Abstract
The chemical-physical processes controlling hydrothermal carbonization (HTC) are still not completely understood. This paper focuses on two aspects: the influence on the hydrochar formation of the particle size of the feedstock and the presence of solved compounds in the feedwater. To address these, brewer’s spent grains were crushed to < 1 mm and separated in three fractions. In addition, residual process water (rPW) from 5-hydroxymethylfurfural (HMF) production instead of bi-distilled H2O was added in a series of experiments for recycling. The results show a transfer limitation of hydrolysis products through pores for the particle size fractions > 250 μm proved by HPLC analysis of liquid byproducts, particularly when rPW, containing readily condensable/polymerizable intermediates, is added. This has a positive effect on the yield and carbon content of the hydrochars caused mainly by an increase in its secondary char fraction. The reaction pathways involved are discussed in detail.

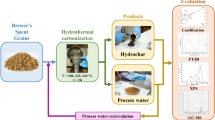
Graphical abstract













Similar content being viewed by others
Abbreviations
- HTC:
-
Hydrothermal carbonization
- HC:
-
Hydrochar
- HMF:
-
5-hydroxymethylfurfural
- C eff :
-
Carbon retention efficiency
- rPW:
-
Residual process water
- FRU:
-
Fructose
- BSG:
-
Brewer’s spent grains
- PW:
-
Process water
- C fix :
-
Fixed carbon
- VM:
-
Volatile matter
- LA:
-
Levulinic acid
- FA:
-
Formic acid
- AA:
-
Acetic acid
- LaA:
-
Lactic acid
- GA:
-
Glycolic acid
- MF:
-
Methylfurfural
- FU:
-
Furfural
- DOC:
-
Dissolved organic carbon
- NDF:
-
Neutral detergent fiber,
- ADF:
-
Acid detergent fiber
- ADL:
-
Acid detergent lignin
- PG:
-
Process gas
- SUC:
-
Sucrose
- GLU:
-
Glucose
- FRU:
-
Fructose
- GIAD:
-
Glyceraldehyde
- GAD:
-
Glycolaldehyde
- PA:
-
Pyruvic acid
- DHA:
-
Dihydroxyacetone
- XYL:
-
Xylose
- ARA:
-
Arabinose
- BDL:
-
Below detection limit
- FAD:
-
Formaldehyde
- BTO:
-
1,2,4-benzenetriol
- EtOH:
-
Ethanol
- DHH:
-
2,5-dioxo-6-hydroxy-hexanal
- ERY:
-
Erythrose
- FUA:
-
Furfuryl-alcohol
- MeOH:
-
Methanol
- AAD:
-
Acetaldehyde
- MAN:
-
Mannose
- OA:
-
Oxalic acid
- PAD:
-
Pyruvaldehyde
- ProA:
-
Propionic acid
- ProeA:
-
Propenoic acid
References
Kruse A, Dahmen N (2018) Hydrothermal biomass conversion: Quo vadis? J Supercrit Fluids 134:114–123
Kim D, Yoshikawa K, Park K (2015) Characteristics of biochar obtained by hydrothermal carbonization of cellulose for renewable energy. Energies 8(12):14040–14048
Lynam JG, Reza MT, Yan W, Vásquez VR, Coronella CJ (2015) Hydrothermal carbonization of various lignocellulosic biomass. Biomass Conv Bioref 5(2):173–181
Sharples A (1957) The hydrolysis of cellulose and its relation to structure. Trans Faraday Soc 53:1003
Vos KD, Burris FO, Riley RL (1966) Kinetic study of the hydrolysis of cellulose acetate in the pH range of 2–10. J Appl Polym Sci 10(5):825–832
Tolonen LK, Zuckerstätter G, Penttilä PA, Milacher W, Habicht W, Serimaa R et al (2011) Structural changes in microcrystalline cellulose in subcritical water treatment. Biomacromolecules 12(7):2544–2551
García-Bordejé E, Pires E, Fraile JM (2017) Parametric study of the hydrothermal carbonization of cellulose and effect of acidic conditions. Carbon 123:421–432
Baugh KD, McCarty PL (1988) Thermochemical pretreatment of lignocellulose to enhance methane fermentation: I. Monosaccharide and furfurals hydrothermal decomposition and product formation rates. Biotechnol Bioeng 31(1):50–61
Qi Y, Song B, Qi Y (2016) The roles of formic acid and levulinic acid on the formation and growth of carbonaceous spheres by hydrothermal carbonization. RSC Adv 6(104):102428–102435
