with the surface of the cell, stimulating an active ion exchange (Na+ , K+ , Ca+ , Cl−, and OH−), when ACV has been acquired sustained or gradually released.
Jung et al. (2018) tried to develop an immunogenic MERS-CoV vaccine using a heterologous prime-boost strategy concerning a recombinant adenovirus serotype 5, which encodes the spike gene of MERS-CoV (Ad5/MERS) and spike protein nanoparticles. The mice groups (female BALB/c mice), which are three times immunized with the prime-boost vaccination, show that the homologous spike protein NPs effectively induced higher antibody titers relative to the Ad5/MERS group alone. However, they suggest that the heterologous one-stage Ad5/MERS prime and two-stage spike protein NPs boosting appeared to be more successful for longer-lasting immunogenicity and an appropriate Th1/Th2 response balance than the homologous prime-boosting scheme using either Ad5/MERS or spike protein nanoparticles alone. Another research revealed that HPIV-3 replication was inhibited, likely because of a blocking role of the cell-virus that leverages AgNPs. The findings of this study show that inhibitory activity depends on both the size of the NPs and the zeta potential (Lee et al. 2015).
Many nano-based solutions are designed to specifically bind the virus particles so that they do not approach the host cell. For example, carbon quantum dots interact with human coronavirus (HCoV-229E strain) S protein to prevent the viral protein interaction with the host cells which will reduce the viral replication (Fig. 2). As boronic acid was used in the carbon quantum dots, they had even greater antiviral activity (Łoczechin et al. 2019).
Carbon quantum dots prevent the interaction of human corona viruses with their receptor(s) (a) and inhibit the replication of virus genome (b) (Łoczechin et al. 2019)
New developments of SARS-CoV-2 nanotherapy
Many newly existing therapists for COVID-19 use nano-based techniques. SARS-coV-2 virus-like particles are being developed by iBio and Beijing CC-Pharming using iBio's Quick Pharma method (ibioinc; visit related links). The virus-like particulate matter (VLPs) is purified from plants and screened as candidates for vaccines. NanoViricides, Inc. has developed SARS-CoV-2 virus particulate nanoviricides (nanomicelle bonded with ligands) that can bind and engrave. Moderna has created an mRNA encapsulating lipid nanoparticle vaccine in which mRNA codes spike protein of SARS-CoV-2 (Moderna, Inc., visit related links). This vaccine based on nanoparticles has already been clinically validated and ready for public.
Examples of biocompatible systems
Nanomedicine is one of the promising platforms for virus detection and neutralization. Several experimental reports of antiviral action of nanoparticles against different viruses like influenza virus H3N2 and H1N1 (Mazurkova et al. 2010; Lysenko et al. 2013), herpes simplex virus (Lysenko et al. 2013; Hu et al. 2014), hepatitis B virus (Lu et al. 2008), HIV-1 (Elechiguerra et al. 2005; Lara et al. 2010), dengue virus type-2 (Sucipto et al. 2017), Foot and Mouth disease virus (Rafiei et al. 2016), vesicular stomatitis virus (Lokshyn et al. 2014). Nanoparticles of silver (Hu et al. 2014), gold (Sucipto et al. 2017; Rafiei et al. 2016), silicon Dioxide (Botequim et al. 2012), copper (Sucipto et al. 2017), titaniumdioxide (Mazurkova et al. 2010), ceriumdioxide (Lokshyn et al. 2014) are the few of the evaluated delivery system for their suppressive properties of viruses. Elechiguerra et al. (2005) and Rafieiet al. (2016) demonstrated the adsorption of nanoparticles on the surface of the virus, which leads to local surface transformations, such as glycoprotein agglutination, thus preventing virus penetration into the cell.
Chemistry, architecture of each nanosystem and its unique properties determine the mechanisms of nanomaterial mediated drug delivery. Many forms of nanoparticles have been suggested as promising for viruses and are divided into three broad categories based on their composition, such as inorganic, organic, and virus-like or self-assembling protein nanoparticles.
Organic nanoparticles
Organic nanoparticles are nanomaterials based on carbon, which are usually characterized by high biocompatibility and increased drug load ability. Organic nanoparticles are the most extensively studied method of drug delivery nanoparticles, and the most widely accepted method for human therapeutic use (Zazo et al. 2016). The use of organic nanoparticles over inorganic nanoparticles is highly recognized in the biomedical sector due to various safety concerns and may prove beneficial in efficiently carrying or supplying the drug load (Mitragotri and Stayton 2014; McClements and Xiao 2017).
Lipid-based nanoparticles, liposomes and niosomes
Nanoparticles made from lipids have proven their potential in advanced medicine as an extremely efficient biocompatible platform for therapeutics. Anomalous bio-absorbable and biocompatible properties of lipid-based NPs have created interesting opportunities for these nanosystems (Joshy et al. 2017). To check drug release parameters for hemocompatibility and non-toxic viral inhibition in cell lines VK2/E6E7 challenged with human papillomavirus (HPV), Gao et al. (2018) developed a nanostructured nanolipid carrier loaded with podophyllotoxin (POD). Both the types of lipid-based NPs that are solid-lipid NPs and nanostuctured lipid nanocarriers have been reported as vehicles for successful antiviral delivery against certain viruses, such as HIV, Hepatitis C virus, and HPV. Still, their therapy efficiency against SARS-CoV-2 has yet to be assessed. Using solid-lipid nanoparticles has additional benefits include increased toxicity compared to synthetic polymer nanoparticles, with better drug release profiles (Patel and Patravale 2011; Xie et al. 2014). Argenta et al. (2014) studied lipid nanoemulsions encapsulating coumestrol as Topical Treatment for herpes simplex virus. They formulated the bioactive compound coumestrol, trapped in a hydroxyethylcellulose gel by fluid or rigid phospholipid nanoemulsions (dioleylphosphocholine, DOPC, and distearoylphosphocholine, DSPC, respectively). Coumestrol formulation using DOPC-based nanoemulsions showed an improved antiviral activity against HSV-1 and HSV-2, which might be considered for advanced therapy studies.
Among the numerous possible applications of lipid-based NP formulations, liposomes composed of phospholipid bilayers containing an aqueous nucleus have been thoroughly researched in vaccine studies because of their ability to function as immunologic adjuvants (Perrie et al. 2008; Kumar et al. 2016). Liposomes provide various benefits, including relatively non-toxic, successful conjugated agent encapsulation and easy alteration to improve further their mucosal and cellular uptake and biodegradable properties (Khan et al. 2020). Like any other form of nanoparticles, surface charge plays a vital role in affecting liposome pharmacokinetic properties. In particular, liposomes were used as carriers for the topical application of acyclovir and the treatment of ocular CMV infections and as carriers for the oral administration of interferon-α (Milovanovic et al. 2017). Another type of vesicle, noisome, is similar to a liposome but formed from nonionic surfactant instead of lipid noisome.
Polymer nanoparticles
As an attractive delivery mechanism, polymeric nanoparticles in colloidal solids have been documented, and their progress in successful antiviral treatment has been going on for over a decade. They can be synthesized by adding many monomers that are approved by the World Health Organization (WHO) and the Food and Drug Administration (FDA) (Uskokovic and Stevanovic 2009). Their small size can promote them for use in medicine and pharmaceuticals by its capillary penetration and absorption by cells leading to increased concentrations at the target sites (Ochekpe et al. 2009). Nanocapsules (are hollow spheres, where the drug is confined) or nanospheres (are matrix structures where the drug is distributed physically or equally) are the two primary forms of polymeric nanoparticles. Chitosan polymer nanoparticles have drawn particular interest in internal administration because of their superior in vivo biocompatibility and biodegradability profiles, and their ability to open strong links between epithelial cells (Sonaje et al. 2012; Zhao et al. 2014). Joshy et al. (2017) conducted a study against HIV using amine-functionalized polymer gelatin NPs loaded with zidovudine. Studies were also conducted on hemolysis and aggregation and found that drug-conjugated NPs displayed promising biocompatibility and antiviral activity.
Dendrimer nanoparticles
A dendrimer is a well-organized hyperbranched, symmetrical molecule, also an outstanding medium for genes, peptide or drugs (natural/synthetic) delivery inside the biological system at the desired site (Xu et al. 2018). Antiviral activity of dendrimer combined with therapeutic agents avoided host infection by inhibiting viral entry into the cells, activation of CD8+ T cell against viruses like influenza virus, ebola virus, zika virus, HSV-1, 2 have already been reported and have become an effective tool for the treatment of HIV viral infections (Chahal et al. 2017; Asgary et al. 2018). Nandy et al. (2015) documented the development of dendrimeric nanoparticles with anionic naphthalene disulphonate surfaces based on Poly-L-lysine (PLL), which may prevent the entry of HIV viruses by binding to the viral envelope protein gp120 and preventing the formation of a complex CD4-gp120.
Nanomicelles
Nanomicelles are the supramolecular assembly of a surfactant molecule size ranging from 10 to 100 nm (Letchford and Burt 2007), having powerful and most attractive nanotechnological properties including effective drug encapsulation, biocompatibility, colloidal stability and prolonged circulation (Talelli et al. 2015). Micelles also exhibit dissociation, thus allowing a longer drug retention period, and therefore a higher accumulation of the medication at the target site (Mahajan et al. 2012). Naseri et al. (2017) developed a nanoformulation using a bioactive compound encapsulated with nanomycelle and reported better bioavailability and adequate antiviral activity.
Inorganic nanoparticles
Metal nanoparticles can be categorized under inorganic nanoparticles, which are smaller than organic nanoparticles, ranging in size from 1 to 100 nm, while their loading efficacy is far greater (Mahajan et al. 2012). Two major regions namely a core containing the inorganic portion (such as gold, quantum dots, silica, or iron oxide) and a shell region consisting organic polymers, providing an adequate substratum for the conjugation of biomacromolecules or shielding the core area against unnecessary physicochemical interactions (Swierczewska et al. 2011; Giner-Casares et al. 2016).This concept of multiple interactions with the targeted molecule at a particular site further leads to the use of these NPs in actively targeted imaging for diagnostics, hyperthermia therapy and medication (Li et al. 2018).
Gold nanoparticles
Gold nanoparticles have shown particular interest in the production of vaccines because of their excellent conductivity, the versatility of surface alteration, biocompatibility and they can easily activate the immune system by internalizing the cells and has a lower toxicity than other metallic nanoparticles (Cui et al. 2012; Ramkumar et al. 2017). There are many studies that biocompatible polymer-stabilized gold nanoparticles demonstrated an active antiviral agent against several viruses, such as HIV-1, H1N1, H3N2, H5N1, dengue virus, bovine viral diarrhea and Foot-and-mouth virus (FMDB) (Rafiei et al. 2016; Vijayakumar and Ganesan 2014; Ahmed et al. 2016). Due to the existence of a negative charge on gold nanoparticles, it quickly functionalized with various biomolecules such as drug molecules, antibiotics, proteins, genes and a range of targeting ligands without displaying any toxicity found in in-vivo investigations on some human cell lines(Ghosh et al. 2008; Sreejivungsa et al. 2016; Verissimo et al. 2016; Kong et al. 2017). MarquesNeto et al. (2017) studied intranasal delivery adaptability and configuration and confirmed that gold nanoparticles are readily disseminated into lymph nodes, triggering CD8 + (T-killer).
Silver nanoparticles
Among metallic nanoparticles, silver ones are the most successfully studied nanoparticles against bacterial and viral diseases and for detection of infection (Gong et al. 2007). Numerous tests of the NPs had already been carried out to establish a novel approach to either destroy or improve the severity of the infection by releasing of silver ions (Rai et al. 2009), destruction of the cell membrane, and DNA damage (Huh and Kwon 2011).
Nanosponge
Nanosponges are made from membranes derived from human cells, which are naturally attacked by SARS-CoV-2 (Verissimo et al. 2016). The new approach suggested by Zhang et al. (2020) for drug production, the priority should be on the infected host cells rather than on the causative agent; coronaviruses can't infect their normal cellular targets while bound with nanosponges. Two types of cellular nanosponges were manufactured by the team, probably human lung epithelial type II cell nanosponge (known as "Epithelial-NS") and human macrophage nanosponge (known as "MΦ-NS"). Zhang et al. (2020) confirmed the presence of ACE2, transmembrane protease serine 2 (TMPRSS2), and dipeptidyl peptidase IV (DPP4) viral receptors by Western blot analysis on the epithelial-based nanosponges. To improve the antiviral efficacy of these nanosponges, more work is needed for testing in appropriate animal models, though.
Nanoparticles-based treatment modalities/Possible nano-based approach for SARS-COV-2 inhibition
Enhanced drug delivery
Drug delivery system is a method used to deliver drugs to desired cells and organs using a different drug carriers (Bheemidi et al. 2011). Its major purpose is to overcome problems of the pharmacological activities of drugs like the lack of selectivity, solubility limitation and drug aggregation, and to reduce the therapeutic undesirable effects (Li et al. 2019). During the past few years, drug delivery systems witnessed a great progress. Recently, nanoparticles were considered to be great drug carriers. Drugs can be encapsulated in nanoparticles, including liposomes, dendrimers, micelles, nano capsules, nanospheres and among others. These encapsulations considerably improved the therapeutic index and reduced side effects (Zhang et al. 2010; Zhu et al. 2014). Throughout the past decades, serious and chronic diseases were mainly treated using a simple compound such as pills, tablets, creams and aerosols (Khan and Irchhaiya 2016). The main drawback of these approaches is related to their uncontrolled release of the drug (Liu et al. 2016; Laffleur and Keckeis 2020). A controlled and efficient drug release with reduced administration cost is needed to control the limitations of the conventional drug delivery systems (Laffleur and Keckeis 2020).
