Abstract
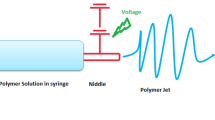
Electrospun nanofibrous mats have recently been employed as drug reservoirs for their unique features, such as high surface-to-volume ratios and easy fabrication process. We describe herein various methods of fabricating drug- and gene-encapsulated nanofibrous meshes, which can be prepared by electrospinning. The electrospinning process of nanofibrous mats is affected by many parameters, including viscosity and ejection speeds of the polymeric solutions and the electrical potential applied to the system. Both single- and dual-nozzle systems are widely employed in the preparation of electrospun nanofibers encapsulating drugs and genes, which are usually incorporated into the electrospun mats either by physical mixing with polymeric solutions before electrospinning or by physical incorporation after electrospinning. Various strategies have been tailored to maintain the bioactivity of proteins for tissue regeneration before and after electrospinning. Nucleic acids, such as DNA and siRNA, are also incorporated into nanofibrous meshes to enhance tissue regeneration by expressing transgenes or silencing domestic genes in specific tissues. Drug- or gene-incorporated nanofibrous meshes can greatly increase tissue regeneration rates and reduce scar formation in normal and diabetic wounds. Hybrid nanofibers, with multiple cell layers or hydrogels, have also been used to improve wound healing efficiency by increasing cell infiltration.

Similar content being viewed by others
References
Rho KS, Jeong L, Lee G, Seo BM, Park YJ, Hong SD, et al. Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials. 2006;27:1452–61.
Chong EJ, Phan TT, Lim IJ, Zhang YZ, Bay BH, Ramakrishna S, et al. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007;3:321–30.
Choi JS, Leong KW, Yoo HS. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials. 2008;29:587–96.
Schneider A, Wang XY, Kaplan DL, Garlick JA, Egles C. Biofunctionalized eletrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 2009;5:2570–8.
Venugopal J, Ramakrishna S. Biocompatible nanofiber matrices for the engineering of a dermal substitute for skin regeneration. Tissue Eng. 2005;11:847–54.
Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev. 2007;59:1413–33.
Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11:101–9.
Park SH, Kim TG, Kim HC, Yang DY, Park TG. Development of dual scale scaffolds via direct polymer melt deposition and electrospinning for applications in tissue regeneration. Acta Biomater. 2008;4:1198–207.
Kumbar SG, James R, Nukavarapu SP, Laurencin CT. Elctrospun nanofiber scaffolds: engineering soft tissues. Biomed Mater. 2008;3:34002–17.
Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res A. 2003;67:531–7.
Grafahrend D, Calvet JL, Klinkhammer K, Salber J, Dalton PD, Möller M, et al. Control of protein adsorption on functionalized electrospun fibers. Biotechnol Bioeng. 2008;101:609–21.
Doshi J, Reneker DH. Electrospinning process and applications of electrospun fibers. J Electrost. 1995;35:151–60.
Zhao Y, Cao X, Jiang L. Bio-mimic multichannel microtubes by a facile method. J Am Chem Soc. 2007;129:764–5.
He CL, Huang ZM, Han XJ. Fabrication of drug-loaded electrospun aligned fibrous threads for suture applications. J Biomed Mater Res A. 2009;89:80–95.
Katti DS, Robinson KW, Ko FK, Laurencin CT. Bioresorbable nanofiber-based systems for wound healing and drug delivery: optimization of fabrication parameters. J Biomed Mater Res B Appl Biomater. 2004;70:286–96.
Tan SH, Inai R, Kotaki M, Ramakrishna S. Systematic parameter study for ultra-fine fiber fabrication via electrospinning process. Polymer. 2005;46:6128–34.
Bhattarai N, Edmondson D, Veiseh O, Matsen FA, Zhang M. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials. 2005;26:6176–84.
Deitzel JM, Kleinmeyer J, Harris D, Beck Tan NC. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer. 2001;42:261–72.
Veleirinho B, Rei MF, Lopez-DA-Silva JA. Solvent and concentration effects on the properties of electrospun poly(ethylene terephthalate) nanofiber mats. J Polym Sci, Part B: Polym Phys. 2008;46:460–71.
Choi JS, Yoo HS. Pluronic/chitosan hydrogels containing epidermal growth factor with wound-adhesive and photo-crosslinkable properties. J Biomed Mater Res A. 2010;95:564–73.
Yoo HS, Oh JE, Lee KH, Park TG. Biodegradable nanoparticles containing doxorubicin-PLGA conjugate for sustained release. Pharm Res. 1999;16:1114–8.
