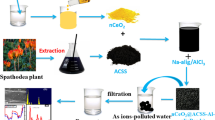
Abstract
H2SO4-activated carbon generated from the leaves of Magnoliaceae plant-(SACM) is doped in zirconium alginate beads-(SACM@Zr). The active carbon and beads are identified to have affinity for toxic chromate ions and hence studied as adsorbents for chromium remediation of water. The sorbents are characterized using conventional methods including FTIR, FESEM and EDX techniques. The sorption nature is investigated and optimized with respect to initial chromium concentration, adsorbent dosage, contact time, pH and temperature. The adsorption capacities are 28.82 mg/g for active carbon and 37.74 mg/g for beads. Thermodynamic parameters are analyzed. Negative values of ΔG∘ and positive values of ΔH∘ and ΔS° indicate that the chromium adsorption ‘onto’ the adsorbents is spontaneous, endothermic and more disorder prevails at solution/solid interface. Langmuir adsorption isotherms and pseudo second-order kinetics are best models for explaining adsorption process. The adsorbents are successfully applied to treat chromium polluted effluents from Ethiopia Tannery Companies and polluted waters of Leyole and Worka rivers around Kombolcha, located in the north-central part of Ethiopia.

Graphical abstract












Similar content being viewed by others
References
Khamis M, Jumean F, Abdo N (2009) Speciation and removal of chromium from aqueous solution by white, yellow and red UAE sand. J Hazard Mater 169:948–952
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: A review. J Environ Manag 92:407–418
Babu AN, Reddy DS, Kumar GS, Ravindhranath K, Mohan GK (2018) Removal of lead and fluoride from contaminated water using exhausted coffee grounds based bio-sorbent. J Environ Manag 218:602–612
Krebsz M, Pasinszki T, Tung TT, Nine MJ, Losic D (2021) Multiple applications of bio-graphene foam for efficient chromate ion removal and oil-water separation. Chemosphere 263:127790. https://doi.org/10.1016/j.chemosphere.2020.127790
Gholami F, Mahvi AH, Omrani GA, Nazmara S (2006) Removal of chromium (VI) from aqueous solution by Ulmus leaves. Iran J Environ Healt 3:97–102
Babu AN, Mohan GK, Ravindhranath K (2016) Removal of Chromium (VI) from polluted waters using adsorbents derived from Chenopodium album and Eclipta prostrate plant materials. Int J Chem Tech Research 9(03):506–516
Goyal N, Jain SC, Banerjee UC (2003) Comparative studies on the microbial adsorption of heavy metals. Adv Environ Res 7:311–319
US, (2009) Department of Health and Human Services, Toxicological profile for chromium, Public health services agency for toxic substances and diseases registry, Washington, DC.
Praveen P, Loh KC (2016) Thermodynamic analysis of Cr(VI) extraction using TOPO impregnated membranes. J Hazard Mater 314:204–210
Ravulapalli S, Kunta R (2018) Enhanced removal of chromium (VI) from wastewater using active carbon derived from Lantana camara plant as adsorbent. Water Sci Technol 78(6):1377–1389
Alloway BJ (1995) Heavy Metals in Soils, Blackie Academic and Professional, London, edn. 2, p. 368.
Kimbrough DE, Cohen Y, Winer AM, Creelman L, MabuniCA (1999) Critical assessment of chromium in the environment critical reviews in environmental science and technology. Crit Rev Environ Sci Technol 29(1):1–46
Singh IB, Singh DR (2002) Cr (VI) removal in acidic aqueous solution using iron-bearing industrial solid wastes and their stabilisation with cement. Environ Technol 23(1):85–95
Gil RA, Cerutti S, G’asquez JA, Olsina RA, Martinez LD (2006) Pre-concentration and speciation of chromium in drinking water samples by coupling of online sorption on activated carbon to ETAAS determination. Talanta 1065(68):1065–1070
World Health Organization (WHO) (2004) Guidelines for drinking-water quality (third ed.). Recommendations. WHO, Geneva 1:334–335
Katz F, Slem H (1994) The biological and environmental chemistry of chromium (pp. 51–58). New York: VCH.