Since COVID-19 is a respiratory disease, inhalation exposure nanoparticles may be a non-invasive means of transmitting anti-SARS-CoV-2 drugs directly to their target site. This will lead to the pre-emptive accumulation of nanoparticles in the lung tissue infected by SARS-CoV-2. Tools such as nebulizers that supply the drug as a solid or liquid embedded in the Gas Medium are used in breathing form to deliver medications (Zhou et al. 2014). They can be conveniently delivered in inhalable form with improved lung deposition and retention in conjunction with nano-delivery systems (Abdelaziz et al. 2018). Nanocarriers are particularly essential for poorly soluble medicines, which are developed into bolus and later intoxicated by lung when used as free medications (Beck-Broichsitter et al. 2012). The physiological hurdles to the production of inhalable nanomedicines are the mucociliar clearance and phagocytosis of alveolar macrophages.
Stimuli-responsive strategy for drug delivery
Currently, stimulus-sensitive transportis considered the most interesting strategy for drug delivery. A wide range of triggers can be used to ensure a controlled and specific delivery. Li et al. (2019) defined the different endogenous and exogenous triggers that can be used to design the stimulus-responsive systems. To ameliorate the penetration ability of drugs into the desired cells, intrinsic and extrinsic stimuli are used to modulate the nanoparticles structure and their surface properties. Canaparo et al. (2019) and Mi (2020) recently provided an overview presenting endogenous and exogenous trigger used to guarantee on antibiotics and antibacterial drug delivery.
Stimuli-responsive strategy showed a great relevance in the field of treating cancer and developing vaccine. However, some limitations need to be controlled in the field of using nonantibiotics such as the limited tissue-penetration depth putting this strategy in the early stages of development. Another issue is the material toxicity, especially for the inorganic materials (Canaparo et al. 2019; Mi 2020).
Co-delivery strategy and theranostic
Among frequent clinical practice to treat a wide range of diseases, researchers use to combine different drugs. However, the difficulty lies in the combination of the individual pharmacokinetic profiles of the used drugs to get the best synergistic effect. Cell-based tests (Zhang et al. 2017a) are essential to get an optimal dose ratio. Co-delivery using carriers having identical pharmacokinetic properties are useful to get a better combination.
The term theranostics was used about ten years ago to describe diagnostic tests developed to conduct distinctive therapies (Upponi et al. 2018). Lately, the term has been used to designate the fusion of diagnostic and therapeutic strategies in the interest of increasing the efficiency and the safety in a better customized form (MacKay and Li 2010). Thereby, nanoparticles were used as co-delivery strategies for specified theranostics (Mura et al. 2013). Stimuli-sensitive polymeric nanoparticles with multifunctional properties qualify a better drug loading and improve in vivo therapeutic molecules stability. Nonetheless, improvements are still needed to finer identify the mechanism of action between the therapeutic agents and their pharmacological activities (Pan et al. 2019).
Biomimetic delivery strategy
Biomimetic is an innovative structure of technology inspired from nature to upgrade human life (Hwang et al. 2015). The intention of seeking inspiration from nature has been used for a long time. This idea has also paved the way for actual profits by reducing waste and spending (Barthelat 2007).
The Biomimetic Drug Delivery System is an advanced strategy for nanosystems. It imitates the biological systems such as cell structure or cell membrane (Zhang et al. 2017b). This delivery system presents good biocompatibility and less immunogenicity (Xue et al. 2017). It is well-established in the field of biomedical applications, in particular for targeted drug delivery in addition to the detoxification of cells and blood (Armstrong et al. 2017). The biomimetic strategy is most applicable in nanoparticles concealment in the cell membrane, extracellular vesicles, nanoparticles coated with lipoproteins, virus-like nanoparticles and others (Li et al. 2019).
The Biomimetic Drug Delivery System guarantied good potential in the biomedical application. But, similar to new emerging technologies, many problems need to be resolved before leading clinical application (Li et al. 2019). These cover incontinent encapsulation of nanoparticles and difficulties in making a normalized protocol for their production and purification (Laffleur and Keckeis 2020).
Specific small interfering RNAs (siRNAs) delivery
Short interfering ribonucleic acids (siRNA) have attracted attention thanks to their effective function in gene silencing (Cooper and Putnam 2016).The science of siRNA has become of great interest to the pharmaceutical industry, designed as a novel drugs delivery system. siRNA inhibits protein synthesis, and reduces the undesirable side effects compared to the conventional pharmacological therapy. Other advantages are the easy manufacturing in a large scale and a well-developed synthesis with reduced costs (Cavallaro et al. 2017).
siRNA structure and mode of action
Approximately, siRNA is a dsRNA of 21–23 pair of complementary bases in a double-stranded nucleic acid molecule in length, with 2-nucleotide extend over the both 3’ ends and complementarity to select mRNA (Jackson and Linsley 2010). When synthesized in the cell nucleus and then transferred in the cytoplasm, it’s called endo-siRNA (Piatek and Werner 2014), and exogenous, when it is formed outside the aimed cell and then delivered into the cytoplasm (Jeang 2012). But commonly, the siRNA designation in higher organisms refers to exogenous synthesized dsRNA (Cavallaro et al. 2017). Already in the cytoplasm, siRNA partake the formation of a multi-protein complex, the RNA-induced silencing complex (RISC), which breaks down the passenger strand and integrates the guide strand. The RISC complex refers to this strand to recognize and split a complementary mRNA to prevent the synthesis of an encoded protein and to selectively silence the gene expression (Kanasty et al. 2013).
siRNA gained significant attention as an encouraging therapeutic system in the field of gene and protein functions(Kang et al. 2017a; Wang et al. 2016). It is studied to theoretically silence any target gene even the undruggable ones. Researchers are currently developing novel siRNA structures to treat cancer, genetic and viral diseases (Aagaard and Rossi 2007; Iranpur Mobarakeh et al. 2019; Patel and Agrawal 2017). Figure 3 represents the siRNA mechanism in breaking down genes.
siRNA-mediated gene silencing from Cavallaro et al. Review (Cavallaro et al. 2017)
In brief, when RNAi is inserted into the cell, it is integrated into a protein complex named the Silencing Complex or RISC (Scaggiante et al. 2011). The multifunctional protein of RISC complex Argonaut 2, unfolds the siRNA. Subsequently, the sense strand of the siRNA is split. Once the RISC with the antisense strand is activated, it starts scanning to find a complementary mRNA. When attached to its founded mRNA, the single-stranded siRNA causes the mRNA 7 division. The mRNA is subsequently perceived as untypical and consequently degraded (Cavallaro et al. 2017).
siRNA therapeutic potential
For a well-designed siRNA, the RNAi system can be used to suppress practically any genes in the body, expanding its therapeutic potential larger than small conventional molecule drugs. With a view to implement these advances in a practical use, delivery systems must be developed in a safe and effective manner. However, non-modified RNAi is contingent to be degraded by endogenous enzymes. Also, its large size (∼13 kDa) and its great negative charge decrease the cellular membranes cross ability. Nevertheless, effectiveness and non-toxicity presents the major challenge that should be studied to use the siRNA technology in a practical therapeutic application (Whitehead et al. 2009).
The biological barriers control in siRNA technology
In vivo delivery of siRNA presents the principal challenge for being applied in clinical therapies. Endogenous nucleases rapidly degrade the unmodified RNAi. One possible technique to inhibit this enzymatic breakdown and to decrease simultaneously the undesirable side effects is the siRNA chemical modification (Shukla et al. 2010). Howbeit, there are some issues that still need further efforts to be solved (Serrano-Sevilla et al. 2019): the quite small siRNA size provoke a fast renal emission regarding the corpuscle pore size. The RNA hydrophilicity and its notable molecular weight prevent its penetration into the hydrophobic cell membrane. Solutions consist of enhancing the siRNA transport into the cytoplasm of targeted cells to successfully use the siRNA-based therapeutics (Hong and Nam 2014).
Currently, viral-based carriers are studied in the field of siRNA delivery research. Although, in spite of their promising efficiency for in vivo transfection, their toxicity, inflammatory and immunogenicity potentials remain questionable (Serrano-Sevilla et al. 2019). Moreover, other limitations exist like difficult manufacturing processes and little cell selectivity (Singh et al. 2014). Withal, current progress in nanotechnology sciences is focused on the development of non-viral vectors as substitute for siRNA delivery. Nanostructured delivery systems improve the absorption ability into a targeted tissue and extend the retention time of siRNA (Kumari et al. 2010).
Basically, RNAi therapeutics is being continuously studied since the first confirmation of gene inhibition. siRNA-based vectors shown on table potential as therapeutic agents for a large range of diseases (Whitehead et al. 2009). However, further work is needed to improve the RNAi therapeutic delivery to carry out their application in the clinic.
Peptide inhibitors
Peptides are active molecules containing at least two amino acids through a peptide bond. Unlike large proteins, peptides are smaller in size and include less than 100 amino acid residues. Their high selectivity and superior safety characteristics make them a good candidate for pharmacological applications (Uhlig et al. 2014). The human body contains peptides for several biological roles mainly to signal and regulate molecules in different physiological processes. Previously, usage of peptides in drug development was limited because they were easily deactivated by many proteases contained in the human body. Successfully, current technological advances have revoked the previous situation and improved the peptide stability (Chew et al. 2017; Gentilucci et al. 2010).
The mechanism of action for therapeutic peptides
Several studies are available to describe the mechanism of action of therapeutic peptides. Among these, some peptides presented physical effects causing disordering of the lipid bilayer of the virus envelope or even the viral aggregation. Others bind to and simulate the synthetic viral membrane. There are inhibitory peptides that prevent viruses to bind and/or to fusion to cells.
Numerous studies submit that some processes are involved at the same time (Badani et al. 1838). Chew et al. (2017) discussed the formation of peptide drugs and their mechanism of antiviral activities. Special attention was given to peptidomimetics (modified peptides) viral activity against dengue virus. They also described the use of peptides as inhibitors for viral infection focusing on three principal targets, specially, host cell receptors, viral structural proteins and viral non-structural proteins.
Implications and future directions
Recently, peptides have gained much attention from the therapeutic drug industry. Several researches were developed to use peptides as therapeutic drugs to treat viral diseases thanks to their high selectivity, low toxicity and little accumulation in tissues. However, peptides have some limitations that could be resolved by chemical modification (Chew et al. 2017; Craik et al. 2013). Advances in the field of materials and chemistry synthesis have improved the purification systems aimed to face challenges in manufacturing peptides in large scale while reducing production cost and making compound modifications.
Prevention approaches
Virus entry into cells
The entry for SARS-CoV in the human body is established through the respiratory system, mostly by droplet transmission. The infection and cells responses are resulted from the spike protein of the surface envelop actions. The interaction between the spike protein (S protein) and the receptor initiate the attachment of the virion to the host cell. Once the attachment and the bonding are established, the S protein conformation is changed and then followed by cathepsin of L-mediated proteolysis within the endosome. The host cell receptor of SARS-CoV, situated in the lower respiratory system in the human body is called the angiotensin converting enzyme 2 (ACE2) which is found in a large variety of tissues (Xian et al. 2020).
Unfortunately, there is currently no vaccine or drugs available for COVID-19.However, significant researches are under development to get a novel vaccine against COVID-19 as soon as possible (Cascella et al. 2020). At the actual situation, several strategies have been adopted worldwide mainly distancing, and wearing masks to reduce the virus spread.
Large amount of research has been performed to search for inhibitors of COVID-19 antiviral agents. Pang et al. (2020) carried out a systematic search to identify the published studies in the field of the fight against coronavirus. A total of 1065 articles were identified in our initial search and 236, 236 and 593 articles related to diagnostics, therapeutics and vaccines, respectively. Equally, Sivasankarapillai et al. (2020) discussed in their recent review the use of nanotechnology to develop novel drugs to treat COVID-19.
Nanoscience to face Coronavirus
Nanomaterials have proven their efficiency as antiviral materials to treat several diseases by different mechanisms. Their immunological advantages are: small size allowing to transfer drugs to targeted zones, large surface area allowing holding further drug cargos (Nasrollahzadeh et al. 2020). Also, nanomaterials are able to deliver viral therapeutics in a modulated system, to imitate viruses regarding their small size and structure without causing actual infection and to induce immune response. Nanoparticles therapeutics are used in several medical domain such as prevention, detection and treatment and they enhance significantly the drug delivery by targeting specified tissues (Petros and Desimone 2010; Roldão et al. 2010).
The respiratory diseases are also the subject of recent researches in nanoscience (Nikazar et al. 2020). However, little literature is available concerning serious and productive nanotechnology-based therapeutic plans against COVID-19.The use of conventional vaccines is limited because of the toxic return that could lead to a defective immune response and weak immunogenicity. Nanoparticles are used to solve these limitations (Sivasankarapillai et al. 2020). The four major nanoparticles described to be viral against respiratory viruses are given below (Sivasankarapillai et al. 2020; Nikazar et al. 2020).
