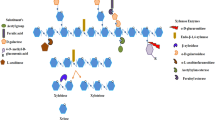
Abstract
Strengthening green alternative energy sources has been enforced due to hydrocarbon fuels’ consequential harmful impacts. Climate change, economic enhancement, and energy security are motivating reasons behind the idea of expansion of biofuel production worldwide. Raw materials like lignocellulose have wide range of complex sugars in the form of cellulose (60–70%) and xylan (30–40%) which further by its hydrolysis and fermentation can be used to produce biofuels. Presently, naturally occurring xylanase enzyme is widely used as a natural resource for biofuel diligence to break down the second abundant β-1,4 xylan sugar into xylooligosaccharide, xylobiose, and xylose subunits. For larger scale, numerous approaches are required to improve or modify its thermostability, specificity, and enzyme activity along with its broad range of substrate to shorten the cost in biofuel production. Therefore, the present review provides a synopsis of present scenario of xylanase as a major participant in biofuel production, along with its various applications and factors affecting xylanase production. This present review provides evidence that narrates its presence in the production of biofuels such as bioethanol and biobutanol. Lastly, the present works discuss the major aspects of biofuel conversion efficiency.







Similar content being viewed by others
Data availability
The authors selected not to share data.
References
Luque R, Herrero-Davila L, Campelo JM, Clark JH, Hidalgo JM, Luna D, Marinas JM, Romero AA (2008) Biofuels: a technological perspective. Energy Environ Sci 1(5):542–564. https://doi.org/10.1039/b807094f
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102(1):100–112. https://doi.org/10.1002/bit.22033
Jeevahan J, Sriramanjaneyulu G, Durairaj RB, Mageshwaran G (2018) Experimental investigation of the suitability of 1-butanol blended with biodiesel as an alternative biofuel in diesel engines. Biocatal Agric Biotechnol 15:72–77. https://doi.org/10.1016/j.bcab.2018.05.013
Paramjeet S, Manasa P, Korrapati N (2018) Biofuels: production of fungal-mediated ligninolytic enzymes and the modes of bioprocesses utilizing agro-based residues. Biocatal Agric Biotechnol 14:57–71. https://doi.org/10.1016/j.bcab.2018.02.007
Xing D, Wang H, Pan A, Wang J, Xue D (2012) Assimilation of corn fiber hydrolysates and lipid accumulation by Mortierellaisabellina. Biomass Bioenerg 39:494–501. https://doi.org/10.1016/j.biombioe.2012.01.024
Yeoman CJ, Han Y, Dodd D, Schroeder CM, Mackie RI, Cann IKO (2010) Thermostable enzymes as biocatalysts in the biofuel industry. Adv Appl Microbiol 70(10):1–55. https://doi.org/10.1016/S0065-2164(10)70001-0
Ji X-J, Huang H, Nie Z-K, Qu L, Xu Q, Tsao GT (2011) Fuels and Chemicals from hemicellulose sugars. Adv Biochem Eng Biotechnol 128:199–224. https://doi.org/10.1007/10_2011_124
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48(8):3713–3729. https://doi.org/10.1021/ie801542g
Zhao X, Dong L, Chen L, Liu D (2013) Batch and multi-step fed-batch enzymatic saccharification of Formiline-pretreated sugarcane bagasse at high solid loadings for high sugar and ethanol titers. Biores Technol 135:350–356. https://doi.org/10.1016/j.biortech.2012.09.074
Zeng Y, Himmel ME, Ding SY (2017) Visualizing chemical functionality in plant cell walls Mike Himmel. Biotechnol Biofuels 10(1):1–16. https://doi.org/10.1186/s13068-017-0953-3
Yang Y, Zhu N, Yang J, Lin Y, Liu J, Wang R, Wang F, Yuan H (2017) A novel bifunctional acetyl xylan esterase/arabinofuranosidase from Penicillium chrysogenum P33 enhances enzymatic hydrolysis of lignocellulose. Microb Cell Fact 16(1):1–12. https://doi.org/10.1186/s12934-017-0777-7
Butt MS, Tahir-Nadeem M, Ahmad Z, Sultan MT (2008) Xylanases and their applications in baking industry. Food Technol Biotechnol 46(1):22–31