Kotas J, Stasicka Z (2000) Chromium occurrence in the environment and methods of its speciation. Environ Pollut 107(3):263–283
Mohan GK, Babu AN, Kalpana K, Ravindhranath K (2019) Removal of chromium (VI) from water using adsorbent derived from spent coffee grounds. Int J Environ Sci Technol 16(1):101–112
Cushnie GC Jr (1985) Electroplating wastewater pollution control technology. Noyes Publications Park Ridge:30–39
Kobya M (2004) Removal of Cr(VI) from aqueous solutions by adsorption onto hazelnut shell activated carbon: kinetic and equilibrium studies. Bioresour Technol 91:317–321
Deveci H, Kar Y (2013) Adsorption of hexavalent chromium from aqueous solutions by bio-chars obtained during biomass pyrolysis. J IndEng Chem 19:190–196
Cetin G, Kocaoba S, Akcin G (2013) Removal and recovery of chromium from solutions simulating tannery wastewater by strong acid cation exchanger. J Chem 7
Ravulapalli S, Kunta R (2017) Defluoridation studies using active carbon derived from the barks of Ficusracemosa plant. J Fluor Chem 193:58–66
Ponou J, Kim J, Wang LP, Dodbiba G, Fujita T (2011) Sorption of Cr (VI) anions in aqueous solution using carbonized or dried pineapple leaves. Chem Eng J 172(2-3):906–913
Cimino G, Passerini A, Toscano G (2000) Removal of toxic cations and Cr(VI) from aqueous solution by hazelnut shell. Water Res 34(11):2955–2962
Dubey SP, Gopal K (2007) Adsorption of chromium (VI) on low cost adsorbents derived from agricultural waste material: a comparative study. J Haza Materials 145(3):465–470
Babel S, Kurniawan TA (2004) Cr (VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 54(7):951–967
Al-Othman ZA, Ali R, Naushad M (2012) Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: adsorption kinetics, equilibrium and thermodynamic studies. Chem Eng J 184:238–247
Garg UK, Kaur MP, GargVK SD (2007) Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. J Hazard Mater 140(1-2):60–68
Ranganathan K (2000) Chromium removal by activated carbons prepared from Casurinaequisetifolia leaves. Bioresour Technol 73(2):99–103
Rai MK, Shahi G, Meena V, Meena R, Chakraborty S, Singh RS, Rai BN (2016) Removal of hexavalent chromium Cr (VI) using activated carbon prepared from mango kernel activated with H3PO4. Resource-Efficient Technol 2:S63–S70
Aparna B, Gupta SK (2016) Removal of Cr(VI) from waste water using root of Eucalyptus tree. Int J Eng Technol Sci Res 3(11):37–42
Velumani K, Kumar PE, Sivakumar V (2016) Adsorption of Chromium (VI) using a nonconventional adsorbent. Rasayan J Chem 9(2):149–159
Metcalf and Eddy (2003) Wastewater Engineering: Treatment of Reuse, McGraw Hill Co., New York, edn 4.
Vogel AI (1961) A Textbook of Quantitative Inorganic Analysis, Including Elementary Instrumental Analysis, 3rd edn. John Wiley and Sons, Inc., New York, NY, USA.
Dehghani MH, Tajik S, Panahi A, Khezri M, Zarei A, Heidarinejad Z, Yousefi M (2018) Adsorptive removal of noxious cadmium from aqueous solutions using poly urea-formaldehyde: A novel polymer adsorbent. MethodsX 5:1148–1155
Babu AN, Krishna Mohan GV, Kalpana K, Ravindhranath K (2018a) Removal of fluoride from water using H2O2-treated fine red mud doped in Zn-alginate beads as adsorbent. J Environ Chem Eng 6(1):906–916. https://doi.org/10.1016/j.jece.2018.01.014
Khalil U, Shakoor MB, Ali S, Ahmad SR, Rizwan M, Alsahli AA, Alyemeni MN (2021) Selective Removal of hexavalent chromium from wastewater by Rice Husk: Kinetic, isotherm and spectroscopic Iivestigation. Water 13:263. https://doi.org/10.3390/w13030263
Amir M, Anwar ul HAS, Salma B (2020) Effective Adsorption of hexavalent chromium and divalent nickel ions from water through Polyaniline, Iron Oxide, and their composites. Appl Sci 10:2882. https://doi.org/10.3390/app10082882
Samson OO, Adedibu CT (2016) Removal of hexavalent chromium from aqueous solutions by adsorption on modified groundnut hull. Beni-suef Univ J Basic Appl Sci 5:377–338
Gorzin F, Abadi MMBR (2018) Adsorption of Cr(VI) from aqueous solution by adsorbent prepared from paper mill sludge: Kinetics and thermodynamics studies. Adsorpt Sci Technol 36(1–2):149–169
Windholz M (1976) The Merck Index, vol. 802, Merck & Company, Whitehouse Station, NJ, USA, 9th edition.