-
Polymeric nanoparticles: They are good candidates for biomedical application because of their controlled properties, easy synthesis and great biocompatibility. Among the well-known members of this category are the Poly (lactic acid-co-glycolic acid) (PLGA) and chitosan (Carter et al. 2020).
-
Self-assembling proteins nanoparticles: Very suitable for biomedical application, produced by monomeric proteins oligomerization (Pillai et al. 2020).
-
Inorganic nanoparticles: A large literature is found to study their biological effects mainly the metal oxide nanoparticles. They presented good antibacterial and antifungal characteristics (Du et al. 2005). However, little study is available for inorganic nanoparticles that present antiviral action against respiratory viruses.
-
Peptide-based nanoparticles: Previously, short sequenced peptide inhibitors as well as amino acid mutations were proved to potentially act against SARS-COV infections (McReynolds et al. 2008). Recently, a peptide inhibitor isolated from ACE2 showed good evidence for blocking SARS-CoV-2. In addition, binding efficacy is estimated to be increased by multiple binding of linked peptides nanocarrier (Mansoor et al. 2015).
COVID-19 is a pandemic spreading rapidly. Novel methods to diagnose and treat it are under development. Some methods can be inspired from already existent nanomaterials used previously to face earlier SARS-COV.
Simulation of cells immune system using virus-like nanoparticles
The infectious threats are mainly treated using vaccines which are the most efficacious antidotes that evoke an immune response against particular pathogens. To improve the immune treatment, scientists are incorporating effectively virus-like agent into vaccine conception (Chen et al. 2016). These vaccines have shown better results in eliciting immune responses compared to the conventional vaccine (Noad and Roy 2003) which encouraged researchers to develop novel nanomaterials to mimic virus characteristics to make new vaccines (Moon et al. 2011). The act of displaying several antigens on a single particle makes the access of the antigens to the immune cells more effective (Moon et al. 2012). Vaccine virus-like nanoparticles have capsid without genetic material and rigid structures holding therapeutic molecules in a compact area of nano-distribution (Singh et al. 2006).
The nanocarrier of drugs with different morphology and sizes varying from 10 nm to a few microns are placed in the empty space of the capsid after removing the genetic material (Ma et al. 2012). To avoid unforeseen genetic mutation, capsids from plant viruses are generally used as nano holders as their genomes are unlike the human and animal ones (Hefferon 2018; Jeevanandam et al. 2019). In addition, Virus-like particles made by baculovirus recombination reveal better immune responses than those particles exhibited by mammals (Quan et al. 2020).
Virus-like particles used as vaccine to fight respiratory viruses like HMPV, RSV infections and influenza have been implemented (Lévy et al. 2013; Cox et al. 2014; Quan et al. 2007). The increasing development in the field of biomedical engineering has increased the use of different virus-like nanoparticles like antigen, drug and vaccine as therapeutic nanocarriers, biosensors and medical imaging (Jeevanandam et al. 2019). As nanoparticles improve the humoral responses versus selected antigens, they equally stimulate the immunity cells and the immunological memory which make them suitable for good vaccine conception (Chattopadhyay et al. 2017). The fact that viruses and nanoparticle vaccine have particularly similar characteristics such size, morphology and immunologic result encourages the scientists to concept nanovaccine and to use it in clinical applications (Singh et al. 2006).
Nano based products (like masks, Filter, Gowns, etc.)
Within a short span of time of its origin, SARS CoV-2 virus has rapidly spread worldwide (WHO 2020). Healthcare experts are at the frontline, working hardly to limit the epidemic expansion of the virus. New protection protocol is essential to protect the health of people in general and healthcare professionals, and patients in particular. Nanoparticles are also used to simulate mask exposure to the SARS-CoV-2 virus. ReSpimask® is a mask which allows the use of copper oxide nanoparticles and has a filtration potential of 99.9% for viruses and bacteria (Quan et al. 2020). Another designed mask was the MVX Nano Mask™, a self-safe guarding mask that can kill 99.9 per cent of all bacteria and viruses that come in contact and that is powerful against coronavirus, according to the manufacturer (Cavalcanti and Cajubá 2020). Lustig et al. (2020) have produced aerosols that include virus-like fluorescent nanoparticles to monitor transfer across materials that significantly contribute to the accuracy of the detection. They also noted that products with permeable hydrophilic layers and filter layers of hydrophobic materials inhibit the virus from spreading. Recently, research groups have focused on nanofilter masks, and developed a mask with multilayered electrostatically charged polyvinylidene fluoride (PVDF) nanofiber filters that can inhibit the transmission of CoV aerosols smaller than 300 nm (Leung and Sun 2020). Their findings show that the 6-layer charged nanofiber can effectively filter nanoaerosols with sizes ranging from 50 to 300 nm.
Challenges associated with the personal protective equipment against COVID-19
The personal protective equipment (PPE) used by healthcare professionals caring for patients with COVID-19 are contains gloves, face masks, spill gowns, protective suits, etc. It is estimated that the healthcare workers spends approximately 30 min to dress up properly and they wear the PPE for many hours per day (Kang et al. 2017b). When undressing, special attention is required to prevent infections (Honda and Iwata 2016). The healthcare experts expressed high level of stress when wearing the current PPE. Further improvements are needed. For example, the anti-fog item in goggle could reduce the risk of infection when removed (Han et al. 2018).
Moreover, other discomfort features exist when using the actual PPE such as the use of elastics to place closely the face masks that can result in great feeling of irritation (Locatelli et al. 2014) and the one-size-fits-all aspirators that do not outfit the all users faces. For these reasons, new materials and designs for the PPE is needed to be more adaptive and comfortable especially when used for long periods (Huang et al. 2020b).
Shortages of PPE have been reported worldwide, particularly in the early stages of the epidemic in various regions (Chaib 2020), preventing front-line healthcare professionals from changing their personal protective equipment and keeping use them for extra time. Consequently, the antiviral coatings of the PPE surface (Si et al. 2018), is considered to be efficient in reducing the pandemic spread.
Solutions to fight against COVID-19
Goel et al. (2020) resumed the three main scenarios that are currently being considered for the control of the COVID-19 disease as indicated below:
-
1)
Study and experiments on vaccine: researches are underway worldwide on new vaccines and accelerated acceptance procedures are being rolled out. However, scientists are concerned about the virus mutation that forms new strains, making the vaccine ineffective.
-
2)
Therapeutic experiences with actual medicine: The re-use of drugs and advanced formulations is getting attention, such as antimalarial drugs and other immunomodulatory biomolecules.
-
3)
Nanotechnology: advanced nanomaterials with low toxicity level are being developed for nanomedicine using previous advances in cancer treatments and cardiovascular therapies for example to treat coronavirus-infected persons.
In this review, the use of nanotechnology to fight against the novel coronavirus is detailed such in the domain of new vaccine development and targeted-drug delivery. Also, nanotechnology is used in filtering blood or in face masks. Recently, the nanotechnology has largely improved the protection effect of the personal protective equipment. Protective performance of these equipment is principally determined by the characteristics of the filtration such the thickness of the nonwoven layer, fiber charge and fiber diameter (Parthasarathi and Thilagavathi 2015). In general, the mask efficacy is conditional to their appropriate usage. Actually, the face protective masks are mainly contaminated with droplets containing infectious contagion. In the actual situation, the coronavirus penetration into the protective layer is established through droplets. Therefore, when removing the masks, the medical care givers hands could get contaminated (Li et al. 2006). For these reasons, nanomaterials are used to make a better filtration system by reducing the infection potential with direct and secondary vectors and producing reusable equipment (Habibzadeh and Stoneman 2020). Antiviral nanomaterials are being incorporated into nonwoven fabrics and into fibers especially in masks (Zhou et al. 2020c).
As being described in the first part of this review, composite, organic and inorganic materials are increasingly being used as functional component. Nevertheless, there are many materials that can be used presenting different antimicrobial resistances and distinct chemical and physical characteristics. The functions are expected to require different applications and manufacturing processes. Researchers are testing different antiviral agents that can be incorporated into the protective equipment all over the globe. Balagna et al. (2020) have proved that face masks with nanocluster/silica composite coating has antiviral effect which reduced the COVID-19 titer, under particular conditions, to zero. The advantage of these coating is the possibility of being deposited on different surfaces like polymeric, ceramic, glass and metallic. Thus, these materials can contribute to the protection against SARS-COV-2. Sportelli et al. (2020) underlined the use of nanotechnology solutions in several fields of the fight against the COVID-19. Nanomaterials showed great efficiency to filtrate coronavirus with diameter ranging from 100 to 60 nm.
Last but not least, development of intelligent PPE with antiviral detection helps to control the transmission rates (Goel et al. 2020). Mutually, they can gather the expanding particles for early detection of contamination (Leung et al. 2020). Several research articles on antibacterial and antiviral agents have been reported using metal particles. For example, they are used for air conditioning filters, packaging products, protective textile, etc. However, their commercialization is still limited for many reasons such their toxicity level, off-target effect, production cost, effectiveness, etc. (Sportelli et al. 2020). In this actual situation of coronavirus epidemic, turning these researches in a practical application is a very pressing need.
Conclusion and perspective
COVID-19 has a much higher infection risk than SARS or MERS. To control this disease in a short period of time, research development using novel and effective methods is needed. In the present circumstances, nanomaterials are needed to counter the actual threat to universal public health, to prepare new solutions for contagious diseases and to rethink the use of more sustainable scientific products. Nanomaterials are examined to be good candidates to fight against viral infections, especially coronavirus, because of their easy entry into cells, their rapid interaction with viruses and their genome replication prevention. In addition, improvements, including increasing the biological availability and decreasing the toxicity of typical antiviral therapeutics, can be achieved through breakthrough advances in nanotechnology. Today, scientists are working on significant studies to develop new nano/bio-materials and composites to achieve targeted delivery and nanovaccine against contagious viruses, in particular for the COVID-19. Due to the complicated situation generated by COVID-19, nanotechnology and nanomedicine could be viable alternatives to improve the efficiency of research.