Uday USP, Choudhury P, Bandyopadhyay TK, Bhunia B (2016) Classification, mode of action and production strategy of xylanase and its application for biofuel production from water hyacinth. Int J Biol Macromol 82:1041–1054. https://doi.org/10.1016/j.ijbiomac.2015.10.086
Walia A, Guleria S, Mehta P, Chauhan A, & Parkash J (2017) Microbial xylanases and their industrial application in pulp and paper biobleaching: a review. 3 Biotech 7(1). https://doi.org/10.1007/s13205-016-0584-6
Busic A, Kundas S, Morzak G, Belskaya H, Mardetko N, Šantek MI, Komes D, Novak S, Šantek B (2018) Recent trends in biodiesel and biogas production. Food Technol Biotechnol 56(2):152–173
Tollefson J (2008) Energy: not your father’s biofuels. Nature 451:880–830. https://doi.org/10.1038/451880a
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Singh J, Gu S (2010) Commercialization potential of microalgae for biofuels production. Renew Sustain Energy Rev 14(9):2596–2610. https://doi.org/10.1016/j.rser.2010.06.014
Robak K, Balcerek M (2018) Review of second generation bioethanol production from residual biomass. Food Technol Biotechnol 56(2):174–187. https://doi.org/10.17113/ftb.56.02.18.5428
Basso A, Serban S (2019) Industrial applications of immobilized enzymes—a review. Molecular Catalysis 479:110607. https://doi.org/10.1016/j.mcat.2019.110607
Bibra M, Kunreddy V, Sani R (2018) Thermostable xylanase production by Geobacillus sp. strain DUSELR13, and its application in ethanol production with lignocellulosic biomass. Microorganisms 6(3):93
Botto E, Gioia L, Menéndez M del P, & Rodríguez P (2019) Pseudozyma sp. isolation from Eucalyptus leaves and its hydrolytic activity over xylan. Biocatalysis Agric Biotechnol 21(June):101282. https://doi.org/10.1016/j.bcab.2019.101282
Sakthiselvan P, Madhumathi R, Partha N (2015) Eco friendly bio-butanol from sunflower oil sludge with production of xylanase. Eng Agric Environ Food 8(4):212–221. https://doi.org/10.1016/j.eaef.2015.01.008
Xin F, He J (2013) Characterization of a thermostable xylanase from a newly isolated Kluyvera species and its application for biobutanol production. Biores Technol 135:309–315. https://doi.org/10.1016/j.biortech.2012.10.002
Dodd D, Cann IKO (2009) Enzymatic deconstruction of xylan for biofuel production. GCB Bioenergy 1(1):2–17. https://doi.org/10.1111/j.1757-1707.2009.01004.x
Rennie EA, Scheller HV (2014) Xylan biosynthesis. Curr Opin Biotechnol 26:100–107. https://doi.org/10.1016/j.copbio.2013.11.013
Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Biol 61(1):263–289. https://doi.org/10.1146/annurev-arplant-042809-112315
Smith PJ, Wang HT, York WS, Peña MJ, Urbanowicz BR (2017) Designer biomass for next-generation biorefineries: leveraging recent insights into xylan structure and biosynthesis. Biotechnol Biofuels 10(1):1–14. https://doi.org/10.1186/s13068-017-0973-z
Hsieh Y, Harris P (2019) Xylans of red and green algae: what is known about their structures and how they are synthesised? Polymers 11(2):354. https://doi.org/10.3390/polym11020354
Harris PV, Xu F, Kreel NE, Kang C, Fukuyama S (2014) New enzyme insights drive advances in commercial ethanol production. Curr Opin Chem Biol 19(1):162–170. https://doi.org/10.1016/j.cbpa.2014.02.015
Juturu V, Wu JC (2012) Microbial xylanases: engineering, production and industrial applications. Biotechnol Adv 30(6):1219–1227. https://doi.org/10.1016/j.biotechadv.2011.11.006
Martínez Cuesta S, Rahman SA, Furnham N, Thornton JM (2015) The classification and evolution of enzyme function. Biophys J 109(6):1082–1086. https://doi.org/10.1016/j.bpj.2015.04.020
Selvarajan E, Veena R (2017) Recent advances and future perspectives of thermostable xylanase. Biomed Pharmacol J 10(1):261–279. https://doi.org/10.13005/bpj/1106
Denisenko YA, Gusakov AV, Rozhkova AM, Zorov IN, Bashirova AV, Matys VY, Nemashkalov VA, Sinitsyn AP (2019) Protein engineering of GH10 family xylanases for gaining a resistance to cereal proteinaceous inhibitors. Biocatal Agric Biotechnol 17:690–695. https://doi.org/10.1016/j.bcab.2019.01.042