Sumerskii IV, Krutov SM, Zarubin MY (2010) Humin-like substances formed under the conditions of industrial hydrolysis of wood. Russ J Appl Chem 83(2):320–327
Sevilla M, Fuertes AB (2009) Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chem Eur J 15(16):4195–4203
Steinbach D, Kruse A, Sauer J (2017) Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production- a review. Biomass Conv Bioref 7(2):247–274
Luo S, Zhou Y, Yi C, Luo Y, Fu J (2014) Influence of the feed moisture, rotor speed, and blades gap on the performances of a biomass pulverization technology. TheScientificWorldJournal 2014:435816
Cocero MJ, Cabeza Á, Abad N, Adamovic T, Vaquerizo L, Martínez CM, Pazo-Cepeda MV (2018) Understanding biomass fractionation in subcritical & supercritical water. J Supercrit Fluids 133:550–565
van Putten R-J, van der Waal JC, de Jong E, Rasrendra CB, Heeres HJ, de Vries JG (2013) Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem Rev 113(3):1499–1597
Rapp KM Process for preparing pure 5-hydroxymethylfurfuraldehyde: United States patent
van Zandvoort I, Wang Y, Rasrendra CB, Van Eck ERH, Bruijnincx PCA, Heeres HJ et al (2013) Formation, molecular structure, and morphology of humins in biomass conversion: influence of feedstock and processing conditions. ChemSusChem 6(9):1745–1758
Kambo HS, Minaret J, Dutta A (2018) Process water from the hydrothermal carbonization of biomass: a waste or a valuable product? Waste Biomass Valor 9(7):1181–1189
Tsilomelekis G, Orella MJ, Lin Z, Cheng Z, Zheng W, Nikolakis V, Vlachos DG (2016) Molecular structure, morphology and growth mechanisms and rates of 5-hydroxymethyl furfural (HMF) derived humins. Green Chem 18(7):1983–1993
Reiche S, Kowalew N, Schlögl R (2015) Influence of synthesis pH and oxidative strength of the catalyzing acid on the morphology and chemical structure of hydrothermal carbon. Chemphyschem 16(3):579–587
Uddin MH, Reza MT, Lynam JG, Coronella CJ (2013) Effects of water recycling in hydrothermal carbonization of loblolly pine. Environ Prog Sustain Energy 34:n/a-n/a
Stemann J, Putschew A, Ziegler F (2013) Hydrothermal carbonization: process water characterization and effects of water recirculation. Bioresour Technol 143:139–146
Kabadayi Catalkopru A, Kantarli IC, Yanik J (2017) Effects of spent liquor recirculation in hydrothermal carbonization. Bioresour Technol 226:89–93
Weiner B, Poerschmann J, Wedwitschka H, Koehler R, Kopinke F-D (2014) Influence of process water reuse on the hydrothermal carbonization of paper. ACS Sustain Chem Eng 2(9):2165–2171
Poerschmann J, Weiner B, Koehler R, Kopinke F-D (2015) Organic breakdown products resulting from hydrothermal carbonization of brewer’s spent grain. Chemosphere 131:71–77
Kruse A, Badoux F, Grandl R, Wüst D (2012) Hydrothermale Karbonisierung: 2. Kinetik der Biertreber-Umwandlung Chemie Ingenieur Technik 84(4):509–512
Lynch KM, Steffen EJ, Arendt EK (2016) Brewers’ spent grain: a review with an emphasis on food and health. J Inst Brew 122(4):553–568
McCarthy AL, O'Callaghan YC, Piggott CO, FitzGerald RJ, O'Brien NM (2013) Brewers’ spent grain; bioactivity of phenolic component, its role in animal nutrition and potential for incorporation in functional foods: a review. Proc Nutr Soc 72(1):117–125
Robertson JA, I'Anson KJA, Treimo J, Faulds CB, Brocklehurst TF, Eijsink VGH, Waldron KW (2010) Profiling brewers’ spent grain for composition and microbial ecology at the site of production. LWT Food Sci Technol 43(6):890–896
Mussatto SI, Roberto IC (2006) Chemical characterization and liberation of pentose sugars from brewer's spent grain. J Chem Technol Biotechnol 81(3):268–274