References
Aagaard L, Rossi JJ (2007) RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev 59:75–86. https://doi.org/10.1016/j.addr.2007.03.005
Abdelaziz HM, Gaber M, Abd-Elwakil MM, Mabrouk MT, Elgohary MM, Kamel NM, Kabary DM, Freag MS, Samaha MW, Mortada SM, Elkhodairy KA, Fang JY, Elzoghby AO (2018) Inhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregates. J Control Release. https://doi.org/10.1016/j.jconrel.2017.11.036
Abou El-Nour KMM, Eftaiha A, Al-Warthan A, Ammar RAA (2010) Synthesis and applications of silver nanoparticles. Arab J Chem 3:135–140. https://doi.org/10.1016/j.arabjc.2010.04.008
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, Sun C, Sylvia S, Rozelle S, Raat H, Zhou H (2020) Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty 9:29. https://doi.org/10.1186/s40249-020-00646-x
Ahmed SR, Kim J, Suzuki T, Lee J, Park EY (2016) Detection of influenza virus using peroxidase-mimic of gold nanoparticles. Biotechnol Bioeng 113:2298–2303. https://doi.org/10.1002/bit.25982
Aiswarya D, Raja RK, Kamaraj C, Balasubramani G, Deepak P, Arul D, Amutha V, Sankaranarayanan C, Hazir S, Perumal P (2019) Biosynthesis of gold and silver nanoparticles from the symbiotic bacterium, Photorhabdus luminescens of Entomopathogenic Nematode: Larvicidal Properties against three mosquitoes and Galleria mellonella Larvae. J Clust Sci 30:1051–1063. https://doi.org/10.1007/s10876-019-01564-1
Alanagreh L, Alzoughool F, Atoum M (2020) The human coronavirus disease covid-19: its origin, characteristics, and insights into potential drugs and its mechanisms. Pathogens 9:331. https://doi.org/10.3390/pathogens9050331
Al-Tawfiq JA, Momattin H, Dib J, Memish ZA (2014) Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis 20:42–46. https://doi.org/10.1016/j.ijid.2013.12.003
Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF (2020) The proximal origin of SARS-CoV-2. Nat Med 26:450–452. https://doi.org/10.1038/s41591-020-0820-9
Ang L, Lee HW, Choi JY, Zhang J, Lee MS (2020) Herbal medicine and pattern identification for treating COVID-19: a rapid review of guidelines. Integr Med Res 9:100407. https://doi.org/10.1016/j.imr.2020.100407
Arabi YM, Shalhoub S, Mandourah Y, Al-Hameed F, Al-Omari A, Al Qasim E, Jose J, Alraddadi B, Almotairi A, Al Khatib K, Abdulmomen A, Qushmaq I, Sindi AA, Mady A, Solaiman O, Al-Raddadi R, Maghrabi K, Ragab A, Mekhlafi GAA, Balkhy HH, Al Harthy A, Kharaba A, Gramish JA, Al-Aithan AM, Al-Dawood A, Merson L, Hayden FG, Fowler R (2020) Ribavirin and interferon therapy for critically ill patients with middle east respiratory syndrome: a multicenter observational study. Clin Infect Dis 70:1837–1844. https://doi.org/10.1093/cid/ciz544
Argenta DF, de Mattos CB, Misturini FD, Koester LS, Bassani VL, SimõEs CMO, Teixeira HF (2014) Factorial design applied to the optimization of lipid composition of topical antiherpetic nanoemulsions containing isoflavone genistein. Int J Nanomed 9:4737–4747. https://doi.org/10.2147/IJN.S67732
Armstrong JPK, Holme MN, Stevens MM (2017) Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano. https://doi.org/10.1021/acsnano.6b07607
Asgary V, Shoari A, Afshar Moayad M, Shafiee Ardestani M, Bigdeli R, Ghazizadeh L, Khosravy MS, Panahnejad E, Janani A, Bashar R, Abedi M, Ahangari Cohan R (2018) Evaluation of G2 citric acid-based dendrimer as an adjuvant in veterinary rabies vaccine. Viral Immunol 31:47–54. https://doi.org/10.1089/vim.2017.0024
Badani H, Garry RF, Wimley WC (1838) Peptide entry inhibitors of enveloped viruses: the importance of interfacial hydrophobicity. Biochim Biophys Acta - Biomembr 2014:2180–2197. https://doi.org/10.1016/j.bbamem.2014.04.015
Balagna C, Perero S, Percivalle E, Nepita EV, Ferraris M (2020) Virucidal effect against Coronavirus SARS-CoV-2 of a silver nanocluster/silica composite sputtered coating. Open Ceram 1:100006. https://doi.org/10.1016/j.oceram.2020.100006
Baram-Pinto D, Shukla S, Perkas N, Gedanken A, Sarid R (2009) Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjug Chem 20:1497–1502. https://doi.org/10.1021/bc900215b
Barthelat F (2007) Biomimetics for next generation materials. Philos Trans R Soc A Math Phys Eng Sci. https://doi.org/10.1098/rsta.2007.0006
Beaudette FR, Hudson CR (1937) Cultivation of the virus of infectious bronchitis. J Am Vet Med Assoc 90:51–60
Beck-Broichsitter M, Merkel OM, Kissel T (2012) Controlled pulmonary drug and gene delivery using polymeric nano-carriers. J Control Release. https://doi.org/10.1016/j.jconrel.2011.12.004
Bhattacharya R, Mukherjee P (2008) Biological properties of “naked” metal nanoparticles. Adv Drug Deliv Rev 60:1289–1306. https://doi.org/10.1016/j.addr.2008.03.013
Bheemidi VS, Tiruckovela M, Varanasi PK (2011) An imperative note on novel drug delivery systems. J Nanomed Nanotechnol. https://doi.org/10.4172/2157-7439.1000125
Botequim D, Maia J, Lino MMF, Lopes LMF, Simões PN, Ilharco LM, Ferreira L (2012) Nanoparticles and surfaces presenting antifungal, antibacterial and antiviral properties. Langmuir 28:7646–7656. https://doi.org/10.1021/la300948n
Bowman MC, Ballard TE, Ackerson CJ, Feldheim DL, Margolis DM, Melander C (2008) Inhibition of HIV fusion with multivalent gold nanoparticles. J Am Chem Soc 130:6896–6897. https://doi.org/10.1021/ja710321g
Bzowka M, Mitusinska K, Raczynska A, Samol A, Tuszynski JA, Gora A (2020) Molecular dynamics simulations indicate the COVID-19 Mpro is not a viable target for small-molecule inhibitors design. BioRxiv. https://doi.org/10.1101/2020.02.27.968008
Cameron MJ, Bermejo-Martin JF, Danesh A, Muller MP, Kelvin DJ (2008) Human immunopathogenesis of severe acute respiratory syndrome (SARS). Virus Res 133:13–19. https://doi.org/10.1016/j.virusres.2007.02.014
Canaparo R, Foglietta F, Giuntini F, Della Pepa C, Dosio F, Serpe L (2019) Recent developments in antibacterial therapy: Focus on stimuli-responsive drug-delivery systems and therapeutic nanoparticles. Molecules 24:1991. https://doi.org/10.3390/molecules24101991
Cao S, Woodrow KA (2019) Nanotechnology approaches to eradicating HIV reservoirs. J Pharm Biopharm Eur. https://doi.org/10.1016/j.ejpb.2018.06.002
Caron J, Reddy LH, Lepêtre-Mouelhi S, Wack S, Clayette P, Rogez-Kreuz C, Yousfi R, Couvreur P, Desmaële D (2010) Squalenoyl nucleoside monophosphate nanoassemblies: new prodrug strategy for the delivery of nucleotide analogues. Bioorganic Med Chem Lett 20:2761–2764. https://doi.org/10.1016/j.bmcl.2010.03.070
Carter DC, Wright B, Jerome WG, Rose JP, Wilson E (2020) A unique protein self-assembling nanoparticle with significant advantages in vaccine development and production. J Nanomater 2020:1–10. https://doi.org/10.1155/2020/4297937
Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R (2020) Features, evaluation and treatment coronavirus (COVID-19). In: StatPearls. StatPearls Publishing, Treasure Island, FL, USA. http://www.ncbi.nlm.nih.gov/books/NBK554776/
Cavalcanti IDL, de Lira Nogueira MCB (2020) Pharmaceutical nanotechnology: which products are been designed against COVID-19? J Nanoparticle Res. https://doi.org/10.1007/s11051-020-05010-6
Cavallaro G, Sardo C, Craparo EF, Porsio B, Giammona G (2017) Polymeric nanoparticles for siRNA delivery: production and applications. Int J Pharm 525:313–333. https://doi.org/10.1016/j.ijpharm.2017.04.008
Chahal JS, Fang T, Woodham AW, Khan OF, Ling J, Anderson DG, Ploegh HL (2017) An RNA nanoparticle Vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci Rep 7:257. https://doi.org/10.1038/s41598-017-00193-w
Chaib F (2020) Shortage of personal protective equipment endangering health workers worldwide. WHO
Chan JFW, Yuan S, Kok KH, To KKW, Chu H, Yang J, Xing F, Liu J, Yip CCY, Poon RWS, Tsoi HW, Lo SKF, Chan KH, Poon VKM, Chan WM, Ip JD, Cai JP, Cheng VCC, Chen H, Hui CKM, Yuen KY (2020) A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395:514–523. https://doi.org/10.1016/S0140-6736(20)30154-9
Chang XJ, Zhang S, Jiang YP, Chen CM, Chen J, Liu BJ, Zhou J, Li W, Wang ZZ, Xiao W (2015) Mechanism of reduning injection on anti-acute lung injury in rats based on cytokine storm. Chinese Tradit Herb Drugs 46:236–239. https://doi.org/10.7501/j.issn.0253-2670.2015.02.016
Channappanavar R, Perlman S (2017) Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. https://doi.org/10.1007/s00281-017-0629-x
Chattopadhyay S, Chen JY, Chen HW, Hu CMJ (2017) Nanoparticle vaccines adopting virus-like features for enhanced immune potentiation. Nanotheranostics. 1:244–260. https://doi.org/10.7150/ntno.19796
Cheever FS, Daniels JB, Pappenheimer AM, Bailey OT (1949) A Murine Virus (JHM) causing disseminated encephalomyelitis with extensive destruction of myelin: I. Isolation and biological properties of the virus. J Exp Med 90:181–194
Chen W, Lim CED, Kang HJ, Liu J (2011) Chinese herbal medicines for the treatment of type A H1N1 influenza: A systematic review of randomized controlled trials. PLoS ONE. https://doi.org/10.1371/journal.pone.0028093
Chen HW, Huang CY, Lin SY, Fang ZS, Hsu CH, Lin JC, Chen YI, Yao BY, Hu CMJ (2016) Synthetic virus-like particles prepared via protein corona formation enable effective vaccination in an avian model of coronavirus infection. Biomaterials 106:111–118. https://doi.org/10.1016/j.biomaterials.2016.08.018
Chen Y, Liu Q, Guo D (2020a) Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. https://doi.org/10.1002/jmv.25681
Chen WH, Strych U, Hotez PJ, Bottazzi ME (2020b) The SARS-CoV-2 vaccine pipeline: an overview. Curr Trop Med Rep. https://doi.org/10.1007/s40475-020-00201-6
Cheng Y, Wong R, Soo YOY, Wong WS, Lee CK, Ng MHL, Chan P, Wong KC, Leung CB, Cheng G (2005) Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. https://doi.org/10.1007/s10096-004-1271-9
Chew MF, Poh KS, Poh CL (2017) Peptides as therapeutic agents for dengue virus. Int J Med Sci 14:1342–1359. https://doi.org/10.7150/ijms.21875
Chudhary SA, Imtiaz S, Iqbal N (2020) Laboratory detection of novel corona virus 2019 using polymerase chain reaction. Int J Front Sci https://doi.org/10.37978/tijfs.v4i2.5.
Cooper BM, Putnam D (2016) Polymers for siRNA delivery: a critical assessment of current technology prospects for clinical application. ACS Biomater Sci Eng 2:1837–1850. https://doi.org/10.1021/acsbiomaterials.6b00363
Cox RG, Erickson JJ, Hastings AK, Becker JC, Johnson M, Craven RE, Tollefson SJ, Boyd KL, Williams JV (2014) Human metapneumovirus virus-like particles induce protective B and T cell responses in a mouse model. J Virol 88:6368–6379. https://doi.org/10.1128/jvi.00332-14
Craik DJ, Fairlie DP, Liras S, Price D (2013) The future of peptide-based drugs. Chem Biol Drug Des 81:136–147. https://doi.org/10.1111/cbdd.12055
Cui Y, Zhao Y, Tian Y, Zhang W, Lü X, Jiang X (2012) The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 33:2327–2333. https://doi.org/10.1016/j.biomaterials.2011.11.057
Cui J, Li F, Shi ZL (2019) Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. https://doi.org/10.1038/s41579-018-0118-9
Curry T, Kopelman R, Shilo M, Popovtzer R (2014) Multifunctional theranostic gold nanoparticles for targeted CT imaging and photothermal therapy. Contrast Media Mol Imaging 9:53–61. https://doi.org/10.1002/cmmi.1563
De Wit E, Van Doremalen N, Falzarano D, Munster VJ (2016) SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. https://doi.org/10.1038/nrmicro.2016.81
Du Q, Wang S, Wei D, Sirois S, Chou KC (2005) Molecular modeling and chemical modification for finding peptide inhibitor against severe acute respiratory syndrome coronavirus main proteinase. Anal Biochem 337:262–270. https://doi.org/10.1016/j.ab.2004.10.003
Dudas G, Rambaut A (2016) MERS-CoV recombination: implications about the reservoir and potential for adaptation. Virus Evol 2:vev023. https://doi.org/10.1093/ve/vev023
Dunn K, Edwards-Jones V (2004) The role of ActicoatTM with nanocrystalline silver in the management of burns. Burns 30:S1–S9. https://doi.org/10.1016/S0305-4179(04)90000-9
Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH, Yacaman MJ (2005) Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol 3:6. https://doi.org/10.1186/1477-3155-3-6
Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, Dold C, Faust SN, Finn A, Flaxman AL, Hallis B, Heath P, Jenkin D, Lazarus R, Makinson R, Minassian AM, Pollock KM, Ramasamy M, Robinson H, Snape M, Tarrant R, Voysey M, Green C, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ, Oxford COVID Vaccine Trial Group (2020) Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396:467–478. https://doi.org/10.1016/S0140-6736(20)31604-4
Forni D, Cagliani R, Clerici M, Sironi M (2017) Molecular evolution of human coronavirus genomes. Trends Microbiol. https://doi.org/10.1016/j.tim.2016.09.001
Fu Y, Cheng Y, Wu Y (2020) Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. https://doi.org/10.1007/s12250-020-00207-4
Fujimori Y, Sato T, Hayata T, Nagao T, Nakayam M, Nakayam T, Sugamat R, Suzuki K (2012) Novel antiviral characteristics of nanosized copper(i) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl Environ Microbiol 78:51–955. https://doi.org/10.1128/AEM.06284-11
Gagliardi M (2017) Biomimetic and bioinspired nanoparticles for targeted drug delivery. Ther Deliv. https://doi.org/10.4155/tde-2017-0013
Gaikwad S, Ingle A, Gade A, Rai M, Falanga A, Incoronato N, Russo L, Galdiero S, Galdiero M (2013) Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomedicine 8:4303–4314. https://doi.org/10.2147/IJN.S50070
Galdiero S, Falanga A, Vitiello M, Cantisani M, Marra V, Galdiero M (2011) Silver nanoparticles as potential antiviral agents. Molecules. https://doi.org/10.3390/molecules16108894
Gao Y, Han K, Wang Q, Hu Z, Liu Q, Liu L, Zeng K (2018) Development of podophyllotoxin-loaded nanostructured lipid carriers for the treatment of condyloma acuminatum. Mol Med Rep 17:6506–6514. https://doi.org/10.3892/mmr.2018.8696
Ge XY, Li JL, Lou Yang X, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, Zhang YJ, Luo CM, Tan B, Wang N, Zhu Y, Crameri G, Zhang SY, Wang LF, Daszak P, Shi ZL (2013) Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 503:535–538. https://doi.org/10.1038/nature12711
Gentilucci L, De Marco R, Cerisoli L (2010) Chemical modifications designed to improve peptide stability: incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr Pharm Des 16:3185–3203. https://doi.org/10.2174/138161210793292555
Ghosh P, Han G, De M, Kim CK, Rotello VM (2008) Gold nanoparticles in delivery applications. Adv Drug Deliv Rev 60:1307–1315. https://doi.org/10.1016/j.addr.2008.03.016
Giner-Casares JJ, Henriksen-Lacey M, Coronado-Puchau M, Liz-Marzán LM (2016) Inorganic nanoparticles for biomedicine: where materials scientists meet medical research. Mater Today 19:19–28. https://doi.org/10.1016/j.mattod.2015.07.004
Girija PLT, Sivan N (2020) Ayurvedic treatment of COVID-19/SARS-CoV-2: a case report. J Ayurveda Integr Med. https://doi.org/10.1016/j.jaim.2020.06.001
Goel S, Hawi S, Goel G, Thakur VK, Agrawal A, Hoskins C, Pearce O, Hussain T, Upadhyaya HM, Cross G, Barber AH (2020) Resilient and agile engineering solutions to address societal challenges such as coronavirus pandemic. Mater Today Chem 17:100300. https://doi.org/10.1016/j.mtchem.2020.100300
Gong P, Li H, He X, Wang K, Hu J, Tan W, Zhang S, Yang X (2007) Preparation and antibacterial activity of Fe3O4@Ag nanoparticles. Nanotechnology 18:285–604. https://doi.org/10.1088/0957-4484/18/28/285604
Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, Zou W, Zhan J, Wang S, Xie Z, Zhuang H, Wu B, Zhong H, Shao H, Fang W, Gao D, Pei F, Li X, He Z, Xu D, Shi X, Anderson VM, Leong ASY (2005) Multiple organ infection and the pathogenesis of SARS. J Exp Med 202:415–424. https://doi.org/10.1084/jem.20050828
Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Luo SW, Li PH, Zhang LJ, Guan YJ, Butt KM, Wong KL, Chan KW, Lim W, Shortridge KF, Yuen KY, Peiris JSM, Poon LLM (2003) Isolation and characterization of viruses related to the SARS coronavirus from animals in Southern China. Science (80) 302:276–278. https://doi.org/10.1126/science.1087139
Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, Liu L, Shan H, Lei C, Hui DSC, Du B, Li L, Zeng G, Yuen KY, Chen R, Tang C, Wang T, Chen P, Xiang J, Li S, Wang JL, Liang Z, Peng Y, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu J, Chen Z, Li G, Zheng Z, Qiu S, Luo J, Ye C, Zhu S, Zhong N (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720. https://doi.org/10.1056/NEJMoa2002032
Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Sen Tan K, Wang DY, Yan Y (2020) The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- A n update on the status. Mil Med Res 7:11. https://doi.org/10.1186/s40779-020-00240-0
Guo L, Ren L, Yang S, Xiao M, Chang D, Yang F, Dela Cruz CS, Wang Y, Wu C, Xiao Y, Zhang L, Han L, Dang S, Xu Y, Yang QW, Xu SY, Zhu HD, Xu YC, Jin Q, Sharma L, Wang L, Wang J (2020) Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. https://doi.org/10.1093/cid/ciaa310
Habibzadeh P, Stoneman EK (2020) The novel coronavirus: a bird’s eye view. Int J Occup Environ Med 11:65–71
Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203:631–637. https://doi.org/10.1002/path.1570
Han Z, Feng X, Guo Z, Niu S, Ren L (2018) Flourishing bioinspired antifogging materials with superwettability: progresses and challenges. Adv Mater 30:1704652. https://doi.org/10.1002/adma.201704652
Han W, Quan B, Guo Y, Zhang J, Lu Y, Feng G, Wu Q, Fang F, Cheng L, Jiao N, Li X, Chen Q (2020) The course of clinical diagnosis and treatment of a case infected with coronavirus disease 2019. J Med Virol 92:461–463. https://doi.org/10.1002/jmv.25711
Hefferon KL (2018) Repurposing plant virus nanoparticles. Vaccines 6:11. https://doi.org/10.3390/vaccines6010011
Helmy YA, Fawzy M, Elaswad A, Sobieh A, Kenney SP, Shehata AA (2020) The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J Clin Med 9:1225. https://doi.org/10.3390/jcm9041225
Hendricks GL, Weirich KL, Viswanathan K, Li J, Shriver ZH, Ashour J, Ploegh HL, Kurt-Jones EA, Fygenson DK, Finberg RW, Comolli JC, Wang JP (2013) Sialylneolacto-N-tetraose c (LSTc)-bearing liposomal decoys capture influenza a virus. J Biol Chem 288:8061–8073. https://doi.org/10.1074/jbc.M112.437202
Honda H, Iwata K (2016) Personal protective equipment and improving compliance among healthcare workers in high-risk settings. Curr Opin Infect Dis 29:400–406. https://doi.org/10.1097/QCO.0000000000000280
Hong CA, Nam YS (2014) Functional nanostructures for effective delivery of small interfering RNA therapeutics. Theranostics 4:1211–1232. https://doi.org/10.7150/thno.8491
Hu RL, Li SR, Kong FJ, Hou RJ, Guan XL, Guo F (2014) Inhibition effect of silver nanoparticles on herpes simplex virus 2. Genet Mol Res 13:7022–7028. https://doi.org/10.4238/2014.March.19.2
Hu B, Zeng LP, Lou Yang X, Ge XY, Zhang W, Li B, Xie JZ, Shen XR, Zhang YZ, Wang N, Luo DS, Zheng XS, Wang MN, Daszak P, Wang LF, Cui J, Shi ZL (2017) Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog 13:e1006698. https://doi.org/10.1371/journal.ppat.1006698
Huang X, Xu Y, Yang Q, Chen J, Zhang T, Li Z, Guo C, Chen H, Wu H, Li N (2015) Efficacy and biological safety of lopinavir/ritonavir based anti-retroviral therapy in HIV-1-infected patients: a meta-analysis of randomized controlled trials. Sci Rep 5:8528. https://doi.org/10.1038/srep08528
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Huang C, Wen T, Shi FJ, Zeng XY, Jiao YJ (2020a) Rapid detection of IgM antibodies against the SARS-CoV-2 virus via colloidal gold nanoparticle-based lateral-flow assay. ACS Omega 5:12550–12556. https://doi.org/10.1021/acsomega.0c01554
Huang H, Fan C, Li M, Nie HL, Wang FB, Wang H, Wang R, Xia J, Zheng X, Zuo X, Huang J (2020b) COVID-19: a call for physical scientists and engineers. ACS Nano. https://doi.org/10.1021/acsnano.0c02618
Huh AJ, Kwon YJ (2011) “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release 156:128–145. https://doi.org/10.1016/j.jconrel.2011.07.002
Hung IFN, To KKW, Lee CK, Lee KL, Chan K, Yan WW, Liu R, Watt CL, Chan WM, Lai KY, Koo CK, Buckley T, Chow FL, Wong KK, Chan HS, Ching CK, Tang BSF, Lau CCY, Li IWS, Liu SH, Chan KH, Lin CK, Yuen KY (2011) Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. https://doi.org/10.1093/cid/ciq106
Hwang J, Jeong Y, Park JM, Lee KH, Hong JW, Choi J (2015) Biomimetics: forecasting the future of science, engineering, and medicine. Int J Nanomed 10:5701–5713. https://doi.org/10.2147/IJN.S83642
Iranpur Mobarakeh V, Modarressi MH, Rahimi P, Bolhassani A, Arefian E, Atyabi F, Vahabpour R (2019) Optimization of chitosan nanoparticles as an anti-HIV siRNA delivery vehicle. Int J Biol Macromol 129:305–315. https://doi.org/10.1016/j.ijbiomac.2019.02.036
Itani R, Tobaiqy M, Al Faraj A (2020) Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics. 10:5932–5942. https://doi.org/10.7150/thno.46691
Jackson AL, Linsley PS (2010) Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov 9:57–67. https://doi.org/10.1038/nrd3010
Jeang KT (2012) RNAi in the regulation of mammalian viral infections. BMC Biol 10:58. https://doi.org/10.1186/1741-7007-10-58
Jeevanandam J, Pal K, Danquah MK (2019) Virus-like nanoparticles as a novel delivery tool in gene therapy. Biochimie 157:38–47. https://doi.org/10.1016/j.biochi.2018.11.001
Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB (2005) ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol 79:14614–14621. https://doi.org/10.1128/jvi.79.23.14614-14621.2005
Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, Duan G (2020) Virology, epidemiology, pathogenesis, and control of covid-19. Viruses 12:372. https://doi.org/10.3390/v12040372
Joshy KS, Snigdha SS, Kalarikkal N, Pothen LA, Thomas S (2017) Gelatin modified lipid nanoparticles for anti- viral drug delivery. Chem Phys Lipids 207:24–37. https://doi.org/10.1016/j.chemphyslip.2017.07.002
Jung SY, Kang KW, Lee EY, Seo DW, Kim HL, Kim H, Kwon TW, Park HL, Kim H, Lee SM, Nam JH (2018) Heterologous prime–boost vaccination with adenoviral vector and protein nanoparticles induces both Th1 and Th2 responses against Middle East respiratory syndrome coronavirus. Vaccine 36:3468–3476. https://doi.org/10.1016/j.vaccine.2018.04.082
Kahn JS, McIntosh K (2005) History and recent advances in coronavirus discovery. Pediatr Infect Dis J 24:S223–S227. https://doi.org/10.1097/01.inf.0000188166.17324.60
Kanasty R, Dorkin JR, Vegas A, Anderson D (2013) Delivery materials for siRNA therapeutics. Nat Mater 12:967–977. https://doi.org/10.1038/nmat3765
Kang SH, Revuri V, Lee SJ, Cho S, Park IK, Cho KJ, Bae WK, Lee YK (2017a) Oral siRNA delivery to treat colorectal liver metastases. ACS Nano 11:10417–10429. https://doi.org/10.1021/acsnano.7b05547
Kang JH, O’Donnell JM, Colaianne B, Bircher N, Ren D, Smith KJ (2017b) Use of personal protective equipment among health care personnel: Results of clinical observations and simulations. Am J Infect Control 45:17–23. https://doi.org/10.1016/j.ajic.2016.08.011
Kato T, Takami Y, Kumar Deo V, Park EY (2019) Preparation of virus-like particle mimetic nanovesicles displaying the S protein of Middle East respiratory syndrome coronavirus using insect cells. J Biotechnol. https://doi.org/10.1016/j.jbiotec.2019.10.007
Kerry RG, Malik S, Redda YT, Sahoo S, Patra JK, Majhi S (2019) Nano-based approach to combat emerging viral (NIPAH virus) infection. Nanomed Nanotechnol Biol Med. https://doi.org/10.1016/j.nano.2019.03.004
Khan R, Irchhaiya R (2016) Niosomes: a potential tool for novel drug delivery. J Pharm Investig 46:195–204. https://doi.org/10.1007/s40005-016-0249-9
Khan AA, Allemailem KS, Almatroodi SA, Almatroudi A, Rahmani AH (2020) Recent strategies towards the surface modification of liposomes: an innovative approach for different clinical applications. 3 Biotech 10:163. https://doi.org/10.1007/s13205-020-2144-3
Kong FY, Zhang JW, Li RF, Wang ZX, Wang WJ, Wang W (2017) Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules 22:1445. https://doi.org/10.3390/molecules22091445