Lee SH, Lim V, Lee CK (2018) Newly isolate highly potential xylanase producer strain from various environmental sources. Biocatal Agric Biotechnol 16:669–676. https://doi.org/10.1016/j.bcab.2018.09.024
Kuancha C, Apiraksakorn J (2012) Cultural condition improvement for xylanase production by Bacillus subtilis GN156. KKU Res J 17(6):933–938
Kumar V, Dangi AK, Shukla P (2018) Engineering thermostable microbial xylanases toward its industrial applications. Mol Biotechnol 60(3):226–235. https://doi.org/10.1007/s12033-018-0059-6
Vimalashanmugam K, Viruthagiri T (2013) Production of xylanase enzyme by Aspergillus terreus under SSF using response surface methodology. Int J ChemTech Res 5(5):2365–2374
Gupta G, Sahai V, Gupta RK (2013) Optimization of xylanase production from Melanocarpusalbomyces using wheat straw extract and its scale up in stirred tank bioreactor. Indian J Chem Technol 20(4):282–289
Liu L, Cheng J, Chen H, Li X, Wang S, Song A, Wand M, Wang B, Shen J (2011) Directed evolution of a mesophilic fungal xylanase by fusion of a thermophilic bacterial carbohydrate-binding module. Process Biochem 46(1):395–398. https://doi.org/10.1016/j.procbio.2010.07.026
Polizeli MLTM, Rizzatti ACS, Monti R, Terenzi HF, Jorge JA, Amorim DS (2005) Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol 67(5):577–591. https://doi.org/10.1007/s00253-005-1904-7
Bhushan B (2012) Isolation, screening and optimized production of extracellular xylanase under submerged condition from Aspergillus flavus Mtcc 9390. Enzyme Eng 01(02):1–6. https://doi.org/10.4172/eeg.1000103
Han N, Miao H, Ding J, Li J, Mu Y, Zhou J, Huang Z (2017) Improving the thermostability of a fungal GH11 xylanase via site-directed mutagenesis guided by sequence and structural analysis. Biotechnol Biofuels 10(1):1–12. https://doi.org/10.1186/s13068-017-0824-y
Kanagasabai V, Thangavelu V (2013) Response surface methodological optimization of the medium components for production of xylanase under SSF by Aspergillus fumigatus. J Adv Sci Res 4(2):13–20
Mathur N, Goswami GK (2015) In silico study of Bacillus brevis xylanase - structure prediction and comparative analysis with other bacterial and fungal xylanase. Int J Biomed Data Mining 04(01):1–5. https://doi.org/10.4172/2090-4924.1000112
Zimbardi ALRL, Sehn C, Meleiro LP, Souza FHM, Masui DC, Nozawa MSF, Guimarães LHS, Jorge JA, Furriel RPM (2013) Optimization of β-glucosidase, β-xylosidase and xylanase production by Colletotrichum graminicola under solid-state fermentation and application in raw sugarcane trash saccharification. Int J Mol Sci 14(2):2875–2902. https://doi.org/10.3390/ijms14022875
Harris AD, Ramalingam C (2010) Xylanases and its application in food industry: a review. J Exp Sci 1(7):1–11
Goswani GK, Rawat S (2015) Microbial xylanase and their applications. Int J Curr Res Acad Rev 3(6):436–450
Ahmed SA, Saleh SAA, Mostafa FA, Abd El Aty AA, Ammar HAM (2016) Characterization and valuable applications of xylanase from endophytic fungus Aspergillus terreus KP900973 isolated from Corchorus olitorius. Biocatal Agric Biotechnol 7:134–144. https://doi.org/10.1016/j.bcab.2016.05.015
Lin XQ, Han SY, Zhang N, Hu H, Zheng SP, Ye YR, Lin Y (2013) Bleach boosting effect of xylanase A from Bacillus halodurans C-125 in ECF bleaching of wheat straw pulp. Enzyme Microb Technol 52(2):91–98. https://doi.org/10.1016/j.enzmictec.2012.10.011
Sharma N (2017) Microbial xylanases and their industrial applications as well as future perspectives: a review. Global J Biol Agric Health Sci 6(3):5–12. https://doi.org/10.24105/gjbahs.6.3.1702
Abd El Aty AA, Saleh SAA, Eid BM, Ibrahim NA, Mostafa FA (2018) Thermodynamics characterization and potential textile applications of Trichoderma longibrachiatum KT693225 xylanase. Biocatal Agric Biotechnol 14:129–137. https://doi.org/10.1016/j.bcab.2018.02.011
