Abstract
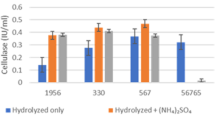
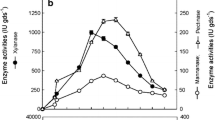
Bioenergy is one of the most promising solutions for the environmental problems of using non-renewable energy resources. Bioethanol is a form of bioenergy produced from food crops, such as corn, wheat, and sugarcane. Distillers’ dried grains with solubles (DDGS) is the by-product of corn and wheat ethanol production in the dry mill process and has a high nutritional profile. The undigested carbohydrate components in DDGS and proteins can be utilized as the feedstock to produce microbial cellulases and xylanases as the value-added products. Currently, several bacterial and fungal strains are used to produce such enzymes by using expensive feedstocks for commercial preparations. In this study, several bacterial and fungal strains have been evaluated to explore the potential of hydrolyzed DDGS as the main feedstock for these hydrolytic enzymes production. Maximum cellulase production of 0.592 IU/ml was observed for Aspergillus niger (NRRL 1956) on the eighteenth day and stable high production of xylanase of 34.8 IU/ml was obtained for Aspergillus niger (NRRL 567) on day twelfth during shake-flask fermentation. Hydrolytic enzyme production by Bacillus subtilis (NRRL NSR352, DSM 17766, and NF1) was relatively lower (0–0.261 IU/ml for cellulase and 1.2–5.2 IU/ml for xylanase) than the fungal enzyme production. In conclusion, this study demonstrated that hydrolyzed DDGS can be an alternative economical substrate for A. niger strains to produce cellulase and xylanase, respectively. The next phases of the study should further optimize the production of cellulase and xylanase in terms of growth parameters and medium components by using bench-top bioreactors.




Similar content being viewed by others
References
AFDC (2019) Global ethanol production. https://afdc.energy.gov/data/10331. Accessed 19 Sep 2019
Yan J (2015) First-generation biofuels. Wiley Online Library, Hoboken
Mohanty SK, Swain MR (2019) Bioethanol production from corn and wheat: food, fuel, and future. In: Bioethanol production from food crops. Academic Press, Cambridge, pp 45–59
Liu K, Rosentrater KA (2016) Distillers grains: production, properties, and utilization. CRC press, Boca Raton
Świątkiewicz S, Koreleski J (2008) The use of distillers dried grains with solubles (DDGS) in poultry nutrition. Worlds Poult Sci J 64:257–266
Lee RA, Lavoie J-M (2013) From first-to third-generation biofuels: challenges of producing a commodity from a biomass of increasing complexity. Anim Front 3:6–11
Jones DS, Searcy SW, Eaton LM (2018) Assessment of perennial grass inventories predicted in the billion-ton studies
McKendry P (2002) Energy production from biomass (part 1): overview of biomass. Bioresour Technol 83:37–46
Mosier N, Wyman C, Dale B, Elander R, Lee YY, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Jäger G, Büchs J (2012) Biocatalytic conversion of lignocellulose to platform chemicals. Biotechnol J 7:1122–1136
Mathew GM, Sukumaran RK, Singhania RR, Pandey A (2008) Progress in research on fungal cellulases for lignocellulose degradation. J Sci Ind Res (India) 67:898–907
Gilkes NR, Kilburn DG, Miller RC Jr, Warren RAJ (1991) Bacterial cellulases. Bioresour Technol 36:21–35
Kaiser CA, Krieger M, Lodish H, Berk A (2007) Molecular cell biology. WH Freeman
Demain AL, Newcomb M, Wu JHD (2005) Cellulase, clostridia, and ethanol. Microbiol Mol Biol Rev 69:124–154
Horn SJ, Vaaje-Kolstad G, Westereng B, Eijsink V (2012) Novel enzymes for the degradation of cellulose. Biotechnol Biofuels 5:45
Hamid SBA, Islam MM, Das R (2015) Cellulase biocatalysis: key influencing factors and mode of action. Cellulose 22:2157–2182
Prajapati AS, Panchal KJ, Pawar VA et al (2018) Review on cellulase and xylanase engineering for biofuel production. Ind Biotechnol 14:38–44