Kumar A, Ma H, Zhang X, Huang K, Jin S, Liu J, Wei T, Cao W, Zou G, Liang XJ (2012) Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials 33:1180–1189. https://doi.org/10.1016/j.biomaterials.2011.10.058
Kumar A, Pandey AN, Jain SK (2016) Nasal-nanotechnology: revolution for efficient therapeutics delivery. Drug Deliv 23:671–683. https://doi.org/10.3109/10717544.2014.920431
Kumari A, Yadav SK, Yadav SC (2010) Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surfaces B BiointeRfaces 75:1–18. https://doi.org/10.1016/j.colsurfb.2009.09.001
Laffleur F, Keckeis V (2020) Advances in drug delivery systems: work in progress still needed? Int J Pharm X. https://doi.org/10.1016/j.ijpx.2020.100050
Lai MMC, Perlman S, Anderson LJ. (2007). Coronaviridae. In: PM Knipe, DM Howley (eds.) Fields virology, 5th ed., Lippincott Williams & Wilkins, Philadelphia, PA, USA, pp 1305–1318
Lammers T, Sofias AM, van der Meel R, Schiffelers R, Storm G, Tacke F, Koschmieder S, Brümmendorf TH, Kiessling F, Metselaar JM (2020) Dexamethasone nanomedicines for COVID-19. Nat Nanotechnol. https://doi.org/10.1038/s41565-020-0752-z
Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C (2010) Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol 8:1–10. https://doi.org/10.1186/1477-3155-8-1
Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, Singh DK (2011) Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnol 9:30. https://doi.org/10.1186/1477-3155-9-30
Lau SKP, Chan JFW (2015) Coronaviruses: emerging and re-emerging pathogens in humans and animals. Virol J. https://doi.org/10.1186/s12985-015-0432-z
Layqah LA, Eissa S (2019) An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchim Acta 186:224. https://doi.org/10.1007/s00604-019-3345-5
Lee YT, Ko EJ, Hwang HS, Lee JS, Kim KH, Kwon YM, Kang SM (2015) Respiratory syncytial virus-like nanoparticle vaccination induces long-term protection without pulmonary disease by modulating cytokines and T-cells partially through alveolar macrophages. Int J Nanomedicine 10:4491–4505. https://doi.org/10.2147/IJN.S83493
Letchford K, Burt H (2007) A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm 65:259–269. https://doi.org/10.1016/j.ejpb.2006.11.009
Letko M, Marzi A, Munster V (2020) Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol 5:562–569. https://doi.org/10.1038/s41564-020-0688-y
Leung WWF, Sun Q (2020) Electrostatic charged nanofiber filter for filtering airborne novel coronavirus (COVID-19) and nano-aerosols. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2020.116886
Leung NHL, Chu DKW, Shiu EYC, Chan KH, McDevitt JJ, Hau BJP, Yen HL, Li Y, Ip DKM, Peiris JSM, Seto WH, Leung GM, Milton DK, Cowling BJ (2020) Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 26:676–680. https://doi.org/10.1038/s41591-020-0843-2
Lévy C, Aerts L, Hamelin MÈ, Granier C, Szécsi J, Lavillette D, Boivin G, Cosset FL (2013) Virus-like particle vaccine induces cross-protection against human metapneumovirus infections in mice. Vaccine 31:2778–2785. https://doi.org/10.1016/j.vaccine.2013.03.051
Li W, Moore MJ, Vasllieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greeneugh TC, Choe H, Farzan M (2003) Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426:450–454. https://doi.org/10.1038/nature02145
Li Y, Leung P, Yao L, Song QW, Newton E (2006) Antimicrobial effect of surgical masks coated with nanoparticles. J Hosp Infect 62:58–63. https://doi.org/10.1016/j.jhin.2005.04.015
Li L, Lu Y, Jiang C, Zhu Y, Yang X, Hu X, Lin Z, Zhang Y, Peng M, Xia H, Mao C (2018) Actively targeted deep tissue imaging and photothermal-chemo therapy of breast cancer by antibody-functionalized drug-loaded x-ray-responsive bismuth sulfide@mesoporous silica core-shell nanoparticles. Adv Funct Mater 28:1704623. https://doi.org/10.1002/adfm.201704623
Li C, Wang J, Wang Y, Gao H, Wei G, Huang Y, Yu H, Gan Y, Wang Y, Mei L, Chen H, Hu H, Zhang Z, Jin Y (2019) Recent progress in drug delivery. Acta Pharm Sin b 9:1145–1162. https://doi.org/10.1016/j.apsb.2019.08.003
Li X, Geng M, Peng Y, Meng L, Lu S (2020a) Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal 10:102–108. https://doi.org/10.1016/j.jpha.2020.03.001
Li X, Zai J, Zhao Q, Nie Q, Li Y, Foley BT, Chaillon A (2020b) Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol 92:602–611. https://doi.org/10.1002/jmv.25731
Lim J, Jeon S, Shin HY, Kim MJ, Seong YM, Lee WJ, Choe KW, Kang YM, Lee B, Park SJ (2020) Case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J Korean Med Sci 35:e79. https://doi.org/10.3346/jkms.2020.35.e79
Liu Y, Chen C (2016) Role of nanotechnology in HIV/AIDS vaccine development. Adv Drug Deliv Rev 103:76–89. https://doi.org/10.1016/j.addr.2016.02.010
Liu J, Wu P, Gao F, Qi J, Kawana-Tachikawa A, Xie J, Vavricka CJ, Iwamoto A, Li T, Gao GF (2010) Novel Immunodominant peptide presentation strategy: a featured HLA-A*2402-Restricted cytotoxic T-lymphocyte epitope stabilized by intrachain hydrogen bonds from severe acute respiratory syndrome coronavirus nucleocapsid protein. J Virol 84:11849–11857. https://doi.org/10.1128/jvi.01464-10
Liu D, Yang F, Xiong F, Gu N (2016) The smart drug delivery system and its clinical potential. Theranostics 6:1306–1323. https://doi.org/10.7150/thno.14858
Liu X, Ai F, Li H, Xu Q, Mei L, Miao J, Wen Q, Zhang C, Zhang S, Zhou J, Chen X, Chu C, Guo J (2019) Anti-inflammatory effects of shenfu injection against acute lung injury through inhibiting HMGB1-NF- B pathway in a rat model of endotoxin shock. Evidence-Based Complement Altern Med 2019:9857683. https://doi.org/10.1155/2019/9857683
Liu T, Hu J, Kang M, Lin L, Zhong H, Xiao J, He G, Song T, Huang Q, Rong Z, Deng A, Zeng W, Tan X, Zeng S, Zhu Z, Li J, Wan D, Lu J, Deng H, He J, Ma W (2020) Transmission dynamics of 2019 novel coronavirus (2019-nCoV). SSRN Electron J. https://doi.org/10.2139/ssrn.3526307
Locatelli SM, LaVela SL, Gosch M (2014) Health care workers’ reported discomfort while wearing filtering face-piece respirators. Work Heal Saf 62:362–368. https://doi.org/10.3928/21650799-20140804-03
Łoczechin A, Séron K, Barras A, Giovanelli E, Belouzard S, Chen YT, Metzler-Nolte N, Boukherroub R, Dubuisson J, Szunerits S (2019) Functional carbon quantum dots as medical countermeasures to human coronavirus. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.9b15032
Lokshyn M, Lozovski V, Lysenko VS, Piatnytsia V, Spivak M, Sterligov V (2014) Nanoparticles in antivirus therapy. Adv Mater Res 854:149–155
Lu L, Sun RWY, Chen R, Hui CK, Ho CM, Luk JM, Lau GKK, Che CM (2008) Silver nanoparticles inhibit hepatitis B virus replication. Antivir Ther 13:253–262
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W (2020) Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395:565–574. https://doi.org/10.1016/S0140-6736(20)30251-8
Lustig SR, Biswakarma JJH, Rana D, Tilford SH, Hu W, Su M, Rosenblatt MS (2020) Effectiveness of common fabrics to block aqueous aerosols of virus-like nanoparticles. ACS Nano 14:7651–7658. https://doi.org/10.1021/acsnano.0c03972
Lysenko V, Lozovski V, Spivak M (2013) Nanophysics and antiviral therapy. Ukr J Phys 58(1):77–90
Ma Y, Nolte RJM, Cornelissen JJLM (2012) Virus-based nanocarriers for drug delivery. Adv Drug Deliv Rev 64:811–825. https://doi.org/10.1016/j.addr.2012.01.005
Ma J, Huo XQ, Chen X, Zhu WX, Yao MC, Qiao YJ, Zhang YL (2020) Study on screening potential traditional Chinese medicines against 2019-nCoV based on Mpro and PLP. Zhongguo Zhong Yao Za Zhi 45:1219–1224
MacKay JA, Li Z (2010) Theranostic agents that co-deliver therapeutic and imaging agents? Adv Drug Deliv Rev 62:1003–1004. https://doi.org/10.1016/j.addr.2010.10.001
Mahajan SD, Aalinkeel R, Law WC, Reynolds JL, Nair BB, Sykes DE, Yong KT, Roy I, Prasad PN, Schwartz SA (2012) Anti-HIV-1 nanotherapeutics: promises and challenges for the future. Int J Nanomed 7:5301. https://doi.org/10.2147/IJN.S25871
Mansoor F, Earley B, Cassidy JP, Markey B, Doherty S, Welsh MD (2015) Comparing the immune response to a novel intranasal nanoparticle PLGA vaccine and a commercial BPI3V vaccine in dairy calves. BMC Vet Res 11:220. https://doi.org/10.1186/s12917-015-0481-y
Marambio-Jones C, Hoek EMV (2010) A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanoparticle Res 12:1531–1551. https://doi.org/10.1007/s11051-010-9900-y
Marques Neto LM, Kipnis A, Junqueira-Kipnis AP (2017) Role of metallic nanoparticles in vaccinology: implications for infectious disease vaccine development. Front Immunol 8:239. https://doi.org/10.3389/fimmu.2017.00239
Mazurkova NA, Spitsyna YE, Shikina NV, Ismagilov ZR, Zagrebel’nyi SN, Ryabchikova EI (2010) Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnologies Russ 5:417–420. https://doi.org/10.1134/S1995078010050174
McClements DJ, Xiao H (2017) Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. Npj Sci Food 1:6. https://doi.org/10.1038/s41538-017-0005-1
McReynolds S, Jiang S, Guo Y, Celigoy J, Schar C, Rong L, Caffrey M (2008) Characterization of the prefusion and transition states of severe acute respiratory syndrome coronavirus S2-HR2. Biochemistry 47:6802–6808. https://doi.org/10.1021/bi800622t
Mi P (2020) Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 10:4557–4588. https://doi.org/10.7150/thno.38069
Milovanovic M, Arsenijevic A, Milovanovic J, Kanjevac T, Arsenijevic N (2017) Nanoparticles in antiviral therapy. Antimicrob Nanoarchitectonics. https://doi.org/10.1016/B978-0-323-52733-0.00014-8
Min CK, Cheon S, Ha NY, Sohn KM, Kim Y, Aigerim A, Shin HM, Choi JY, Inn KS, Kim JH, Moon JY, Choi MS, Cho NH, Kim YS (2016) Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep 6:1–12. https://doi.org/10.1038/srep25359
Misra SK, Dighe K, Schwartz-Duval AS, Shang Z, Labriola LT, Pan D (2018) In situ plasmonic generation in functional ionic-gold-nanogel scaffold for rapid quantitative bio-sensing. Biosens Bioelectron 120:77–84. https://doi.org/10.1016/j.bios.2018.08.019
Mitragotri S, Stayton P (2014) Organic nanoparticles for drug delivery and imaging. MRS Bull 39:219–223. https://doi.org/10.1557/mrs.2014.11
Moitra P, Alafeef M, Alafeef M, Alafeef M, Dighe K, Frieman MB, Pan D, Pan D, Pan D (2020) Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano 14:7617–7627. https://doi.org/10.1021/acsnano.0c03822
Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, Sohail M, Luo S, Ho Um S, Khant H, Goodwin JT, Ramos J, Chiu W, Irvine DJ (2011) Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater 10:243–251
Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ (2012) Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand T fh cells and promote germinal center induction. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1112648109
Mura S, Nicolas J, Couvreur P (2013) Stimuli-responsive nanocarriers for drug delivery. Nat Mater 12:991–1003. https://doi.org/10.1038/nmat3776
Nadeem MS, Zamzami MA, Choudhry H, Murtaza BN, Kazmi I, Ahmad H, Shakoori AR (2020) Origin, potential therapeutic targets and treatment for coronavirus disease (COVID-19). Pathogens 9:307. https://doi.org/10.3390/pathogens9040307
Nandy B, Saurabh S, Sahoo AK, Dixit NM, Maiti PK (2015) The SPL7013 dendrimer destabilizes the HIV-1 gp120-CD4 complex. Nanoscale 7:18628–18641. https://doi.org/10.1039/c5nr04632g
Naseri S, Darroudi M, Aryan E, Gholoobi A, Rahimi HR, Ketabi K, Movaqar A, Abdoli M, Gouklani H, Teimourpour R, Meshkat Z (2017) The antiviral effects of curcumin nanomicelles on the attachment and entry of hepatitis C virus. Iran J Virol 11:29–35
Nasrollahzadeh M, Sajjadi M, Soufi GJ, Iravani S, Varma RS (2020) Nanomaterials and nanotechnology-associated innovations against viral infections with a focus on coronaviruses. Nanomaterials. https://doi.org/10.3390/nano10061072