Nagar S, Jain RK, Thakur VV, Gupta VK (2013) Biobleaching application of cellulase poor and alkali stable xylanase from Bacillus pumilus SV-85S. 3 Biotech 3(4):277–285. https://doi.org/10.1007/s13205-012-0096-y
Sharma D, Chaudhary R, Kaur J, Arya SK (2020) Greener approach for pulp and paper industry by xylanase and laccase. Biocatal Agric Biotechnol 25:101604. https://doi.org/10.1016/j.bcab.2020.101604
Bajpai P, Bajpai PK, & Kondo R (1999) Pulp bleaching with xylanases. Biotechnol Environ Protect Pulp Paper Ind 49–64. https://doi.org/10.1007/978-3-642-60136-1_4
Bedford MR (2018) The evolution and application of enzymes in the animal feed industry: the role of data interpretation. Br Poult Sci 59(5):486–493. https://doi.org/10.1080/00071668.2018.1484074
Barabote RD, Parales JV, Guo YY, Labavitch JM, Parales RE, Berry AM (2010) Xyn10A, a thermostable endoxylanase from acidothermuscellulolyticus 11B. Appl Environ Microbiol 76(21):7363–7366. https://doi.org/10.1128/AEM.01326-10
Sanghi A, Garg N, Sharma J, Kuhar K, Kuhad RC, Gupta VK (2008) Optimization of xylanase production using inexpensive agro-residues by alkalophilic Bacillus subtilis ASH in solid-state fermentation. World J Microbiol Biotechnol 24(5):633–640. https://doi.org/10.1007/s11274-007-9521-5
AkhavanSepahy A, Ghazi S, AkhavanSepahy M (2011) Cost-effective production and optimization of alkaline xylanase by indigenous Bacillus mojavensis AG137 fermented on agricultural waste. Enzyme Res 2011(1):1–9. https://doi.org/10.4061/2011/593624
Raj A, Kumar S, Singh SK (2013) A highly thermostable xylanase from Stenotrophomonas maltophilia: purification and partial characterization. Enzyme Res 2013:1–8. https://doi.org/10.1155/2013/429305
Murugan S, Arnold D, Pongiya UD, & Narayanan PM (2011) Production of xylanase from arthrobacter sp. MTCC 6915 using saw dust as substrate under solid state fermentation. Enzyme Research 2011(1). https://doi.org/10.4061/2011/696942
Boonchuay P, Takenaka S, Kuntiya A, Techapun C, Leksawasdi N, Seesuriyachan P, Chaiyaso T (2016) Purification, characterization, and molecular cloning of the xylanase from Streptomyces thermovulgaris TISTR1948 and its application to xylooligosaccharide production. J Mol Catal B Enzym 129:61–68. https://doi.org/10.1016/j.molcatb.2016.03.014
Kapoor M, Nair LM, Kuhad RC (2008) Cost-effective xylanase production from free and immobilized Bacillus pumilus strain MK001 and its application in saccharification of Prosopis juliflora. Biochem Eng J 38(1):88–97. https://doi.org/10.1016/j.bej.2007.06.009
Kumar V, Satyanarayana T (2013) Biochemical and thermodynamic characteristics of thermo-alkali-stable xylanase from a novel polyextremophilic Bacillus halodurans TSEV1. Extremophiles 17(5):797–808. https://doi.org/10.1007/s00792-013-0565-1
Guha S, Bhutty S, Khurana SP, Kohli UK (2013) Optimization of cultural conditions for production of thermo-alkali tolerant xylanase from Bacillus sp. Int J Res Pure Appl Microbiol 3:116–120
Porsuk I, Özakin S, Bali B, Ince Yilmaz E (2013) A cellulase-free, thermoactive, and alkali xylanase production by terrestrial Streptomyces sp. CA24. Turkish J Biol 37(3):370–375. https://doi.org/10.3906/biy-1208-40
Abdel-Sater M, El-Said A (2001) Xylan-decomposing fungi and xylanolytic activity in agricultural and industrial wastes. Int Biodeterior Biodegradation 47(1):15–21. https://doi.org/10.1016/s0964-8305(00)00113-x
Hoq M, Hempel C, Deckwer WD (1994) Cellulase-free xylanase by Thermomyces lanuginosus RT9: Effect of agitation, aeration, and medium components on production. J Biotechnol 37(1):49–58. https://doi.org/10.1016/0168-1656(94)90202-x
Sharma M, & Chadha BS (2011) Production of hemicellulolytic enzymes for hydrolysis of lignocellulosic biomass. Biofuels 203-228. https://doi.org/10.1016/B978-0-12-385099-7.00009-7
Roy S, Dutta T, Sarkar TS, Ghosh S (2013) Novel xylanases from Simplicilliumobclavatum MTCC 9604: comparative analysis of production, purification and characterization of enzyme from submerged and solid state fermentation. Springerplus 2(1):1–10. https://doi.org/10.1186/2193-1801-2-382