Sanchez-Polo M, Rivera-Utrilla J (2002) Adsorbent− adsorbate interactions in the adsorption of Cd (II) and Hg (II) on ozonized activated carbons. Environ Sci Technol 36(17):3850–3854
Argun ME, Dursun S, Ozdemir C, Karatas M (2007) Heavy metal adsorption by modified oak sawdust: thermodynamics and kinetics. J Hazard Mater 141(1):77–85
Simha P, Yadav A, Pinjari D, PanditAB (2016) On the behaviour, mechanistic modelling and interaction of biochar and crop fertilizers in aqueous solutions. Resource-Efficient Technol 2(3):133–142
Jung C, Heo J, Han J, Her N, Lee SJ, Oh J, Yoon Y (2013) Hexavalent chromium removal by various adsorbents: powdered activated carbon, chitosan, and single/multi-walled carbon nanotubes. Sep Purif Technol 106:63–71
Yu B, ZhangY SA, Shukla SS, Dorris KL (2000) The removal of heavy metal from aqueous solutions by sawdust adsorption—removal of copper. J Hazard Mater 80(1-3):33–42
Krishna Mohan GV, Naga Babu A, Kalpana K, Ravindhranath K (2017) Removal of chromium (VI) from water using adsorbent derived from spent coffee grounds. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-017-1593-7
Inglezakis VJ, Loizidou MM, Grigoropoulou HP (2004) Ion exchange studies on natural and modified zeolites and the concept of exchange site accessibility. J Colloid Interface Sci 275(2):570–576
Mansooreh D, Simin N, Mojtaba K (2014) Removal of 2,4-Dichlorophenolyxacetic acid (2,4-D) herbicide in the aqueous phase using modified granular activated carbon. J Environ Health Sci Eng 12:28
Sujitha R, Ravindhranath K (2017) Extraction of phosphate from polluted waters using calcium alginate beads doped with active carbon derived from A. aspera plant as adsorbent. J Anal Method Chem 2017:1–13. https://doi.org/10.1155/2017/3610878
Fan C, Zhang Y (2018) Adsorption isotherms, kinetics and thermodynamics of nitrate and phosphate in binary systems on a novel adsorbent derived from corn stalks. J Geochem Explor 188:95–100
Biftu WK, Ravulapalli S, Kunta R (2020) Effective de-fluoridation of water using Leucaenaluecocephala active carbon as adsorbent. Int J Environ Res 14:415–426. https://doi.org/10.1007/s41742-020-00268-z
Ngah WW, Hanafiah MAKM (2008) Adsorption of copper on rubber (Heveabrasiliensis) leaf powder: Kinetic, equilibrium and thermodynamic studies. Biochem Eng J 39(3):521–530
RavulapalliS RK (2019) Novel adsorbents possessing cumulative sorption nature evoked from Al2O3 nanoflakes, C. urens seeds active carbon and calcium alginate beads for defluoridation studies. J Taiwan Ins Chemical Eng 101:50–63
Sun C, Li C, Wang C, Qu R, Niu Y, Geng H (2012) Comparison studies of adsorption properties for Hg(II) and Au(III) on polystyrene-supported bis-8-oxyquinoline-terminated open-chain crown ether. Chem Eng J 200(202):291–299
Altundogan HS, Altundogan S, Tümen F, Bildik M (2000) Arsenic removal from aqueous solutions by adsorption on red mud. Waste Manag 20(8):761–767
Biftu WK, Ravindhranath K (2020a) Synthesis of nanoZrO2 via simple new green routes and its effective application as adsorbent in phosphate remediation of water with or without immobilization in Al-alginate beads. Water Sci Technol 81:2617–2633. https://doi.org/10.2166/wst.2020.318
Atkins P (1999) Physical chemistry, 6th edn. Oxford University press, London, pp 857–864
Onyango MS, Kojima Y, Aoyi O, Bernardo EC, Matsuda H (2004) Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent cation-exchanged zeolite F-9. J Colloid Interface Sci 279:341–350
Baral SS, Das SN, Rath P (2006) Cr(VI) removal from aqueous solution by adsorption on treated sawdust. Biochem Eng J 31(3):216–222
Anandkumar J, Mandal B (2009) Removal of Cr (VI) from aqueous solution using Bael fruit (Aeglemarmeloscorrea) shell as an adsorbent. J Hazardous Mat 168(2-3):633–640
Dakiky M, Khamis M, Manassra AMerEb M (2002) Selective adsorption of chromium (VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv Environ Res 6(4):533–540
Aditya GVV, Pujitha BP, Babu NC, Venkateswarlu P (2012) Biosorption of chromium onto Erythrina Variegata Orientalis leaf powder. Korean J Chem Eng 29(1):64–71
Alaerts GJ, Jitjaturant V, Kelderman P (1989) Use of coconut shell-based activated carbon for chromium (VI) removal. Water Sci Technol 21(12):1701–1704
Selvi K, Pattabhi S, Kadirvelu K (2001) Removal of Cr (VI) from aqueous solution by adsorption onto activated carbon. Bioresour Technol 80(1):87–89
Feng B, Shen W, Shi L, Qu S (2018) Adsorption of hexavalent chromium by polyacrylonitrile-based porous carbon from aqueous solution. R Soc Open Sci 5:171662
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Biftu, W.K., Suneetha, M. & Ravindhranath, K. Zirconium-alginate beads doped with H2SO4-activated carbon derived from leaves of Magnoliaceae plant as an effective adsorbent for the removal of chromate. Biomass Conv. Bioref. 13, 5991–6006 (2023). https://doi.org/10.1007/s13399-021-01568-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01568-w