Ng DL, Al Hosani F, Keating MK, Gerber SI, Jones TL, Metcalfe MG, Tong S, Tao Y, Alami NN, Haynes LM, Mutei MA, Abdel-Wareth L, Uyeki TM, Swerdlow DL, Barakat M, Zaki SR (2014) Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of middle east respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am J Pathol 186:652–658. https://doi.org/10.1016/j.ajpath.2015.10.024
Nikazar S, Sivasankarapillai VS, Rahdar A, Gasmi S, Anumol PS, Shanavas MS (2020) Revisiting the cytotoxicity of quantum dots: an in-depth overview. Biophys Rev 12:703–718. https://doi.org/10.1007/s12551-020-00653-0
Noad R, Roy P (2003) Virus-like particles as immunogens. Trends Microbiol 11:438–444. https://doi.org/10.1016/S0966-842X(03)00208-7
Ochekpe NA, Olorunfemi PO, Ngwuluka NC (2009) Nanotechnology and drug delivery part 2: nanostructures for drug delivery. Trop J Pharm Res. https://doi.org/10.4314/tjpr.v8i3.44547
Pan D, Pramanik M, Senpan A, Ghosh S, Wickline SA, Wang LV, Lanza GM (2010) Near infrared photoacoustic detection of sentinel lymph nodes with gold nanobeacons. Biomaterials 31:4088–4093. https://doi.org/10.1016/j.biomaterials.2010.01.136
Pan D, Schirra CO, Wickline SA, Lanza GM (2014) Multicolor computed tomographic molecular imaging with noncrystalline high-metal-density nanobeacons. Contrast Media Mol Imaging 9:13–25. https://doi.org/10.1002/cmmi.1571
Pan J, Rostamizadeh K, Filipczak N, Torchilin VP (2019) Polymeric co-delivery systems in cancer treatment: an overview on component drugs’ dosage ratio effect. Molecules. https://doi.org/10.3390/molecules24061035
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JI-P, Gutierrez RA, Gwee SXW, Chua PEY, Yang Q, Ng XY, Yap RKS, Tan HY, Teo YY, Tan CC, Cook AR, Yap JC-H, Hsu LY (2020) Potential rapid diagnostics, vaccine and therapeutics for 2019 novel Coronavirus (2019-nCoV): a systematic review. J Clin Med 9:623. https://doi.org/10.3390/jcm9030623
Parboosing R, Maguire GEM, Govender P, Kruger HG (2012) Nanotechnology and the treatment of HIV infection. Viruses 4:488–520. https://doi.org/10.3390/v4040488
Park M, Cook AR, Lim JT, Sun Y, Dickens BL (2020) A systematic review of COVID-19 epidemiology based on current evidence. J Clin Med 9:967. https://doi.org/10.3390/jcm9040967
Parthasarathi V, Thilagavathi G (2015) Development of plasma enhanced antiviral surgical gown for healthcare workers. Fash Text 2:4. https://doi.org/10.1186/s40691-015-0028-7
Patel P, Agrawal YK (2017) Targeting nanocarriers containing antisense oligonucleotides to cancer cell. J Drug Deliv Sci Technol 37:97–114. https://doi.org/10.1016/j.jddst.2016.12.001
Patel PA, Patravale VB (2011) AmbiOnp: solid lipid nanoparticles of amphotericin B for oral administration. J Biomed Nanotechnol 7:632–639. https://doi.org/10.1166/jbn.2011.1332
Peng L, Li BL, Zhou CW, Li NB, Setyawati MI, Zou HL (2018) “Naked-eye” recognition: emerging gold nano-family for visual sensing. Appl Mater Today 11:166–188. https://doi.org/10.1016/j.apmt.2018.02.007
Perrie Y, Mohammed AR, Kirby DJ, McNeil SE, Bramwell VW (2008) Vaccine adjuvant systems: enhancing the efficacy of sub-unit protein antigens. Int J Pharm 364:272–280. https://doi.org/10.1016/j.ijpharm.2008.04.036
Petros RA, Desimone JM (2010) Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 9:615–627. https://doi.org/10.1038/nrd2591
Piatek MJ, Werner A (2014) Endogenous siRNAs: regulators of internal affairs. Biochem Soc Trans 42:1174–1179. https://doi.org/10.1042/BST20140068
Pillai AM, Sivasankarapillai VS, Rahdar A, Joseph J, Sadeghfar F, Anuf R, Rajesh AK, Kyzas GZ (2020) Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J Mol Struct 1211:128107. https://doi.org/10.1016/j.molstruc.2020.128107
Prajapati NKS (2020) SARS-CoV-2 pandemic: an opportunity for Indian traditional medicines (AYUSH). Int J Complement Altern Med 13:103–105
Qamar MT, Maryam A, Muneer I, Xing F, Ashfaq UA, Khan FA, Anwar F, Geesi MH, Khalid RR, Rauf SA, Siddiqi AR (2019) Computational screening of medicinal plant phytochemicals to discover potent pan-serotype inhibitors against dengue virus. Sci Rep 9:1433. https://doi.org/10.1038/s41598-018-38450-1
Qamar MT, Shahid F, Aslam S, Ashfaq UA, Aslam S, Fatima I, Fareed MM, Zohaib A, Chen LL (2020) Reverse vaccinology assisted designing of multiepitope-based subunit vaccine against SARS-CoV-2. Infect Dis Poverty. https://doi.org/10.1186/s40249-020-00752-w
Qiu G, Gai Z, Tao Y, Schmitt J, Kullak-Ublick GA, Wang J (2020) Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. https://doi.org/10.1021/acsnano.0c02439
Quan F-S, Huang C, Compans RW, Kang S-M (2007) Virus-Like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol 81:3514–3524. https://doi.org/10.1128/jvi.02052-06
Quan FS, Basak S, Chu KB, Kim SS, Kang SM (2020) Progress in the development of virus-like particle vaccines against respiratory viruses. Expert Rev Vaccines 19:11–24. https://doi.org/10.1080/14760584.2020.1711053
Rafiei S, Rezatofighi SE, Ardakani MR, Rastegarzadeh S (2016) Gold nanoparticles impair foot-and-mouth disease virus replication. IEEE Trans Nanobioscience 15:34–40. https://doi.org/10.1109/TNB.2015.2508718
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83. https://doi.org/10.1016/j.biotechadv.2008.09.002
Ramkumar R, Balasubramani G, Raja RK, Raja M, Govindan R, Girija EK, Perumal P (2017) Lantana camara Linn root extract-mediated gold nanoparticles and their in vitro antioxidant and cytotoxic potentials. Artif Cells Nanomedicine Biotechnol 45:748–757. https://doi.org/10.1080/21691401.2016.1276923
Rastogi S, Pandey DN, Singh RH (2020) COVID-19 pandemic: a pragmatic plan for ayurveda intervention. J Ayurveda Integr Med. https://doi.org/10.1016/j.jaim.2020.04.002
Rogers JV, Parkinson CV, Choi YW, Speshock JL, Hussain SM (2008) A preliminary assessment of silver nanoparticle inhibition of monkeypox virus plaque formation. Nanoscale Res Lett 3:129. https://doi.org/10.1007/s11671-008-9128-2
Roldão A, Mellado MCM, Castilho LR, Carrondo MJT, Alves PM (2010) Virus-like particles in vaccine development. Expert Rev Vaccines 9:1149–1176. https://doi.org/10.1586/erv.10.115
Saha K, Agasti SS, Kim C, Li X, Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem Rev 112:2739–2779. https://doi.org/10.1021/cr2001178
Sanvicens N, Marco MP (2008) Multifunctional nanoparticles—properties and prospects for their use in human medicine. Trends Biotechnol 26:425–433. https://doi.org/10.1016/j.tibtech.2008.04.005
Scaggiante B, Dapas B, Farra R, Grassi M, Pozzato G, Giansante C, Grassi G (2011) Improving siRNA bio-distribution and minimizing side effects. Curr Drug Metab 12:11–23. https://doi.org/10.2174/138920011794520017
Schmitt V, Kesch C, Jackson JK, Bidnur S, Beraldi E, Yago V, Bowden M, Gleave ME (2020) Design and characterization of injectable Poly(Lactic-Co-Glycolic Acid) pastes for sustained and local drug release. Pharm Res 37:36. https://doi.org/10.1007/s11095-019-2730-4
Seo G, Lee G, Kim MJ, Baek SH, Choi M, Ku KB, Lee CS, Jun S, Park D, Kim HG, Kim SJ, Lee JO, Kim BT, Park EC, Il Kim S (2020) Rapid Detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. https://doi.org/10.1021/acsnano.0c02823
Serrano-Sevilla I, Artiga Á, Mitchell SG, De Matteis L, de la Fuente JM (2019) Natural polysaccharides for siRNA delivery: nanocarriers based on chitosan, hyaluronic acid, and their derivatives. Molecules. https://doi.org/10.3390/molecules24142570
Shukla S, Sumaria CS, Pradeepkumar PI (2010) Exploring chemical modifications for siRNA therapeutics: a structural and functional outlook. ChemMedChem 5:328–349. https://doi.org/10.1002/cmdc.200900444
Si Y, Zhang Z, Wu W, Fu Q, Huang K, Nitin N, Ding B, Sun G (2018) Daylight-driven rechargeable antibacterial and antiviral nanofibrous membranes for bioprotective applications. Sci Adv. https://doi.org/10.1126/sciadv.aar5931
Singh P, Gonzalez MJ, Manchester M (2006) Viruses and their uses in nanotechnology. Drug Dev Res 67:23–41. https://doi.org/10.1002/ddr.20064
Singh B, Choi YJ, Park IK, Akaike T, Cho CS (2014) Chemical modification of chitosan with ph-sensitive molecules and specific ligands for efficient DNA transfection and siRNA silencing. J Nanosci Nanotechnol 14:564–576. https://doi.org/10.1166/jnn.2014.9079
Sivasankarapillai VS, Pillai AM, Rahdar A, Sobha AP, Das SS, Mitropoulos AC, Mokarrar MH, Kyzas GZ (2020) On facing the SARS-cov-2 (COVID-19) with combination of nanomaterials and medicine: possible strategies and first challenges. Nanomaterials 10:852. https://doi.org/10.3390/nano10050852
Sonaje K, Chuang EY, Lin KJ, Yen TC, Su FY, Tseng MT, Sung HW (2012) Opening of epithelial tight junctions and enhancement of paracellular permeation by chitosan: microscopic, ultrastructural, and computed-tomographic observations. Mol Pharm 9:1271–1279. https://doi.org/10.1021/mp200572t
Sportelli MC, Izzi M, Kukushkina EA, Hossain SI, Picca RA, Ditaranto N, Cioff N (2020) Can nanotechnology and materials science help the fight against sars-cov-2? Nanomaterials. https://doi.org/10.3390/nano10040802
Sreejivungsa K, Suchaichit N, Moosophon P, Chompoosor A (2016) Light-regulated release of entrapped drugs from photoresponsive gold nanoparticles. J Nanomater 2016:4964693. https://doi.org/10.1155/2016/4964693
Staroverov SA, Vidyasheva IV, Gabalov KP, Vasilenko OA, Laskavyi VN, Dykman LA (2011) Immunostimulatory effect of gold nanoparticles conjugated with transmissible gastroenteritis virus. Bull Exp Biol Med 151:436. https://doi.org/10.1007/s10517-011-1350-8
Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF (2016) Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 24:490–502. https://doi.org/10.1016/j.tim.2016.03.003
Sucipto TH, Churrotin S, Setyawati H, Kotaki T, Martak F, Soegijanto S (2017) Antiviral activity of copper(II)chloride dihydrate against dengue virus type-2 in vero cell. Indones J Trop Infect Dis. 6:84–87
Sun RWY, Chen R, Chung NPY, Ho CM, Lin CLS, Che CM (2005) Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chem Commun. https://doi.org/10.1039/b510984a
Sun L, Singh AK, Vig K, Pillai SR, Singh SR (2008) Silver nanoparticles inhibit replication of respiratory syncytial virus. J Biomed Nanotechnol 4:149–158. https://doi.org/10.1166/jbn.2008.012
Swierczewska M, Lee S, Chen X (2011) Inorganic nanoparticles for multimodal molecular imaging. Mol Imaging. https://doi.org/10.2310/7290.2011.00001
Talelli M, Barz M, Rijcken CJF, Kiessling F, Hennink WE, Lammers T (2015) Core-crosslinked polymeric micelles: principles, preparation, biomedical applications and clinical translation. Nano Today 10:93–117. https://doi.org/10.1016/j.nantod.2015.01.005
Teengam P, Siangproh W, Tuantranont A, Vilaivan T, Chailapakul O, Henry CS (2017) Multiplex paper-based colorimetric dna sensor using pyrrolidinyl peptide nucleic acid-induced AgNPs aggregation for detecting MERS-CoV, MTB, and HPV oligonucleotides. Anal Chem 89:5428–5435. https://doi.org/10.1021/acs.analchem.7b00255
Thompson BT, Chambers RC, Liu KD (2017) Acute respiratory distress syndrome. N Engl J Med 377:562–572. https://doi.org/10.1056/NEJMra1608077
Tian X, Li C, Huang A, Xia S, Lu S, Shi Z, Lu L, Jiang S, Yang Z, Wu Y, Ying T (2020) Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 9:382–385. https://doi.org/10.1080/22221751.2020.1729069
Ton AT, Gentile F, Hsing M, Ban F, Cherkasov A (2020) Rapid identification of potential inhibitors of SARS-CoV-2 main protease by deep docking of 1.3 billion compounds. Mol Inform. https://doi.org/10.1002/minf.202000028
Uhlig T, Kyprianou T, Martinelli FG, Oppici CA, Heiligers D, Hills D, Calvo XR, Verhaert P (2014) The emergence of peptides in the pharmaceutical business: from exploration to exploitation. EuPA Open Proteom 4:58–69. https://doi.org/10.1016/j.euprot.2014.05.003
Upponi JR, Jerajani K, Nagesha DK, Kulkarni P, Sridhar S, Ferris C, Torchilin VP (2018) Polymeric micelles: theranostic co-delivery system for poorly water-soluble drugs and contrast agents. Biomaterials 170:26–36. https://doi.org/10.1016/j.biomaterials.2018.03.054
Uskokovic D, Stevanovic M (2009) Poly(lactide-co-glycolide)-based micro and nanoparticles for the controlled drug delivery of vitamins. Curr Nanosci 5:1–14. https://doi.org/10.2174/157341309787314566