Subramaniyan S (2012) Isolation, purification and characterisation of low molecular weight xylanase from Bacillus pumilus SSP-34. Appl Biochem Biotechnol 166(7):1831–1842. https://doi.org/10.1007/s12010-012-9600-4
Shang SM, Qian L, Zhang X, Li KZ, Chagan I (2013) Themoanaerobacteriumcalidifontis sp. nov., a novel anaerobic, thermophilic, ethanol-producing bacterium from hot springs in China. Arch Microbiol 195(6):439–445. https://doi.org/10.1007/s00203-013-0895-5
Dheeran P, Nandhagopal N, Kumar S, Jaiswal YK, Adhikari DK (2012) A novel thermostable xylanase of Paenibacillusmacerans IIPSP3 isolated from the termite gut. J Ind Microbiol Biotechnol 39(6):851–860. https://doi.org/10.1007/s10295-012-1093-1
Goswami GK, Krishnamohan M, Nain V, Aggarwal C, Ramesh B (2014) Cloning and heterologous expression of cellulose free thermostable xylanase from Bacillus brevis. Springerplus 3(1):1–6. https://doi.org/10.1186/2193-1801-3-20
Irfan M, Nadeem M, Syed Q, Baig S (2012) Effect of medium composition on xylanase production by Bacillus subtilis using various agricultural wastes. J Agric Environ Sci 12(5):561–565
Nagar S, Mittal A, Kumar D, Gupta VK (2012) Production of alkali tolerant cellulase free xylanase in high levels by Bacillus pumilus SV-205. Int J Biol Macromol 50(2):414–420
Sunkar B, Kannoju B, & Bhukya B (2020) Optimized production of xylanase by Penicillium purpurogenum and ultrasound impact on enzyme kinetics for the production of monomeric sugars from pretreated corn cobs. Front Microbiol 11. https://doi.org/10.3389/fmicb.2020.00772
Azzouz Z, Bettache A, Boucherba N, Prieto A, Martinez MJ, Benallaoua S, de Eugenio LI (2021) Optimization of β-1,4-endoxylanase production by an Aspergillus niger strain growing on wheat straw and application in xylooligosaccharides production. Molecules 26(9):2527. https://doi.org/10.3390/molecules26092527
Evelyn Amraini SZ, Pratiwi ED, & Ismala UN (2020) Production of cellulase and xylanase from Eupenicillium javanicum by solid-state fermentation utilizing pineapple crown leaves waste as the substrate. J Phys Conf Series 1655:012113. https://doi.org/10.1088/1742-6596/1655/1/012113
Sikarwar VS, Zhao M, Fennell PS, Shah N, Anthony EJ (2017) Progress in biofuel production from gasification. Prog Energy Combust Sci 61:189–248. https://doi.org/10.1016/j.pecs.2017.04.001
Petrova P, Ivanova V (2010) Perspectives for the production of bioethanol from lignocellulosic materials. Biotechnol Biotechnol Equip 24:529–546. https://doi.org/10.1080/13102818.2010.10817894
Gabra FA, Abd-Alla MH, Danial AW, Abdel-Basset R, Abdel-Wahab AM (2019) Production of biofuel from sugarcane molasses by diazotrophic Bacillus and recycle of spent bacterial biomass as biofertilizer inoculants for oil crops. Biocatal Agric Biotechnol 19(January):101112. https://doi.org/10.1016/j.bcab.2019.101112
Basit A, Liu J, Miao T, Zheng F, Rahim K, Lou H, Jiang W (2018) Characterization of two endo-β-1, 4-xylanases from Myceliophthorathermophila and their saccharification efficiencies, synergistic with commercial cellulase. Front Microbiol 9:1–11. https://doi.org/10.3389/fmicb.2018.00233
Choudhary J (2014) Enhanced saccharification of steam-pretreated rice straw by commercial cellulases supplemented with xylanase. J Bioprocess Biotech 04(07). https://doi.org/10.4172/2155-9821.1000188
Ramanjaneyulu G, Sridevi A, Seshapani P, Ramyan A, Dileep Kumar K, Praveen Kumar Reddy G, Rajasekhar Reddy B (2017) Enhanced production of xylanase by Fusarium sp. BVKT R2 and evaluation of its biomass saccharification efficiency. 3 Biotech 7(5):1–17. https://doi.org/10.1007/s13205-017-0977-1
Ritter CET, Camassola M, Zampieri D, Silveira MM, Dillon AJP (2013) Cellulase and xylanase production by Penicillium echinulatumin submerged media containing cellulose amended with sorbitol. Enzyme Res 2013:1–9. https://doi.org/10.1155/2013/240219