Berge ND, Ro KS, Mao J, Flora JRV, Chappell MA, Bae S (2011) Hydrothermal carbonization of municipal waste streams. Environ Sci Technol 45(13):5696–5703
Dinjus E, Kruse A, Tröger N (2011) Hydrothermal carbonization - 1. Influence of lignin in lignocelluloses. Chem Eng Technol 34(12):2037–2043
van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74(10):3583–3597
Muthusamy N (2014) Chemical composition of brewer’s spent grains - a review. Int J Sci Environ Technol (3):2109–2112
Fărcaş AC, Socaci SA, Dulf FV, Tofană M, Mudura E, Diaconeasa Z (2015) Volatile profile, fatty acids composition and total phenolics content of brewers' spent grain by-product with potential use in the development of new functional foods. J Cereal Sci 64:34–42
Poerschmann J, Weiner B, Wedwitschka H, Baskyr I, Koehler R, Kopinke F-D (2014) Characterization of biocoals and dissolved organic matter phases obtained upon hydrothermal carbonization of brewer’s spent grain. Bioresour Technol 164:162–169
Santos M, Jiménez JJ, Bartolomé B, Gómez-Cordovés C, del Nozal MJ (2003) Variability of brewer’s spent grain within a brewery. Food Chem 80(1):17–21
Hardwick WA (ed.). Handbook of Brewing; 1994
Yang H, Wang G, Ding N, Yin C, Chen Y (2016) Size-controllable synthesis of carbon spheres with assistance of metal ions. Synth Met 214:1–4
Jung D, Zimmermann M, Kruse A (2018) Hydrothermal carbonization of fructose: growth mechanism and kinetic model. ACS Sustainable Chem Eng
Falco C, Baccile N, Titirici M-M (2011) Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem 13(11):3273
Chen W-H, Ye S-C, Sheen H-K (2012) Hydrolysis characteristics of sugarcane bagasse pretreated by dilute acid solution in a microwave irradiation environment. Appl Energy 93:237–244
Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM (2010) The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 110(6):3552–3599
Bhatty RS (1986) The potential of hull-less barley - a review. Cereal Chem 62:97–103
Fincher GB (1975) Morphology and chemical composition of barley endosperm cell walls. J Inst Brew 81(2):116–122
Krawielitzki S, Kläusli TM (2015) Modified hydrothermal carbonization process for producing biobased 5-HMF platform chemical. Ind Biotechnol 11(1):6–8
Asghari SF, Yoshida H (2006) Acid-catalyzed production of 5-hydroxymethyl furfural from d-fructose in subcritical water. Ind Eng Chem Res 45(7):2163–2173
Kritzer P (1998) Die Korrosion der Nickel-Basis-Legierung 625 unter hydrothermalen Bedingungen: Einfluss der Parameter Temperatur, Druck, pH-Wert und Anwesenheit von Sauerstoff sowie der Anionen Chlorid, Sulfat, Nitrat und Phosphat auf das Korroionsverhalten
Wegertseder GmbH. Microsoft Word - TechDat.doc
ARS. National Nutrient Database for Standard Reference
Funke A, Ziegler F (2010) Hydrothermal carbonization of biomass: a summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod Bioref 4(2):160–177
Titirici M-M (ed) (2013) Sustainable carbon materials from hydrothermal processes. Wiley, Hoboken
Patil SKR, Lund CRF (2011) Formation and growth of humins via aldol addition and condensation during acid-catalyzed conversion of 5-hydroxymethylfurfural. Energy Fuel 25(10):4745–4755
Patil SKR, Heltzel J, Lund CRF (2012) Comparison of structural features of humins formed catalytically from glucose, fructose, and 5-hydroxymethylfurfuraldehyde. Energy Fuel 26(8):5281–5293
Volpe M, Fiori L (2017) From olive waste to solid biofuel through hydrothermal carbonisation: the role of temperature and solid load on secondary char formation and hydrochar energy properties. J Anal Appl Pyrolysis 124:63–72
Aida TM, Sato Y, Watanabe M, Tajima K, Nonaka T, Hattori H, Arai K (2007) Dehydration of d-glucose in high temperature water at pressures up to 80MPa. J Supercrit Fluids 40(3):381–388