Aro N, Pakula T, Penttilä M (2005) Transcriptional regulation of plant cell wall degradation by filamentous fungi. FEMS Microbiol Rev 29:719–739
Aro N, Ilmén M, Saloheimo A, Penttilä M (2003) ACEI of Trichoderma reesei is a repressor of cellulase and xylanase expression. Appl Environ Microbiol 69:56–65
Haltrich D, Nidetzky B, Kulbe KD et al (1996) Production of fungal xylanases. Bioresour Technol 58:137–161
Margolles-Clark E, Ihnen M, Penttilä M (1997) Expression patterns of ten hemicellulase genes of the filamentous fungus Trichoderma reesei on various carbon sources. J Biotechnol 57:167–179
Polizeli M, Rizzatti ACS, Monti R, Terenzi HF, Jorge JA, Amorim DS (2005) Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol 67:577–591
Anuradha P, Rani DJ, Lakshmi KV, Pasha SA (2009) Bacterial cellulases. Asian J Bio Sci 4:113–119
Maki M, Leung KT, Qin W (2009) The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int J Biol Sci 5:500–516a
Adney B, Baker J (1996) Measurement of cellulase activities. National Renewable Energy Laboratory Golden, CO
Adsul MG, Ghule JE, Singh R et al (2004) Polysaccharides from bagasse: applications in cellulase and xylanase production. Carbohydr Polym 57:67–72
Gao J, Weng H, Zhu D, Yuan M, Guan F, Xi Y (2008) Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal Aspergillus terreus M11 under solid-state cultivation of corn Stover. Bioresour Technol 99:7623–7629
Gruno M, Väljamäe P, Pettersson G, Johansson G (2004) Inhibition of the Trichoderma reesei cellulases by cellobiose is strongly dependent on the nature of the substrate. Biotechnol Bioeng 86:503–511
Ximenes EA, Dien BS, Ladisch MR et al (2007) Enzyme production by industrially relevant fungi cultured on coproduct from corn dry grind ethanol plants. ABAB Symposium. Humana Press, pp 171–183
Tsuchiya HM, Montgomery EM, Corman J (1949) Hydrolysis of the α-1, 6-glucosidic linkage in isomaltose by culture filtrate of Aspergillus niger NRRL 330. J Am Chem Soc 71:3265
Ghori MI, Ahmed S, Malana MA, Jamil A (2011) Corn stover-enhanced cellulase production by Aspergillus niger NRRL 567. Afr J Biotechnol 10:5878–5886
Izmirlioglu G, Demirci A (2016) Strain selection and medium optimization for glucoamylase production from industrial potato waste by Aspergillus Niger. J Sci Food Agric 96:2788–2795
Ahamed A, Vermette P (2008) Enhanced enzyme production from mixed cultures of Trichoderma reesei RUT-C30 and Aspergillus niger LMA grown as fed batch in a stirred tank bioreactor. Biochem Eng J 42:41–46. https://doi.org/10.1016/j.bej.2008.05.007
Ahamed A, Vermette P (2008) Culture-based strategies to enhance cellulase enzyme production from Trichoderma reesei RUT-C30 in bioreactor culture conditions. Biochem Eng J 40:399–407
Manivannan A, Narendhirakannan R (2014) Response surface optimization for co-production of cellulase and xylanase enzymes by Trichoderma reesei NRRL–3652. Int J ChemTech Res 6:3883
Friedrich J, Cimerman A, Perdih A (1986) Comparison of different cellulolytic fungi for bioconversion of apple distillery waste. Appl Microbiol Biotechnol 24:432–434
Kurt AS (2019) Bacterial cellulase production using grape pomace hydrolysate and synthetic sugar as sole carbon sources by shake-flask submerged fermentation
Mahdinia E, Demirci A, Berenjian A (2018) Utilization of glucose-based medium and optimization of Bacillus subtilis natto growth parameters for vitamin K (menaquinone-7) production in biofilm reactors. Biocatal Agric Biotechnol 13:219–224
Iram A, Cekmecelioglu D, Demirci A (2019) Optimization of dilute sulfuric acid, aqueous ammonia, and steam explosion as the pretreatments steps for distillers’ dried grains with solubles as a potential fermentation feedstock. Bioresour Technol 282:475–481
Bailey MJ, Buchert J, Viikari L (1993) Effect of pH on production of xylanase by Trichoderma reesei on xylan-and cellulose-based media. Appl Microbiol Biotechnol 40:224–229