Chiranjeevi T, Rani GB, Chandel AK, Sekhar PVS, Prakasham RS, Addepally U (2012) Optimization of holocellulolytic enzymes production by cladosporiumcladosporioides using taguchi-L’16 orthogonal array. J Biobased Mater Bioenergy 6(2):148–157. https://doi.org/10.1166/jbmb.2012.1201
Amore A, Parameswaran B, Kumar R, Birolo L, Vinciguerra R, Marcolongo L, Ionata E, La Cara F, Pandey A, Faraco V (2015) Application of a new xylanase activity from Bacillus amyloliquefaciens XR44A in brewer’s spent grain saccharification. J Chem Technol Biotechnol 90(3):573–581. https://doi.org/10.1002/jctb.4589
Ellis JT, Hengge NN, Sims RC, Miller CD (2012) Acetone, butanol, and ethanol production from wastewater algae. Biores Technol 111:491–495. https://doi.org/10.1016/j.biortech.2012.02.002
Menon V, Prakash G, Prabhune A, Rao M (2010) Biocatalytic approach for the utilization of hemicellulose for ethanol production from agricultural residue using thermostable xylanase and thermotolerant yeast. Biores Technol 101(14):5366–5373. https://doi.org/10.1016/j.biortech.2010.01.150
Wang Z, Cao X, Li N, Yang Z, Lei M, Zhao Y, Wang L, Li Z, Liu D, Niu H, Ying H (2020) Production of butanol directly from hemicellulose through secretory expression of a xylanase in Clostridium acetobutylicum. Energy Fuels 34(3):3376–3382. https://doi.org/10.1021/acs.energyfuels.9b04489
Qureshi N, Li XL, Hughes S, Saha BC, Cotta MA (2006) Butanol production from corn fiber xylan using Clostridium acetobutylicum. Biotechnol Prog 22(3):673–680. https://doi.org/10.1021/bp050360w
Rajagopalan G, He J, Yang KL (2014) Direct fermentation of xylan by clostridium strain BOH3 for the production of butanol and hydrogen using optimized culture medium. Biores Technol 154:38–43. https://doi.org/10.1016/j.biortech.2013.11.094
Xin F, Chen T, Jiang Y, Dong W, Zhang W, Zhang M, Wu H, Ma J, Jiang M (2017) Strategies for improved isopropanol-butanol production by a Clostridium strain from glucose and hemicellulose through consolidated bioprocessing. Biotechnol Biofuels 10(1):1–13. https://doi.org/10.1186/s13068-017-0805-1
Sharma M, Kumar A (2013) Xylanases: an overview. British Biotechnol J 3(1):1–28
Yasuda M, Nagai H, Takeo K, Ishii Y, Ohta K (2014) Bio-ethanol production through simultaneous saccharification and co-fermentation (SSCF) of a low-moisture anhydrous ammonia (LMAA)-pretreated napiegrass (Pennisetum purpureum Schumach). Springerplus 3(1):1–8. https://doi.org/10.1186/2193-1801-3-333
Horisawa S, Inoue A, & Yamanaka Y (2019) Direct ethanol production from lignocellulosic materials by mixed culture of wood rot fungi schizophyllum commune, Bjerkanderaadusta, and fomitopsis palustris. Fermentation 5(1). https://doi.org/10.3390/fermentation5010021
Shariq M, Sohail M (2019) Application of Candida tropicalis MK-160 for the production of xylanase and ethanol. J King Saud Univ - Sci 31(4):1189–1194. https://doi.org/10.1016/j.jksus.2018.04.009
Tabka MG, Herpoël-Gimbert I, Monod F, Asther M, Sigoillot JC (2006) Enzymatic saccharification of wheat straw for bioethanol production by a combined cellulase xylanase and feruloyl esterase treatment. Enzyme Microb Technol 39(4):897–902. https://doi.org/10.1016/j.enzmictec.2006.01.021
Zheng Y, Yu X, Zeng J, Chen S (2012) Feasibility of filamentous fungi for biofuel production using hydrolysate from dilute sulfuric acid pretreatment of wheat straw. Biotechnol Biofuels 5(3):1–10. https://doi.org/10.1186/1754-6834-5-50
Ranjan A, Moholkar VS (2011) Biobutanol: science, engineering, and economics. Int J Energy Res 36(3):277–323. https://doi.org/10.1002/er.1948
Birgen C, Dürre P, Preisig HA, Wentzel A (2019) Butanol production from lignocellulosic biomass: revisiting fermentation performance indicators with exploratory data analysis. Biotechnol Biofuels 12(1):1–15. https://doi.org/10.1186/s13068-019-1508-6