Vashist SK (2020) In vitro diagnostic assays for COVID-19: recent advances and emerging trends. Diagnostics. https://doi.org/10.3390/diagnostics10040202
Verissimo TV, Santos NT, Silva JR, Azevedo RB, Gomes AJ, Lunardi CN (2016) In vitro cytotoxicity and phototoxicity of surface-modified gold nanoparticles associated with neutral red as a potential drug delivery system in phototherapy. Mater Sci Eng C 65:199–204. https://doi.org/10.1016/j.msec.2016.04.030
Vijayakumar S, Ganesan S (2014) Gold nanoparticles as an HIV entry inhibitor. Curr HIV Res 10:643–646. https://doi.org/10.2174/157016212803901383
Villarreal A, Rangel G, Zhang X, Wong D, Britton G, Fernandez PL, Pérez A, Oviedo D, Restrepo C, Carreirra MB, Sambrano D, Eskildsen GA, De La Guardia C, Flores-Cuadra J, Carrera JP, Zaldivar Y, Franco D, López-Vergès S, Zhang D, Fan F, Wang B, Sáez-Llorens X, DeAntonio R, Torres-Atencio I, Blanco I, Subía FD, Mudarra L, Benzadon A, Valverde W, López L, Hurtado N, Rivas N, Jurado J, Carvallo A, Rodriguez J, Perez Y, Morris J, Luque O, Cortez D, Ortega-Barria E, Kosagisharaf R, Lleonart R, Li C, Goodridge A (2021) Performance of a point of care test for detecting IgM and IgG antibodies against SARS-CoV-2 and seroprevalence in blood donors and health care workers in Panama. Front Med. https://doi.org/10.3389/fmed.2021.616106
Volz E, Hill V, McCrone JT, Price A, Jorgensen D, O’Toole Á, Southgate J, Johnson R, Jackson B, Nascimento FF, Rey SM, Nicholls SM, Colquhoun RM, da Silva Filipe A, Shepherd J, Pascall DJ, Shah R, Jesudason N, Li K, Jarrett R, Pacchiarini N, Bull M, Geidelberg L, Siveroni I, Goodfellow I, Loman NJ, Pybus OG, Robertson DL, Thomson EC, Rambaut A, Connor TR (2020) Evaluating the effects of SARS-CoV-2 Spike mutation D614G on transmissibility and pathogenicity. Cell. https://doi.org/10.1016/j.cell.2020.11.020
Xiang J, Yan M, Li H, Liu T, Lin C, Huang S et al (2020) Evaluation of enzyme-linked immunoassay and colloidal gold- immunochromatographic assay kit for detection of novel coronavirus (SARS-Cov-2) causing an outbreak of pneumonia (COVID-19). MedRxiv. https://doi.org/10.1101/2020.02.27.20028787
Wang LF, Shi Z, Zhang S, Field H, Daszak P, Eaton BT (2006) Review of bats and SARS. Emerg Infect Dis 12:1834–1840. https://doi.org/10.3201/eid1212.060401
Wang J, Qiao LF, Yang GT (2008) Role of Shenfu injection in rats with systemic inflammatory response syndrome. Chin J Integr Med 14:51–55. https://doi.org/10.1007/s11655-008-0051-2
Wang Y, Liu D, Shi W, Lu R, Wang W, Zhao Y, Deng Y, Zhou W, Ren H, Wu J, Wang Y, Wu G, Gao GF, Tana W (2015) Origin and possible genetic recombination of the middle east respiratory syndrome coronavirus from the first imported case in china: phylogenetics and coalescence analysis. Mbio 6:e01280-e1315. https://doi.org/10.1128/mBio.01280-15
Wang BK, Yu XF, Wang JH, Bin Li Z, Li PH, Wang H, Song L, Chu PK, Li C (2016) Gold-nanorods-siRNA nanoplex for improved photothermal therapy by gene silencing. Biomaterials 78:27–39
Wang H, Zhu W, Feng L, Chen Q, Chao Y, Dong Z, Liu Z (2018) Nanoscale covalent organic polymers as a biodegradable nanomedicine for chemotherapy-enhanced photodynamic therapy of cancer. Nano Res 11:3244–3257. https://doi.org/10.1007/s12274-017-1858-y
Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W (2020) Detection of SARS-CoV-2 in different types of clinical specimens. JAMA - J Am Med Assoc 323:1843–1844. https://doi.org/10.1001/jama.2020.3786
Whitehead KA, Langer R, Anderson DG (2009) Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov 8:129–138. https://doi.org/10.1038/nrd2742
WHO (2020) WHO Director-Generals opening remarks at the mission briefing on COVID-19 - 26 Feb 2020, https://www.Who.Int/Dg/Speeches/Detail/Who-Director-General-S-Opening-Remarks-At-the-Media-Briefing-on-Covid-19---11-March-2020
World Health Organization (2015) 19th WHO model list of essential medicines https://doi.org/10.1016/S1473-3099(14)70780-7
World Health Organization (2020) Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases, 2020. https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117
Williams AE, Chambers RC (2014) The mercurial nature of neutrophils: still an enigma in ARDS? Am J Physiol—Lung Cell Mol Physiol 306:217–230. https://doi.org/10.1152/ajplung.00311.2013
Woo PCY, Lau SKP, Lam CSF, Lau CCY, Tsang AKL, Lau JHN, Bai R, Teng JLL, Tsang CCC, Wang M, Zheng B-J, Chan K-H, Yuen K-Y (2012) Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and Avian coronaviruses as the gene source of gammacoronavirus and deltacoronavi. J Virol 86:3995–4008. https://doi.org/10.1128/jvi.06540-11
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science (80) 367:1260–1263. https://doi.org/10.1126/science.aax0902
Xia S, Zhu Y, Liu M, Lan Q, Xu W, Wu Y, Ying T, Liu S, Shi Z, Jiang S, Lu L (2020) Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol Immunol 17:765–767. https://doi.org/10.1038/s41423-020-0374-2
Xian Y, Zhang J, Bian Z, Zhou H, Zhang Z, Lin Z, Xu H (2020) Bioactive natural compounds against human coronaviruses: a review and perspective. Acta Pharm Sin b. https://doi.org/10.1016/j.apsb.2020.06.002
Xiao XSK, Zhai J, Feng Y, Zhou N, Zhang X, Zou J-J, Li N, Guo Y, Li X (2020) Isolation and characterization of 2019-nCoV-like coronavirus from Malayan pangolins. BioRxiv. https://doi.org/10.1101/2020.02.17.951335
Xiaoyan L, Lundborg CS, Banghan D, Bojun C, Hong Z, Jiqiang L, Aihua O, Wenwei O, Zehuai W, Chuanjian L, Marrone G (2018) Clinical outcomes of influenza-like illness treated with Chinese herbal medicine: an observational study. J Tradit Chinese Med 38:107–116. https://doi.org/10.1016/j.jtcm.2018.02.011
Xie S, Tao Y, Pan Y, Qu W, Cheng G, Huang L, Chen D, Wang X, Liu Z, Yuan Z (2014) Biodegradable nanoparticles for intracellular delivery of antimicrobial agents. J Control Release 187:101–117. https://doi.org/10.1016/j.jconrel.2014.05.034
Xu B, Li A, Hao X, Guo R, Shi X, Cao X (2018) PEGylated dendrimer-entrapped gold nanoparticles with low immunogenicity for targeted gene delivery. RSC Adv 8:1265–1273. https://doi.org/10.1039/c7ra11901a
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 8:420–422. https://doi.org/10.1016/S2213-2600(20)30076-X
Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y, Wei Z, Wang L, Kong L, Sun H, Ping Q, Mo R, Zhang C (2017) Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol 12:692–700. https://doi.org/10.1038/nnano.2017.54
Yadavalli T, Ames J, Agelidis A, Suryawanshi R, Jaishankar D, Hopkins J, Thakkar N, Koujah L, Shukla D (2019) Drug-encapsulated carbon (DECON): a novel platform for enhanced drug delivery. Sci Adv 5:0780. https://doi.org/10.1126/sciadv.aax0780
Yang L, Wu Z, Ren X, Yang F, He G, Zhang J, Dong J, Sun L, Zhu Y, Du J, Zhang S, Jin Q (2011) Novel SARS-like betacoronaviruses in bats, China. Emerg Infect Dis 19(2013):989–991. https://doi.org/10.3201/eid1906.121648
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y (2020) Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis 94:91–95. https://doi.org/10.1016/j.ijid.2020.03.017
Yang Y, Islam MS, Wang J, Li Y, Chen X (2020) Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci 16:1708–1717. https://doi.org/10.7150/ijbs.45538
Zaki AM, Van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM (2012) Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 367:1814–1820. https://doi.org/10.1056/NEJMoa1211721
Zazo H, Colino CI, Lanao JM (2016) Current applications of nanoparticles in infectious diseases. J Control Release 224:86–102. https://doi.org/10.1016/j.jconrel.2016.01.008
Zhang L, Pornpattananangkul D, Hu C-M, Huang C-M (2010) Development of nanoparticles for antimicrobial drug delivery. Curr Med Chem 17:585–594. https://doi.org/10.2174/092986710790416290
Zhang M, Liu E, Cui Y, Huang Y (2017a) Nanotechnology-based combination therapy for overcoming multidrug-resistant cancer. Cancer Biol Med 14:212–227
Zhang P, Liu G, Chen X (2017b) Nanobiotechnology: cell membrane-based delivery systems. Nano Today 13:7–9. https://doi.org/10.1016/j.nantod.2016.10.008
Zhang L, Shen FM, Chen F, Lin Z (2020) Origin and evolution of the 2019 novel coronavirus. Clin Infect Dis 71:882–883
Zhang Q, Honko A, Zhou J, Gong H, Downs SN, Vasquez JH, Fang RH, Gao W, Griffiths A, Zhang L (2020) Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett 20:5570–5574. https://doi.org/10.1021/acs.nanolett.0c02278
Zhao X, Cui Z, Song H, Ru W, Zhou X, Yu W (2020) A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. MedRxiv. https://doi.org/10.1101/2020.02.22.961268
Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C, Middelberg APJ (2014) Nanoparticle vaccines. Vaccine 32:327–337. https://doi.org/10.1016/j.vaccine.2013.11.069
Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z (2020) Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa344
Zhou QT, Tang P, Leung SSY, Chan JGY, Chan HK (2014) Emerging inhalation aerosol devices and strategies: where are we headed? Adv Drug Deliv Rev. https://doi.org/10.1016/j.addr.2014.03.006
Zhou P, Lou Yang X, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Di Jiang R, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL (2020a) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. https://doi.org/10.1038/s41586-020-2012-7
Zhou SS, Li WN, Ai ZZ, Wang LQ, Ba YM (2020b) Investigating mechanism of Qingfei Dayuan Granules for treatment of COVID-19 based on network pharmacology and molecular docking. Chinese Tradit Herb Drugs 51:1804–1813. https://doi.org/10.7501/j.issn.0253-2670.2020.07.014
Zhou J, Hu Z, Zabihi F, Chen Z, Zhu M (2020c) Progress and perspective of antiviral protective material. Adv Fiber Mater 2:123–139. https://doi.org/10.1007/s42765-020-00047-7
Zhu X, Radovic-Moreno AF, Wu J, Langer R, Shi J (2014) Nanomedicine in the management of microbial infection—overview and perspectives. Nano Today 9:478–498. https://doi.org/10.1016/j.nantod.2014.06.003
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W (2019) A novel coronavirus from patients with pneumonia in China. N Engl J Med 382(2020):727–733. https://doi.org/10.1056/NEJMoa2001017
Zhu X, Wang X, Han L, Chen T, Wang L, Li H, Li S, He L, Fu X, Chen S, Xing M, Chen H, Wang Y (2020) Reverse transcription loop-mediated isothermal amplification combined with nanoparticles-based biosensor for diagnosis of COVID-19. Biosens Bioelectron. https://doi.org/10.1101/2020.03.17.20037796
Zou X, Chen K, Zou J, Han P, Hao J, Han Z (2020) Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 14:185–192. https://doi.org/10.1007/s11684-020-0754-0
Zylka-Menhorn GGV (2020) Coronavirus 2019-nCoV: Der Steckbrief des Virus ist im Fluss. Dtsch Arztebl 117:A-250/B-219/C-215
Acknowledgements
This work was funded by various financial organisms: RUSA-Phase 2.0 grant [Letter No. F.24-51/2014-U, Policy (TN Multi-Gen), Dept. of Edn. Govt. of India, Dt. 09.10.2018]; Department of Science and Technology-Promotion of University Research and Scientific Excellence (DST-PURSE) (DST letter No.SR/PURSE phase 2/38(G), dt.21.02.2017), India; Scheme for Promotion of Academic and Research Collaboration (SPARC) (No. SPARC/2018-2019/P485/SL; Dated: 15.03.2019) and Institut de Recherche Robert-Sauvé en Santé et Sécurité au Travail (IRSST), Montreal, Canada (Project number 2020-0094). The Natural Sciences and Engineering Research Council of Canada (NSERC); We thank Dr. Harry K. Kaya, University of California, Davis, for review and editorial comments on a penultimate draft of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding authors state that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raja, R.K., Nguyen-Tri, P., Balasubramani, G. et al. SARS-CoV-2 and its new variants: a comprehensive review on nanotechnological application insights into potential approaches. Appl Nanosci 13, 65–93 (2023). https://doi.org/10.1007/s13204-021-01900-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-021-01900-w