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Esterbauer H, Steiner W, Labudova I et al (1991) Production of Trichoderma cellulase in laboratory and pilot scale. Bioresour Technol 36:51–65
Cekmecelioglu D, Demirci A (2017) Feasibility of distillers dried grain with solubles (DDGS) for production of cellulase and xylanase enzymes cocktail. NABEC Papers, pp 17–013
Baldwin EL (2017) Enhancing cellulase production of Aureobasidium pullulans for use in converting dried distillers ’ grains with solubles into a higher protein feed. South Dakota State University
Seo J, Park TS, Kim JN et al (2014) Production of endoglucanase, beta-glucosidase and xylanase by Bacillus licheniformis grown on minimal nutrient medium containing agriculture residues. Asian-Australasian J Anim Sci 27:946–950
Santos DA, Oliveira MM, Curvelo AAS et al (2017) Hydrolysis of cellulose from sugarcane bagasse by cellulases from marine-derived fungi strains. Int Biodeterior Biodegradation 121:66–78. https://doi.org/10.1016/j.ibiod.2017.03.014
Rastogi G, Bhalla A, Adhikari A, Bischoff KM, Hughes SR, Christopher LP, Sani RK (2010) Characterization of thermostable cellulases produced by Bacillus and Geobacillus strains. Bioresour Technol 101:8798–8806
Yang S, Lio J, Wang T (2012) Evaluation of enzyme activity and fiber content of soybean cotyledon fiber and distiller’s dried grains with solubles by solid state fermentation. Appl Biochem Biotechnol 167:109–121
Kheng PP, Omar IC (2005) Xylanase production by a local fungal isolate, Aspergillus niger USM AI 1 via solid state fermentation using palm kernel cake (PKC) as substrate. Songklanakarin J Sci Technol 27:325–336
Kang, SW Park, YS Lee, JS Hong, SI Kim S (2004) Production of cellulases and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass. Bioresour Technol 91:153–156. https://doi.org/10.1016/S0960-8524(03)00172-X
Okafor UA, Okochi VI, Onyegeme-Okerenta BM, Nwodo-Chinedu S (2007) Xylanase production by Aspergillus niger ANL 301 using agro-wastes. Afr J Biotechnol 6
Park Y, Kang S, Lee J, Hong SI, Kim SW (2002) Xylanase production in solid state fermentation by Aspergillus niger mutant using statistical experimental designs. Appl Microbiol Biotechnol 58:761–766
Tenkanen M, Puls J, Poutanen K (1992) Two major xylanases of Trichoderma reesei. Enzym Microb Technol 14:566–574
Saxena S, Bahadur J, Varma A (1991) Production and localisation of carboxymethylcellulase, xylanase and β-glucosidase from Cellulomonas and Micrococcus spp. Appl Microbiol Biotechnol 34:668–670
Peterson R, Nevalainen H (2012) Trichoderma reesei RUT-C30–thirty years of strain improvement. Microbiology 158:58–68
Grassick A, Murray PG, Thompson R, Collins CM, Byrnes L, Birrane G, Higgins TM, Tuohy MG (2004) Three-dimensional structure of a thermostable native cellobiohydrolase, CBH IB, and molecular characterization of the cel7 gene from the filamentous fungus, Talaromyces emersonii. Eur J Biochem 271:4495–4506
Choi J, Lee SY (1999) Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl Microbiol Biotechnol 51:13–21
Acknowledgments
The authors also gratefully acknowledge Pennsylvania Grain Processing, LLC® (Clearfield, PA, USA) for providing DDGS.
Funding
This work was supported in part by the FULBRIGHT Student Program by providing a scholarship to Attia Iram, USDA Northeast Sun Grant Initiative (NE-SGI) Competitive Grants Program, and the USDA National Institute of Food and Agriculture Federal Appropriations under Project PEN04671 and Accession number 1017582.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iram, A., Cekmecelioglu, D. & Demirci, A. Screening of bacterial and fungal strains for cellulase and xylanase production using distillers’ dried grains with solubles (DDGS) as the main feedstock. Biomass Conv. Bioref. 11, 1955–1964 (2021). https://doi.org/10.1007/s13399-019-00588-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00588-x