Wang Y, Blaschek HP (2011) Optimization of butanol production from tropical maize stalk juice by fermentation with Clostridium beijerinckii NCIMB 8052. Biores Technol 102(21):9985–9990. https://doi.org/10.1016/j.biortech.2011.08.038
Xin F, Yan W, Zhou J, Wu H, Dong W, Ma J, Zhang W, Jiang M (2018) Exploitation of novel wild type solventogenic strains for butanol production. Biotechnol Biofuels 11(1):1–8. https://doi.org/10.1186/s13068-018-1252-3
Yang M, Zhang J, Kuittinen S, Vepsäläinen J, Soininen P, Keinänen M, Pappinen A (2015) Enhanced sugar production from pretreated barley straw by additive xylanase and surfactants in enzymatic hydrolysis for acetone-butanol-ethanol fermentation. Biores Technol 189:131–137. https://doi.org/10.1016/j.biortech.2015.04.008
Garg S (2016) Xylanase: applications in biofuel production. Curr Metabolomics 4(1):23–37. https://doi.org/10.2174/2213235x03666150915211224
Sun J, Wen F, Si T, Xu JH, Zhao H (2012) Direct conversion of xylan to ethanol by recombinant Saccharomyces cerevisiae strains displaying an engineered minihemicellulosome. Appl Environ Microbiol 78(11):3837–3845. https://doi.org/10.1128/AEM.07679-11
Mathimani T, Pugazhendhi A (2019) Utilization of algae for biofuel, bio-products and bio-remediation. Biocatal Agric Biotechnol 17:326–330. https://doi.org/10.1016/j.bcab.2018.12.007
Singh G, Kaur S, Khatri M, Arya SK (2019) Biobleaching for pulp and paper industry in India: emerging enzyme technology. Biocatal Agric Biotechnol 17:558–565. https://doi.org/10.1016/j.bcab.2019.01.019
Santos MP, Reinoso FAM, Távilla V, Ferraz A, Milagres AMF (2019) On-site produced and commercially available alkali-active xylanases compared for xylan extraction from sugarcane bagasse. Biocatal Agric Biotechnol 18:101081. https://doi.org/10.1016/j.bcab.2019.101081
Hu S, Zheng H, Gu Y, Zhao J, Zhang W, Yang Y, Wang S, Zhao G, Yang S, & Jiang W (2011) Comparative genomic and transcriptomic analysis revealed genetic characteristics related to solvent formation and xylose utilization in Clostridium acetobutylicum EA 2018. BMC Genomics 12. https://doi.org/10.1186/1471-2164-12-93
Tasneem M, Khan A, Ashraf HHI (2003) Xylanase biosynthesis by chemically mutated strain of Aspergillus niger. J Food Sci Technol 1:178–181
Ho H, Chor X (2015) Improvement of xylanase production by Bacillus subtilis in submerged fermentation after UV and chemicals mutagenesis. J Adv Biol Biotechnol 3(2):42–57. https://doi.org/10.9734/jabb/2015/16356
Gibbs MD, Reeves RA, Bergquist PL (1995) Cloning, sequencing, and expression of a xylanase gene from the extreme thermophile Dictyoglomusthermophilum Rt46B.1 and activity of the enzyme on fiber-bound substrate. Appl Environ Microbiol 61(12):4403–4408. https://doi.org/10.1128/aem.61.12.4403-4408.1995
Yi X, Shi Y, Xu H, Li W, Xie J, Yu R, Zhu J, Cao Y, Qiao D (2010) Hyperexpression of two Aspergillus niger xylanase genes in Escherichia coli and characterization of the gene products. Braz J Microbiol 41(3):778–786. https://doi.org/10.1590/S1517-83822010000300030
Valenzuela SV, Díaz P, Javier Pastor FI (2010) Recombinant expression of an alkali stable GH10 Xylanase from paenibacillusbarcinonensis. J Agric Food Chem 58(8):4814–4818. https://doi.org/10.1021/jf9045792
Verma D, Anand A, Satyanarayana T (2013) Thermostable and alkalistableendoxylanase of the extremely thermophilic bacterium geobacillusthermodenitrificans TSAA1: cloning, expression, characteristics and itsapplicability in generating xylooligosaccharides and fermentable sugars. Appl Biochem Biotechnol 170(1):119–130. https://doi.org/10.1007/s12010-013-0174-6
Verma D, Kawarabayasi Y, Miyazaki K, & Satyanarayana T (2013) Cloning, expression and characteristics of a novel alkalistable and thermostable xylanase encoding gene (Mxyl) retrieved from compost-soil metagenome. PLoS ONE 8(1). https://doi.org/10.1371/journal.pone.0052459