Möller M, Nilges P, Harnisch F, Schröder U (2011) Subcritical water as reaction environment: fundamentals of hydrothermal biomass transformation. ChemSusChem 4(5):566–579
Jin F, Zhou Z, Moriya T, Kishida H, Higashijima H, Enomoto H (2005) Controlling hydrothermal reaction pathways to improve acetic acid production from carbohydrate biomass. Environ Sci Technol 39(6):1893–1902
Chuntanapum A, Matsumura Y (2009) Formation of tarry material from 5-HMF in subcritical and supercritical water. Ind Eng Chem Res 48(22):9837–9846
Srokol Z, Bouche A-G, van Estrik A, Strik RCJ, Maschmeyer T, Peters JA (2004) Hydrothermal upgrading of biomass to biofuel; studies on some monosaccharide model compounds. Carbohydr Res 339(10):1717–1726
Fang Y, Zeng X, Yan P, Jing Z, Jin F (2012) An acidic two-step hydrothermal process to enhance acetic acid production from carbohydrate biomass. Ind Eng Chem Res 51(12):4759–4763
Kabyemela BM, ADSCHIRI T, Malaluan RM, ARAI K (1999) Glucose and fructose decomposition in subcritical and supercritical water: detailed reaction pathway, mechanisms, and kinetics. Ind Eng Chem Res 38(8):2888–2895
Akgül G, Kruse A (2013) Hydrothermal disproportionation of formaldehyde at subcritical conditions. J Supercrit Fluids 73:43–50
Koido K, Ishida Y, Kumabe K, Matsumoto K, Hasegawa T (2010) Kinetics of ethanol oxidation in subcritical water. J Supercrit Fluids 55(1):246–251
Aydıncak K, Yumak T, Sınağ A, Esen B (2012) Synthesis and characterization of carbonaceous materials from saccharides (glucose and lactose) and two waste biomasses by hydrothermal carbonization. Ind Eng Chem Res 51(26):9145–9152
Girisuta B, Janssen LPBM, Heeres HJ (2006) A kinetic study on the decomposition of 5-hydroxymethylfurfural into levulinic acid. Green Chem 8(8):701
Gökkaya DS, Sağlam M, Yüksel M, Madenoğlu TG, Ballice L (2016) Characterization of products evolved from supercritical water gasification of xylose (principal sugar in hemicellulose). Energy Sources Part A 38(11):1503–1511
Karagöz S, Bhaskar T, MUTO A, SAKATA Y (2005) Comparative studies of oil compositions produced from sawdust, rice husk, lignin and cellulose by hydrothermal treatment. Fuel 84(7–8):875–884
Peterson AA, Vogel F, Lachance RP, Fröling M, Antal JMJ, Tester JW (2008) Thermochemical biofuel production in hydrothermal media: a review of sub- and supercritical water technologies. Energy Environ Sci 1(1):32
Yang L, Tsilomelekis G, Caratzoulas S, Vlachos DG (2015) Mechanism of Brønsted acid-catalyzed glucose dehydration. ChemSusChem 8(8):1334–1341
Zhuang X, Zhan H, Song Y, He C, Huang Y, Yin X, Wu C (2019) Insights into the evolution of chemical structures in lignocellulose and non-lignocellulose biowastes during hydrothermal carbonization (HTC). Fuel 236:960–974
Ryu J, Suh Y-W, Suh DJ, Ahn, Dong JA (2010) Hydrothermal preparation of carbon microspheres from mono-saccharides and phenolic compounds. Carbon 48(7):1990–1998
Koch H, Pein J (1985) Condensation reactions between phenol, formaldehyde and 5-hydroxymethylfurfural, formed as intermediate in the acid catalyzed dehydration of starchy products. Polym Bull 13:525–532
Lucian M, Volpe M, Fiori L (2019) Hydrothermal carbonization kinetics of lignocellulosic agro-wastes: experimental data and modeling. Energies 12(3):516
Dussan K, Girisuta B, Lopes M, Leahy JJ, Hayes MHB (2015) Conversion of hemicellulose sugars catalyzed by formic acid: kinetics of the dehydration of D-xylose, L-arabinose, and D-glucose. ChemSusChem 8(8):1411–1428
Poerschmann J, Weiner B, Koehler R, Kopinke F-D (2017) Hydrothermal carbonization of glucose, fructose, and xylose—identification of organic products with medium molecular masses. ACS Sustain Chem Eng 5(8):6420–6428