Lee JI, Yoo HS. Biodegradable microspheres containing poly(ε-caprolactone)-pluronic block copolymers for temperature-responsive release of proteins. Colloids Surf B Biointerfaces. 2008;61:81–7.
Shenoy SL, Bates WD, Frisch HL, Wnek GE. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: good solvent, non-specific polymer–polymer interaction limit. Polymer. 2005;46:3372–84.
Maretschek S, Greiner A, Kissel T. Electrospun biodegradable nanofiber nonwovens for controlled release of proteins. J Control Release. 2008;127:180–7.
Chew SY, Wen J, Yim EKF, Leong KW. Sustained release of proteins from electrospun biodegradable fibers. Biomacromolecules. 2005;6:2017–24.
Chew SY, Hufnagel TC, Lim CT, Leong KW. Mechanical properties of single electrospun drug-encapsulated nanofibers. Nanotechnology. 2006;17:3880–91.
Zeng J, Aigner A, Czubayko F, Kissel T, Wendorff JH, Greiner A. Poly(vinyl alcohol) nanofibers by electrospinning as a protein delivery system and the retardation of enzyme release by additional polymer coatings. Biomacromolecules. 2005;6:1484–8.
Chen JP, Chang GY, Chen JK. Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids Surf A Physicochem Eng Asp. 2008;313–314:183–8.
Kim KH, Jeong L, Park HN, Shin SY, Park WH, Lee SC, et al. Biological efficacy of silk fibroin nanofiber membranes for guided bone regeneration. J Biotechnol. 2005;120:327–39.
Zhou Y, Yang D, Chen X, Xu Q, Lu F, Nie J. Electrospun water-soluble carboxyethyl chitosan/poly(vinyl alcohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules. 2008;9:349–54.
Yang Y, Li X, Cui W, Zhou S, Tan R, Wang C. Structural stability and release profiles of proteins from core–shell poly (dl-lactide) ultrafine fibers prepared by emulsion electropsinning. J Biomed Mater Res A. 2008;86:374–85.
Zhang Y, Huang ZM, Xu X, Lim CT, Ramakrishna S. Preparation of core–shell structured PCL-r-gelatin bi-component nanofibers by coaxial electrospinning. Chem Mater. 2004;16:3406–9.
Jiang H, Hu Y, Zhao P, Li Y, Zhu K. Modulation of protein release from biodegradable core–shell structured fibers prepared by coaxial electrospinning. J Biomed Mater Res B Appl Biomater. 2006;79:50–7.
Zhang YZ, Wang X, Feng Y, Li J, Lim CT, Ramakrishna S. Coaxial electrospinning of (fluorescein isothiocyanate-conjugated bovine serum albumin)-encapsulated poly(ε-caprolactone) nanofibers for sustained release. Biomacromolecules. 2006;7:1049–57.
Liao IC, Chew SY, Leong KW. Aligned core–shell nanofibers delivering bioactive proteins. Nanomedicine. 2006;1:465–71.
Huang ZM, He CL, Yang A, Zhang Y, Han XJ, Yin J, et al. Encapsulating drugs in biodegradable ultrafine fibers through co-axial electrospinning. J Biomed Mater Res A. 2006;77:169–79.
Yoshida M, Langer R, Lendlein A, Lahann J. From advanced biomedical coatings to multi-functionalized biomaterials. Polym Rev. 2006;46:347–75.
Rajangam K, Behanna HA, Hui MJ, Han X, Hulvat JF, Lomasney JW, et al. Heparin binding nanostructures to promote growth of blood vessels. Nano Lett. 2006;6:2086–90.
Casper CL, Yamaguchi N, Kiick KL, Rabolt JF. Functionalizing electrospun fibers with biologically relevant macromolecules. Biomacromolecules. 2005;6:1998–2007.
Cho YI, Choi JS, Jeong SY, Yoo HS. Nerve growth factor (NGF)-conjugated electrospun nanostructures with topographical cues for neuronal differentiation of mesenchymal stem cells. Acta Biomater. 2010;6:4725–33.
Choi JS, Choi SH, Yoo HS. Coaxial electrospun nanofibers for treatment of diabetic ulcers with binary release of multiple growth factors. J Mater Chem. 2011;21:5258–67.
Byrnes CK, Khan FH, Nass PH, Hatoum C, Ducan MD, Harmon JW. Success and limitations of a naked plasmid transfection protocol for keratinocyte growth factor-1 to enhance cutaneous wound healing. Wound Repair Regen. 2001;9:341–6.
Andree C, Swain WF, Page CP, Macklin MD, Slama J, Hatzis D, et al. In vivo transfer and expression of a human epidermal growth factor gene accelerates wound repair. Proc Natl Acad Sci U S A. 1994;91:12188–92.