Helianti I, Ulfah M, Nurhayati N, Suhendar D, Finalissari AK, Wardani AK (2016) Production of xylanase by recombinant Bacillus subtilis DB104 cultivated in agroindustrial waste medium. HAYATI J Biosci 23(3):125–131. https://doi.org/10.1016/j.hjb.2016.07.002
Khandeparker K, Parab P, Amberkar U (2017) Recombinant xylanase from Bacillus tequilensis BT21: biochemical characterization and its application in production of xylobiose from agricultural residues. Food Technol Biotechnol 55(2):164–172. https://doi.org/10.17113/ftb.55.02.17.4896
Aftab MN, Zafar A, Iqbal I, Kaleem A, Zia KM, Awan AR (2018) Optimization of saccharification potential of recombinant xylanase from bacillus licheniformis. Bioengineered 9(1):159–165
Mendonça EHM, Avanci NC, Romano LH, Branco DL, de Pádua AX, Ward RJ, de Baptista Neto Á, Lourenzoni MR (2020) Recombinant xylanase production by Escherichia coli using a non-induced expression system with different nutrient sources. Braz J Chem Eng 37(1):29–39. https://doi.org/10.1007/s43153-019-00004-x
Panbangred W, Kondo T, Negoro S, Shinmyo A, Okada H (1983) Molecular cloning of the genes for xylan degradation of Bacillus pumilus and their expression in Escherichia coli. Mol Gen Genet MGG 192(3):335–341. https://doi.org/10.1007/bf00392172
Lisov AV, Belova OV, Andreeva-Kovalevskaya ZI, Budarina ZI, Solonin AA, Vinokurova NG, Leontievsky AA (2013) Recombinant xylanase from Streptomyces coelicolor Ac-738: characterization and the effect on xylan-containing products. World J Microbiol Biotechnol 30(3):801–808. https://doi.org/10.1007/s11274-013-1480-4
Bai Y, Wang J, Zhang Z, Yang P, Shi P, Luo H, Meng K, Huang H, Yao B (2009) A new xylanase from thermoacidophilic Alicyclobacillus sp. A4 with broad-range pH activity and pH stability. J Ind Microbiol Biotechnol 37(2):187–194. https://doi.org/10.1007/s10295-009-0662-4
Chen X, Xu S, Zhu M, Cui L, Zhu H, Liang Y, Zhang Z (2010) Site-directed mutagenesis of an Aspergillus niger xylanase B and its expression, purification and enzymatic characterization in Pichia pastoris. Process Biochem 45(1):75–80. https://doi.org/10.1016/j.procbio.2009.08.009
Ghazi S, Akhavan A, Azin M, Khajeh K, Khavarinejad R (2014) UV mutagenesis for the overproduction of xylanase from Bacillus mojavensis PTCC 1723 and optimization of the production condition. Iran J Basic Med Sci 17:844–853
Rahim T, Ray AL, Beauty SP, Gomes DJ (1970) Induction of mutation in Neurospora crassa with ultraviolet radiation and evaluation of cellulase and xylanase activities. Bangladesh J Botany 38(2):201–203. https://doi.org/10.3329/bjb.v38i2.5149
Kulkarni N, Shendye A, Rao M (1999) Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev 23(4):411–456. https://doi.org/10.1016/S0168-6445(99)00006-6
Bhardwaj N, Kumar B, & Verma P (2019) A detailed overview of xylanases: an emerging biomolecule for current and future prospective. Bioresour Bioprocess 6(1). https://doi.org/10.1186/s40643-019-0276-2
Song L, Tsang A, Sylvestre M (2015) Engineering a thermostable fungal GH10 xylanase, importance of N-terminal amino acids. Biotechnol Bioeng 112(6):1081–1091. https://doi.org/10.1002/bit.25533
Zhang H, Li J, Wang J, Yang Y, Wu M (2014) Determinants for the improved thermostability of a mesophilic family 11 xylanase predicted by computational methods. Biotechnol Biofuels 7(1):3. https://doi.org/10.1186/1754-6834-7-3
Author information
Authors and Affiliations
Contributions
S. K. Arya and G. Singh designed the concept; R. Chaudhary wrote the manuscript along with T. Kuthiala who reviewed the manuscript. All of the authors discussed, commented on, and revised the manuscript.
Corresponding author
Ethics declarations
Animal research
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chaudhary, R., Kuthiala, T., Singh, G. et al. Current status of xylanase for biofuel production: a review on classification and characterization. Biomass Conv. Bioref. 13, 8773–8791 (2023). https://doi.org/10.1007/s13399-021-01948-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01948-2