van Dam HE, Kieboom APG, van Bekkum H (1986) The conversion of fructose and glucose in acidic media: formation of hydroxymethylfurfural. Starch/Stärke 38(3):95–101
Cottier L, Descotes G. 5-Hydroxymethylfurfural syntheses and chemical transformations 1991(2):233–248
Speck JC The Lobry De Bruyn-Alberda Van Ekenstein Transformation 13:63–103
Kuster BFM, Temmink HMG (1977) The influence of ph and weak-acid anions on the dehydration of d-fructose. Carbohydr Res 54:185–191
Cao X, Peng X, Sun S, Zhong L, Chen W, Wang S, Sun RC (2015) Hydrothermal conversion of xylose, glucose, and cellulose under the catalysis of transition metal sulfates. Carbohydr Polym 118:44–51
Antal MJ, Mok WSL, Richards GN (1990) Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from d-fructose and sucrose. Carbohydr Res 199(1):91–109
Körner P, Jung D, Kruse A (2018) The effect of different Brønsted acids on the hydrothermal conversion of fructose to HMF. Green Chem 20(10):2231–2241
Luijkx GCA, van Rantwijk F, van Bekkum H (1993) Hydrothermal formation of 1,2,4-benzenetriol from 5-hydroxymethyl-2-furaldehyde and d-fructose. Carbohydr Res 242:131–139
Kruse A, Gawlik A (2003) Biomass conversion in water at 330−410 °C and 30−50 MPa. Identification of key compounds for indicating different chemical reaction pathways. Ind Eng Chem Res 42(2):267–279
Antal MJ, Mok WSL, Richards GN (1990) Four-carbon model compounds for the reactions of sugars in water at high temperature. Carbohydr Res 199(1):111–115
Horvat J, Klaic B, Metelko B, Sunjic V (1985) Mechanism of levulinic acid formation. Tetrahedron Lett 26:2111–2114
Flannelly T, Lopes M, Kupiainen L, Dooley S, Leahy JJ (2016) Non-stoichiometric formation of formic and levulinic acids from the hydrolysis of biomass derived hexose carbohydrates. RSC Adv 6(7):5797–5804
Baccile N, Laurent G, Babonneau F, Fayon F, Titirici M-M, Antonietti M (2009) Structural characterization of hydrothermal carbon spheres by advanced solid-state MAS 13 C NMR investigations. J Phys Chem C 113(22):9644–9654
Cheng Z, Everhart JL, Tsilomelekis G, Nikolakis V, Saha B, Vlachos DG (2018) Structural analysis of humins formed in the Brønsted acid catalyzed dehydration of fructose. Green Chem 20(5):997–1006
Li M, Li W, Liu S (2012) Control of the morphology and chemical properties of carbon spheres prepared from glucose by a hydrothermal method. J Mater Res 27(08):1117–1123
Funke A, Reebs F, Kruse A (2013) Experimental comparison of hydrothermal and vapothermal carbonization. Fuel Process Technol 115:261–269
Rasrendra CB, Windt M, Wang Y, Adisasmito S, Makertihartha IGBN, van Eck ERH, Meier D, Heeres HJ (2013) Experimental studies on the pyrolysis of humins from the acid-catalysed dehydration of C6-sugars. J Anal Appl Pyrolysis 104:299–307
Sykes P (ed) (1988) Reaktionsmechanismen der organischen Chemie: Eine Einführung, 9th edn. VCH, Weinheim
Castello D, Kruse A, Fiori L (2015) Low temperature supercritical water gasification of biomass constituents: glucose/phenol mixtures. Biomass Bioenergy 73:84–94
Kruse A (2008) Supercritical water gasification. Biofuels Bioprod Bioref 2:415–437
Chuntanapum A, Yong TL-K, Miyake S, Matsumura Y (2008) Behavior of 5-HMF in subcritical and supercritical water. Ind Eng Chem Res 47(9):2956–2962
Williams PT, Onwudili J (2005) Composition of products from the supercritical water gasification of glucose: a model biomass compound. Ind Eng Chem Res 44(23):8739–8749
Nikolov PY, Yaylayan VA (2011) Thermal decomposition of 5-(hydroxymethyl)-2-furaldehyde (HMF) and its further transformations in the presence of glycine. J Agric Food Chem 59(18):10104–10113
