Abstract
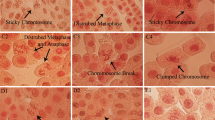
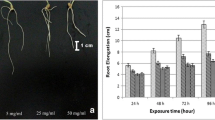
In the present study, we examined the effects of aluminium oxide nanoparticles (Al2O3 NPs) and bulk aluminium oxide (Al2O3) on Allium cepa in terms of genotoxicity and oxidative stress responses under in planta conditions. The concentrations ranged from 1.25 to 5 µM for NP and its bulk form. Results from this investigation indicated steady rise in chromosome aberrations, micronuclei and DNA strand breaks with increase in concentration of the NPs, effects of Al2O3 NPs being significantly higher than those treated with bulk Al2O3. The divisional frequency was affected by the NP treatment. Oxidative damage such as lipid peroxidation and cell death to Allium under Al2O3 NPs treatments was greater than that observed in bulk Al2O3. A significant increase in activity of guiacol peroxidase was observed in accordance with the depletion in catalase activity in both Al2O3 NPs and bulk Al2O3 treated plants as compared to control. In conclusion, the present study indicated that Al2O3 NPs treatment exerted higher genotoxicity and oxidative stress responses on A. cepa than bulk Al2O3 under in planta conditions.



Similar content being viewed by others
Abbreviations
- Al2O3 NPs:
-
Aluminium oxide nanoparticles
- CA:
-
Chromosome aberrations
- MN:
-
Micro nucleus
- MI:
-
Mitotic index
- GPOD:
-
Guiacol peroxidise
- CAT:
-
Catalase
- IPCS, WHO:
-
International Programme on Chemical Safety, World Health Organisation
- UNEP:
-
United Nations Environment Programme
- TTC:
-
2, 3, 5-Triphenyl-2H- tetrazolium chloride
- NaOH:
-
Sodium hydroxide
- NMPA:
-
Normal melting point agarose
- LMPA:
-
Low melting point agarose
- EDTA:
-
Di-sodium salt of ethylene diamine tetra acetic acid
- EtBr:
-
Ethidium bromide
- DLS:
-
Dynamic light scattering
- ZP:
-
Zeta potential
- PDI:
-
Poly dispersity index
- LPO:
-
Lipid peroxidation
- MDA:
-
Malondialdehyde
- ROS:
-
Reactive oxygen species
- SD:
-
Standard deviation
- ANOVA:
-
One-way analysis of variance
References
Achary VM, Jena S, Panda KK, Panda BB. Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotoxicol Environ Saf. 2008;70:300–10.
Achary VM, Parinandi LN, Panda BB. Calcium channel blockers protect against aluminium- induced DNA damage and block adaptive response to genotoxic stress in plant cells. Mutat Res. 2013;751:130–8.
Aebi H. Catalase in vitro. In: Packer L, editor. Methods in enzymology, vol. 105. New York: Academic Press Inc; 1984. p. 121–6.
Boscolo PR, Menossi M, Jorge RA. Aluminum-induced oxidative stress in maize. Phytochemistry. 2003;62:181–9.
Burklew CE, Ashlock J, Winfrey WB, Zhang B. Effects of aluminium oxide nanoparticles on the growth, development, and microRNA expression of tobacco (Nicotiana tabacum). PloS One. 2012;7:e34783.
Cabrera GL, Rodriguez DM. Genotoxicity of soil from farmland irrigated with wastewater using three plant bioassays. Mutat Res. 1999;426:211–4.
Chakraborty R, Mukherjee AK, Mukherjee A. Evaluation of genotoxicity of coal fly ash in Allium cepa root cells by combining comet assay with the Allium test. Environ Monit Assess. 2009;153:351–7.
Chang W, Chen M, Lee T. 2, 3, 5-Triphenyltetrazolium reduction in the viability assay of Ulva fasciata (Chlorophyta) in response to salinity stress. Bot Bull Acad Sin. 1999;40:207–12.
Delhaize E, Gruber BD, Ryan PR. The roles of organic anion permeases in aluminium resistance and mineral nutrition. FEBS Lett. 2007;581:2255–62.
Fernandes TCC, Mazzeo DEC, Marin-Morales MA. Mechanism of micronuclei formation in polyploidizated cells of Allium cepa exposed to trifluralin herbicide. Pestic Biochem Physiol. 2007;88:252–9.
Firbas P, Amon T. Allium chromosome aberration test for evaluation effect of cleaning municipal water with Constructed Wetland (CW) in Sveti Tomaž, Slovenia. J Bioremed Biodegrad. 2013;4:189–93.
Ghosh M, Bandyopadhyay M, Mukherjee A. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: plant and human lymphocytes. Chemosphere. 2010;81:1253–62.
Ghosh M, Bhadra S, Adegoke A, Bandyopadhyay M, Mukherjee A. MWCNT uptake in Allium cepa root cells induces cytotoxic and genotoxic responses and results in DNA hyper—methylation. Mutat Res. 2015;774:49–58.