Davison JM, Krieg T, Eming SA. Particle-mediated gene therapy of wounds. Wound Repair Regen. 2000;8:452–9.
Williams RS, Johnston SA, Riedy M, Devit MJ, McElligott SG, Sanford JC. Introduction of foreign genes into tissues of living mice by DNA-coated microprojectiles. Proc Natl Acad Sci U S A. 1991;88:2726–30.
Pakhomova ON, Gregory BW, Khorokhorina VA, Bowman AM, Xiao S, Pakhomov AG. Electroporation-induced electrosensitization. PLoS One. 2011;6:e17100.
Lee RC, Kolodney MS. Electrical injury mechanisms: electrical breakdown of cell membranes. Plast Reconstr Surg. 1987;80:672–9.
Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177:437–47.
Zhang J, Duan Y, Wei D, Wang L, Wang H, Gu Z, et al. Co-electrospun fibrous scaffold–adsorbed DNA for substrate-mediated gene delivery. J Biomed Mater Res A. 2011;96:212–20.
Luu YK, Kim K, Hsiao BS, Chu B, Hadjiargyrou M. D evelopment of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA-PEG block copolymers. J Control Release. 2003;89:341–53.
Rujitanaroj P, Wang Y, Wang J, Chew SY. Nanofiber-mediated controlled release of siRNA complexes for long term gene-silencing applications. Biomaterials. 2011;32:5915–23.
Ji W, Sun Y, Yang F, Beucken J, Fan M, Chen Z, et al. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm Res. 2011;28:1259–72.
Dehai L, Luu YK, Kim K, Hsiao BS, Hadjiargyrou M, Chu B. In vitro non-viral gene delivery with nanofibrous scaffolds. Nucleic Acids Res. 2005;33:e170.
Jang J, Shea LD. Controllable delivery of non-viral DNA from porous scaffolds. J Control Release. 2003;86:157–68.
Maruyama A, Katoh M, Ishihara T, Akaike T. Comb-type polycations effectively stabilize DNA triplex. Bioconjug Chem. 1997;8:3–6.
Hong K, Zheng W, Baker A, Papahadjopoulos D. Stabilization of cationic liposome-plasmid DNA complexes by polyamines and poly(ethylene glycol)-phospholipid conjugates for efficient in vivo gene delivery. FEBS Lett. 1997;400:233–7.
Werth S, Urban-Klein B, Dai L, Hobel S, Grzelinski M, Bakowsky U, et al. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J Control Release. 2006;122:257–70.
Urban-Kelin B, Werth S, Abuharbeid S, Czubayko F, Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12:461–6.
Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine) and its role in gene delivery. J Control Release. 1999;60:149–60.
Kunath K, Harpe A, Fischer D, Petersen H, Bickel U, Voigt K, et al. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J Control Release. 2003;89:113–25.
Lungwitz U, Breunig M, Blunk T, Gopferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60:247–66.
Yang Y, Li X, Cheng L, He S, Zou J, Chen F, et al. Core–sheath structured fibers with pDNA polyplex loadings for the optimal release profile and transfection efficiency as potential tissue engineering scaffolds. Acta Biomater. 2011;7:2533–43.
El-Aneed A. An overview of current delivery systems in cancer gene therapy. J Control Release. 2004;94:1–14.
Saraf A, Baggett LS, Raphael RM, Kasper FK, Mikos AG. Regulated non-viral gene delivery from coaxial electrospun fiber mesh scaffolds. J Control Release. 2010;143:95–103.
Sill TJ, Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006.
Kim HS, Yoo HS. MMPs-responsive release of DNA from electrospun nanofibrous matrix for local gene therapy: in vitro and in vivo evaluation. J Control Release. 2010;145:264–71.
Kim HS, Yoo HS. Matrix metalloproteinase-inspired suicidal treatments of diabetic ulcers with siRNA-decorated nanofibrous meshes. Gene Ther. 2012. doi:10.1038/gt.2012.49.
Sakai S, Yamada Y, Yamaguchi T, Ciach T, Kawakami K. Surface immobilization of poly(ethyleneimine) and plasmid DNA on electrospun poly(l-lactic acid) fibrous mats using a layer-by-layer approach for gene delivery. J Biomed Mater Res A. 2009;88:281–7.
Godbey WT, Wu KK, Mikos A. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials. 2011;22:471–80.
Fischer D, Bieber T, Li Y, Elsasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16:1273–9.