Gökkaya DS, Sağlam M, Yüksel M, Ballice L (2016) Hydrothermal gasification of xylose: effects of reaction temperature, pressure, and K2CO3 as a catalyst on product distribution. Biomass Bioenergy 91:26–36
Rawn JD (ed) (2014) Organic chemistry: structure, mechanism and synthesis. Elsevier
Bicker M, Endres S, Ott L, Vogel H (2005) Catalytical conversion of carbohydrates in subcritical water: a new chemical process for lactic acid production. J Mol Catal A Chem 239(1–2):151–157
Hirosaka K, Koido K, Fukayama M, Ouryoji K, Hasegawa T (2008) Experimental and numerical study of ethanol oxidation in sub-critical water. J Supercrit Fluids 44(3):347–355
Zhai Z, Li X, Tang C, Peng J, Jiang N, Bai W, Gao H, Liao Y (2014) Decarbonylation of lactic acid to acetaldehyde over aluminum sulfate catalyst. Ind Eng Chem Res 53(25):10318–10327
Ma XJ, Yang XF, Zheng X, Lin L, Chen LH, Huang LL, Cao SL (2014) Degradation and dissolution of hemicelluloses during bamboo hydrothermal pretreatment. Bioresour Technol 161:215–220
Bajpai P (ed) Pretreatment of lignocellulosic biomass for biofuel production: structure of lignocellulosic biomass. Springer, Singapore
Román S, Nabais JMV, Laginhas C, Ledesma B, González JF (2012) Hydrothermal carbonization as an effective way of densifying the energy content of biomass. Fuel Process Technol 103:78–83
Bobleter O (1994) Hydrothermal degradation of polymers derived from plants. Prog Polym Sci 19(5):797–841
Reza MT, Yan W, Uddin MH, Lynam JG, Hoekman SK, Coronella CJ, Vásquez VR (2013) Reaction kinetics of hydrothermal carbonization of loblolly pine. Bioresour Technol 139:161–169
Rissanen JV, Grénman H, Xu C, Willför S, Murzin DY, Salmi T (2014) Obtaining spruce hemicelluloses of desired molar mass by using pressurized hot water extraction. ChemSusChem 7(10):2947–2953
Lucian M, Fiori L (2017) Hydrothermal carbonization of waste biomass: process design, modeling, energy efficiency and cost analysis. Energies 10(2):211
Yan W, Hoekman SK, Broch A, Coronella CJ (2014) Effect of hydrothermal carbonization reaction parameters on the properties of hydrochar and pellets. Environ Prog Sustain Energy 33(3):676–680
Abdilla RM, Rasrendra CB, Heeres HJ (2018) Kinetic studies on the conversion of Levoglucosan to glucose in water using Brønsted acids as the catalysts. Ind Eng Chem Res 57(9):3204–3214
Zan Y, Sun Y, Kong L, Miao G, Bao L, Wang H, Li S, Sun Y (2018) Formic acid-induced controlled-release hydrolysis of microalgae (Scenedesmus) to lactic acid over Sn-beta catalyst. ChemSusChem 11(15):2492–2496
Cabeza A, Piqueras CM, Sobrón F, García-Serna J (2016) Modeling of biomass fractionation in a lab-scale biorefinery: solubilization of hemicellulose and cellulose from holm oak wood using subcritical water. Bioresour Technol 200:90–102
Choudhary V, Mushrif SH, Ho C, Anderko A, Nikolakis V, Marinkovic NS, Frenkel AI, Sandler SI, Vlachos DG (2013) Insights into the interplay of Lewis and Brønsted acid catalysts in glucose and fructose conversion to 5-(hydroxymethyl)furfural and levulinic acid in aqueous media. J Am Chem Soc 135(10):3997–4006
Klingler D, Berg J, Vogel H (2007) Hydrothermal reactions of alanine and glycine in sub- and supercritical water. J Supercrit Fluids 43(1):112–119
Sato N, Quitain AT, Kang K, Daimon H, Fujie K (2004) Reaction kinetics of amino acid decomposition in high-temperature and high-pressure water. Ind Eng Chem Res 43(13):3217–3222
Piqueras CM, Cabeza Á, Gallina G, Cantero DA, García-Serna J, Cocero MJ (2017) Online integrated fractionation-hydrolysis of lignocellulosic biomass using sub- and supercritical water. Chem Eng J 308:110–125
BIO-RAD. guide to Aminex HPLC columns for food and beverage, biotechnology, and bio-organic analysis. MP 2016;82(1)