Gonzalez-Fernandez A, Hernandez P, Lopez-Saez JF. Effect of caffeine and adenosine on G2 repair: mitotic delay and chromosome damage. Mutat Res. 1985;149:275–81.
Grant WF. Chromosome aberration assays in Allium. A report of the U.S. environmental protection agency gene-tox program. Mutat Res. 1982;99:273–91.
Halliwell B, Gutteridge J. Lipid peroxidation in brain homogenates: the role of iron and hydroxyl radicals. J Neurochem. 1997;69:1330–1.
Kim YJ, Choi HS, Song MK, Youk DY, Kim JH, Ryu JC. Genotoxicity of aluminum oxide (Al2O3) nanoparticle in mammalian cell lines. Mol Cell Toxicol. 2009;5:172–8.
Kumari M, Khan SS, Pakrashi S, Mukherjee A, Chandrasekaran N. Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J Hazard Mater. 2011;190:613–21.
Kumari M, Mukherjee A, Chandrasekaran N. Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ. 2009;407:5243–6.
Lee CW, Mahendra S, Zodrow K, Li D, Tsai YC, Braam J, Alvarez PJ. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ Toxicol Chem. 2010;29:669–75.
Leme DM, Marin-Moralis MA. Allium cepa test in environmental monitoring: a review on its application. Mutat Res. 2009;682:71–81.
Liman R. Genotoxic effects of Bismuth (III) oxide nanoparticles by Allium and Comet assay. Chemosphere. 2013;93:269–73.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75.
Ma TH, Xu Z, Xu C, McConnell H, Rabago EV, Arreola GA, Zhang H. The improved Allium/Vicia root tip micronucleus assay for clastogenicity of environmental pollutants. Mutat Res. 1995;334:185–95.
Matagne R. Duration of mitotic cycle and patterns of DNA replication in chromosomes of Allium cepa. Caryologia. 1968;21:209–24.
Matsumoto H, Motoda H. Aluminum toxicity recovery processes in root apices. Possible association with oxidative stress. Plant Sci. 2012;185:1–8.
Okhawa H, Ohishi N, Yagi K. Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–5.
Panda KK, Achary VM, Krishnaveni R, Padhi BK, Sarangi SN, Sahu SN, Panda BB. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol In Vitro. 2011;25:1097–105.
Panda KK, Lenka M, Panda BB. Monitoring and assessment of mercury pollution in the vicinity of a chloralkali plant I. Distribution, availability and genotoxicity of sediment mercury in the Rushikulya estuary, India. Sci Total Environ. 1990;96:281–96.
Patra J, Sahoo MK, Panda BB. Persistence and prevention of aluminium-and paraquat-induced adaptive response to methyl mercuric chloride in plant cells in vivo. Mutat Res. 2003;538:51–61.
Prabhakar PV, Reddy UA, Singh SP, Balasubramanyam A, Rahman MF, Indu Kumari S, Agawane SB, Murty US, Grover P, Mahboob M. Oxidative stress induced by aluminum oxide nanomaterials after acute oral treatment in Wistar rats. J Appl Toxicol. 2012;32(6):436–45.
Rajeshwari A, Kavitha S, Alex SA, Kumar D, Mukherjee A, Chandrasekaran N, Mukherjee A. Cytotoxicity of aluminum oxide nanoparticles on Allium cepa root tip—effects of oxidative stress generation and biouptake. Environ Sci Pollut Res. 2015;22:11057–66.
Ranjbar GH, Cheraghi SAM, Banakar MH. Salt sensitivity of wheat at germination stage. In: Kafi M, Ajmal-Khan M, editors. Crop and forage production using saline waters in dry areas. New Delhi: Daya Publishing; 2008. p. 200–4.
Schickler H, Caspi H. Response of antioxidative enzymes to nickel and stress in hyperaccumulator plants of the genus Alyssum. Physiol Plant. 1999;105:39–44.
Sharma AK, Sharma A. Chromosome techniques- theory and practice. 3rd ed. London: Butterworths Ltd; 1980.
Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91.
Stroinski A, Kubis J, Zielezinska M. Effect of cadmium on glutathione reductase potato tubers. Acta Physiol Plant. 1999;21:201–7.
Yanik F, Vardar F. Toxic Effects of Aluminum Oxide (Al2O3) Nanoparticles on root growth and development in Triticum aestivum. Water Air Soil Pollut. 2015;226:1–13.
Zhang J, Bao SD, Furumai R, Kueera KS, Ali A, Dean NA. Protein phosphate 5 is required for ATR mediated check point activation. Mol Cell Biol. 2005;25:9910–9.
Acknowledgments
The authors would like to acknowledge the University Grant Commission for financial assistance [UGC-MRP (41-1116/2012[SR] dt.01.07.2012] and thank the reviewers for their valuable suggestion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
De, A., Chakrabarti, M., Ghosh, I. et al. Evaluation of genotoxicity and oxidative stress of aluminium oxide nanoparticles and its bulk form in Allium cepa . Nucleus 59, 219–225 (2016). https://doi.org/10.1007/s13237-016-0179-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-016-0179-y