Godbey WT, Wu KK, Hirasaki GJ, Mikos AG. Improved packing of poly(ethylenimine)/DNA complexes increases transfection efficiency. Gene Ther. 1999;6:1380–8.
Shin HJ, Lee CH, Cho IH, Kim Y, Lee Y, Kim IA, et al. Electrospun PLGA nanofiber scaffolds for articular cartilage reconstruction: mechanical stability, degradation and cellular responses under mechanical stimulation in vitro. J Biomater Sci Polym Ed. 2006;17:103–19.
Zhong SP, Zhang YZ, Lim CT. Tissue scaffolds for skin wound healing and dermal reconstruction. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:510–25.
Nam J, Huang Y, Agarwal S, Lannutti J. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng. 2007;13:2249–57.
Vaguette C, Copper-White JJ. Increasing electrospun scaffold pore size with tailored collectors for improved cell penetration. Acta Biomater. 2011;7:2544–57.
Ekaputra AK, Prestwich GD, Cool SM, Hutmacher DW. Combining electrospun scaffolds with electrosprayed hydrogels leads to three-dimensional cellularization of hybrid constructs. Biomacromolecules. 2008;9:2097–103.
Yang XC, Shah JD, Wang HJ. Nanofiber enabled layer-by-layer approach toward three-dimensional tissue formation. Tissue Eng Part A. 2009;15:945–56.
Kai D, Prabhakaran M, Stahl B, Eblenkamp M, Wintermantel E, Ramakrishna S. Mechanical properties and in vitro behavior of nanofiber–hydrogel composites for tissue engineering applications. Nanotechnology. 2010;23:095705–14.
Ionescu LC, Lee GC, Sennett BJ, Burdick JA, Mauck RL. An anisotropic nanofiber/microsphere composite with controlled release of biomolecules for fibrous tissue engineering. Biomaterials. 2010;31:4113–20.
Barakat NAM, Abadir MF, Sheikh FA, Kanjwal MA, Park SJ, Kim HY. Polymeric nanofibers containing solid nanoparticles prepared by electrospinning and their applications. Chem Eng J. 2010;156:487–95.
Wei G, Jin Q, Giannobile WV, Ma PX. Nano-fibrous scaffold for controlled delivery of recombinant human PDGF-BB. J Control Release. 2006;112:103–10.
Wei G, Jin Q, Giannobile WV, Ma PX. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28:2087–96.
Min X, Hussain M, Mao JJ. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials. 2007;28:316–25.
Meng ZX, Zeng QT, Sun ZZ, Xu XX, Wang YS, Zheng W, et al. Immobilizing natural macromolecule on PLGA electrospun nanofiber with surface entrapment and entrapment-graft techniques. Colloids Surf B Biointerfaces. 2012;94:44–50.
Meng ZX, Li HF, Sun ZZ, Zheng W, Zheng YF. Fabrication of mineralized electrospun PLGA and PLGA/gelatin nanofibers and their potential in bone tissue engineering. Mater Sci Eng C. 2013;33:699–706.
Borjigin M, Stouse B, Niamat RA, Bialk P, Eskridge C, Xie J, et al. Proliferation of genetically modified human cells on electrospun nanofiber scaffolds. Mol Ther-Nucleic Acids. 2012;1:e59–67.
Liu X, Lin TL, Yao G, Zhao H, Dodson M, Wang X. In vivo wound healing and antibacterial performances of electrospun nanofiber membranes. J Biomed Mater Res A. 2010;94:499–508.
Alves da Silva ML, Martins A, Costa-Pinto AR, Costa P, Faria S, Gomes M, et al. Cartilage tissue engineering using electrospun PCL nanofiber meshes and MSCs. Biomacromolecules. 2010;11:3228–36.
Choi WS, Bae JW, Lim HR, Joung YK, Park JC, Kwon IK, et al. RGD peptide-immobilized electrospun matrix of polyurethane for enhanced endothelial cell affinity. Biomed Mater. 2008;3:44104–12.
Lee CH, Shin HJ, Cho IH, Kang YM, Kim IA, Park KD, et al. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials. 2005;26:1261–70.
Acknowledgment
This work was supported by a grant from the National Research Foundation (NRF) grant funded by the Korea government (MEST) (grant #: 2012005857R1A2A2A01) and Kangwon National University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ji Suk Choi and Hye Sung Kim contributed equally to this work.
Rights and permissions
About this article
Cite this article
Choi, J.S., Kim, H.S. & Yoo, H.S. Electrospinning strategies of drug-incorporated nanofibrous mats for wound recovery. Drug Deliv. and Transl. Res. 5, 137–145 (2015). https://doi.org/10.1007/s13346-013-0148-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-013-0148-9