Novotný O, Cejpek K, Velíšek J (2008) Formation of carboxylic acids during degradation of monosaccharides. Czech JFood Sci 26(2):113–131
Smith AM, Singh S, Ross AB Fate of inorganic material during hydrothermal carbonisation of biomass: influence of feedstock on combustion behaviour of hydrochar. Fuel 169:135–145
Volpe M, Wüst D, Merzari F, Lucian M, Andreottola G, Kruse A, Fiori L (2018) One stage olive mill waste streams valorisation via hydrothermal carbonisation. Waste Manag 80:224–234
Faboya OO (1990) The interaction between oxalic acid and divalent ions - Mg2+, Zn2+ and Ca2+ - in aqueous medium. Food Chem 38:179–187
Bell DJ, Blake JD, Prazak M, Rowell D, Wilson PN (1991) Studies on yeast differentiation using organic acid metabolites part 1. Development of methodology using highperformance liquid chromatography. J Inst Brew 97:297–305
Jin F, Zhou Z, Enomoto H, Moriya T, Higashijima H (2004) Conversion mechanism of cellulosic biomass to lactic acid in subcritical water and acid–base catalytic effect of subcritical water. Chem Lett 33(2):126–127
Li Y, Lu X, Yuan L, Liu X (2009) Fructose decomposition kinetics in organic acids-enriched high temperature liquid water. Biomass Bioenergy 33(9):1182–1187
Kumalaputri AJ, Randolph C, Otten E, Heeres HJ, Deuss PJ (2018) Lewis acid catalyzed conversion of 5-hydroxymethylfurfural to 1,2,4-benzenetriol, an overlooked biobased compound. ACS Sustain Chem Eng 6(3):3419–3425
Rodríguez Correa C, Stollovsky M, Hehr T, Rauscher Y, Rolli B, Kruse A (2017) Influence of the carbonization process on activated carbon properties from lignin and lignin-rich biomasses. ACS Sustain Chem Eng 5(9):8222–8233
Kang S, Li X, Fan J, Chang J (2012) Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, d-xylose, and wood meal. Ind Eng Chem Res 51(26):9023–9031
Ravber M (2015) Hydrothermal degradation of fats, carbohydrates and proteins in sunflower seeds after treatment with subcritical water. Chem Biochem Eng Q 29(3):351–355
Abdelmoez W, Nakahasi T, Yoshida H (2007) Amino acid transformation and decomposition in saturated subcritical water conditions. Ind Eng Chem Res 46(16):5286–5294
Titirici M-M, Antonietti M, Baccile N (2008) Hydrothermal carbon from biomass: a comparison of the local structure from poly- to monosaccharides and pentoses/hexoses. Green Chem 10(11):1204
Peterson AA, Lachance RP, Tester JW (2010) Kinetic evidence of the Maillard reaction in hydrothermal biomass processing: glucose−glycine interactions in high-temperature, high-pressure water. Ind Eng Chem Res 49(5):2107–2117
Fan Y, Hornung U, Dahmen N, Kruse A. Formation of N-containing heterocycles from hydrothermal liquefaction of modell compounds and sewage sludge. Proceedings of the 24th European Biomass Conference 2017
Fan Y, Hornung U, Dahmen N, Kruse A (2018) Hydrothermal liquefaction of protein-containing biomass: study of model compounds for Maillard reactions. Biomass Conv Bioref 8(4):909–923
Wang T, Zhai Y, Zhu Y, Peng C, Xu B, Wang T, Li C, Zeng G (2018) Influence of temperature on nitrogen fate during hydrothermal carbonization of food waste. Bioresour Technol 247:182–189
Teri G, Luo L, Savage PE (2014) Hydrothermal treatment of protein, polysaccharide, and lipids alone and in mixtures. Energy Fuel 28(12):7501–7509
Qiao L, Chen J, Ying Y, Zheng J-W, Jiang L (2013) Influence of NH4 + on the preparation of carbonaceous spheres by a hydrothermal process. J Mater Sci 48(9):3341–3346
Biller P, Riley R, Ross AB (2011) Catalytic hydrothermal processing of microalgae: decomposition and upgrading of lipids. Bioresour Technol 102(7):4841–4848
Broch A, Hoekman S, Jena U, Langford J (2014) Analysis of solid and aqueous phase products from hydrothermal carbonization of whole and lipid-extracted algae. Energies 7(1):62–79
Heilmann SM, Jader LR, Harned LA, Sadowsky MJ, Schendel FJ, Lefebvre PA, von Keitz MG, Valentas KJ (2011) Hydrothermal carbonization of microalgae II. Fatty acid, char, and algal nutrient products. Appl Energy 88(10):3286–3290
Lucian M, Volpe M, Gao L, Piro G, Goldfarb JL, Fiori L (2018) Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 233:257–268
Gao L, Volpe M, Lucian M, Fiori L, Goldfarb JL (2019) Does hydrothermal carbonization as a biomass pretreatment reduce fuel segregation of coal-biomass blends during oxidation? Energy Convers Manag 181:93–104
Mumme J, Eckervogt L, Pielert J, Diakité M, Rupp F, Kern J (2011) Hydrothermal carbonization of anaerobically digested maize silage. Bioresour Technol 102(19):9255–9260
Rathsack P, Reichel D, Krzack S, Otto M (2014) Komprehensive Gaschromatographie-Massenspektrometrie von Alkylbenzolen in Pyrolyseölen aus Biomasse und Kohle. Chem Ing Tech 86(10):1779–1789
Oka H, Yamago S, Yoshida J, Kajimoto O (2002) Evidence for a hydroxide ion catalyzed pathway in ester hydrolysis in supercritical water. Angew Chem Int Ed 41(4):623–625
Simsir H, Eltugral N, Karagöz S (2017) Hydrothermal carbonization for the preparation of hydrochars from glucose, cellulose, chitin, chitosan and wood chips via low-temperature and their characterization. Bioresour Technol 246:82–87
Álvarez-Murillo A, Sabio E, Ledesma B, Román S, González-García CM (2016) Generation of biofuel from hydrothermal carbonization of cellulose. Kinetics modelling. Energy 94:600–608
Liu X, Song P, Hou J, Wang B, Xu F, Zhang X (2017) Revealing the dynamic formation process and mechanism of hollow carbon spheres: from bowl to sphere. ACS Sustain Chem Eng 6(2):2797–2805
Li R, Wang L, Shahbazi A (2015) A review of hydrothermal carbonization of carbohydrates for carbon spheres preparation. Tr Ren Energy 1(1):43–56
Zhang M, Yang H, Liu Y, Sun X, Zhang D, Xue D (2012) Hydrophobic precipitation of carbonaceous spheres from fructose by a hydrothermal process. Carbon 50(6):2155–2161
Acknowledgments
The authors would like to thank the students Johannes Winkler and Markus Götz from the University of Hohenheim for their efforts in experimentation and analytics as well as Sonja Habicht, Hermann Köhler, and Armin Lautenbach from the Institute of Catalysis Research and Technology of the Karlsruhe Institute of Technology for the technical support regarding the analysis of solid and liquid samples.
Funding
The work was financially supported by the Federal Ministry of Education and Research within the project Humboldt Reloaded during the winter semester 2017/2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Detailed understanding of the reaction pathways originated from oligosaccharides during the hydrothermal carbonization of soft lignocellulosic biomass
• Control of mass transfer during hydrolysis of oligosaccharides from small biomass particle sizes through the fast condensation and polymerization of reactive dehydration products
• Enhancement of yields and carbon retention efficiencies during hydrothermal carbonization by recirculation of residual process water from HMF synthesis instead of using water
Electronic supplementary material
ESM 1
(DOCX 288 kb)
Rights and permissions
About this article
Cite this article
Wüst, D., Correa, C.R., Jung, D. et al. Understanding the influence of biomass particle size and reaction medium on the formation pathways of hydrochar. Biomass Conv. Bioref. 10, 1357–1380 (2020). https://doi.org/10.1007/s13399-019-00488-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00488-0