Abstract
Background
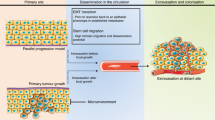
Retinoblastoma (Rb) is a progressive cancer which mainly occurs in children, and which is caused by different genetic or epigenetic alterations that lead to inactivation of both alleles of the RB1 gene. Hereditary and non-hereditary forms of Rb do exist, and the hereditary form is associated with an increased risk of secondary malignancies. Metastasis to distant organs is a critical feature of many tumors, and may be caused by various molecular alterations at different stages. Recognition of these alterations and, thus, insight into the processes underlying the development of metastases may result in novel preventive as well as effective targeted treatment options. Rb is associated with metastases to various organs and tissues, including the bone marrow (BM).
Methods
Here, we provide an overview of mutations and other molecular changes known to be involved in Rb development and metastasis to the BM. This overview is based on a literature search ranging from 1990 to 2015.
Conclusions
The various BM metastasis-related molecular changes identified to date may be instrumental for a better diagnosis, prognosis and classification of Rb patients, as well as for the development of novel comprehensive (targeted) therapies.


Similar content being viewed by others
Abbreviations
- VEGF:
-
Vascular endothelial growth factor
- VEGF-R:
-
Vascular endothelial growth factor receptor
- FGF:
-
Fibroblast growth factor
- KIF14:
-
Kinesin family member 14
- MYCN:
-
V-myc myelocytomatosis viral oncogene neuroblastoma-derived homolog
- KiSS1:
-
KiSS-1 metastasis suppressor
- FGF-R:
-
Fibroblast growth factor receptor
- N-cadherin:
-
Neural-cadherin
- ECM:
-
Extracellular matrix
- MMPs:
-
Matrix metalloproteinases
- LOX:
-
Lysyl oxidase.
References
M. Kuruva, B.R. Mittal, R. Kashyap, A. Bhattacharya, R.K. Marwaha, Bilateral retinoblastoma presenting as metastases to forearm bones four years after the initial treatment. Indian J. Nucl. Med. 26, 115–116 (2011)
M.J. Ali, S.G. Honavar, V.A. Reddy, Distant metastatic retinoblastoma without central nervous system involvement. Indian J. Ophthalmol. 61, 357–159 (2013)
S.N. Zafar, S.Q. Ahmad, N. Zafar, Retinoblastoma in an adult. BMC Res. Notes. 6, 1–3 (2013)
J. Yun, Y. Li, C.-T. Xu, B.-R. Pan, Epidemiology and Rb1 gene of retinoblastoma. Int. J. Ophthalmol. 4, 103–109 (2011)
G.L. Chantada and I.J. Dunkel, in Retinoblastoma, ed. by C. Rodriguez-Galindo and M.W. Wilson (Springer US, 2010), p. 103–114
G.L. Chantada, C. Sampor, A. Bosaleh, V. Solernou, A. Fandiño, M.T. de Dávila, Comparison of staging systems for extraocular retinoblastoma: analysis of 533 patients. JAMA Ophthalmol. 131, 1127–1134 (2013)
Y. Tabe, M. Konopleva, Advances in understanding the leukaemia microenvironment. Br. J. Haematol. 164, 767–778 (2014)
S. Azizidoost, S. Babashah, F. Rahim, M. Shahjahani, N. Saki, Bone marrow neoplastic niche in leukemia. Hematology 19, 232–238 (2014)
V.B.F. Vallone, H. Choi, V. Labovsky, L.M. Martinez, V. Milovic, G. Jaimovich, E. Batagelj, F. Dimase, A.R. Villafañe, N.A. Chasseing, Bone marrow mesenchymal stem cells: pre-metastatic niche for breast cancer. Blood 122, 4859–4859 (2013)
P.T. Finger, J.W. Harbour, Z.A. Karcioglu, Risk factors for metastasis in retinoblastoma. Surv. Ophthalmol. 47, 1–16 (2002)
A. Ray, D.S. Gombos, Retinoblastoma: an overview. Indian J. Pediatr. 79, 916–921 (2012)
G. Bunin, M. Orjuela et al., in Clinical Ophthalmic Oncology, ed. by A.D. Singh, A.L. Murphree, B.E. Damato (Springer, Berlin, 2015), pp. 39–50
L. Castéra, C. Dehainault, D. Michaux, L. Lumbroso-Le Rouic, I. Aerts, F. Doz, A. Pelet, J. Couturier, D. Stoppa-Lyonnet, M. Gauthier-Villars, Fine mapping of whole RB1 gene deletions in retinoblastoma patients confirms PCDH8 as a candidate gene for psychomotor delay. Eur. J. Med. Gen. 21, 460–464 (2013)
M.-y. He, Y. An, Y.-j. Gao, X.-w. Qian, G. Li, J. Qian, Screening of RB1 gene mutations in Chinese patients with retinoblastoma and preliminary exploration of genotype–phenotype correlations. Mol. Vis. 20, 545–552 (2014)
M. Sabir, R.M. Baig, K. Ali, I. Mahjabeen, M. Saeed, M.A. Kayani, Retinoblastoma (RB1) pocket domain mutations and promoter hyper-methylation in head and neck cancer. Cell. Oncol. 37, 203–213 (2014)
J. Sage, The retinoblastoma tumor suppressor and stem cell biology. Genes Dev. 26, 1409–1420 (2012)
S.L. Anwar, T. Krech, B. Hasemeier, E. Schipper, N. Schweitzer, A. Vogel, H. Kreipe, U. Lehmann, Deregulation of RB1 expression by loss of imprinting in human hepatocellular carcinoma. J. Pathol. 233, 392–401 (2014)
C. Marouf, A. Tazzite, B. Diakité, H. Jouhadi, A. Benider, S. Nadifi, Association of TP53 PIN3 polymorphism with breast cancer in Moroccan population. Tumour Biol. 35, 12403–12408 (2014)
M. Wade, Y.-C. Li, G.M. Wahl, MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat. Rev. Cancer 13, 83–96 (2013)
A. Agrawal, J. Yang, R.F. Murphy, D.K. Agrawal, Regulation of the p14ARF-Mdm2-p53 pathway: an overview in breast cancer. Exp. Mol. Pathol. 81, 115–122 (2006)
N.A. Laurie, S.L. Donovan, C.-S. Shih, J. Zhang, N. Mills, C. Fuller, A. Teunisse, S. Lam, Y. Ramos, A. Mohan, Inactivation of the p53 pathway in retinoblastoma. Nature 444, 61–66 (2006)
N.A. Laurie, M.A. Dyer, Targeting MDM2 and MDMX in retinoblastoma. Curr. Cancer Drug Targets 7, 689–695 (2007)
B.L. Thériault, H. Dimaras, B.L. Gallie, T.W. Corson, The genomic landscape of retinoblastoma: a review. Clin. Exp. Ophthalmol. 42, 33–52 (2014)
J.-Y. Choe, J.Y. Yun, Y.K. Jeon, S.H. Kim, H.-K. Choung, S. Oh, M. Park, J.E. Kim, Sonic hedgehog signalling proteins are frequently expressed in retinoblastoma and are associated with aggressive clinicopathological features. J. Clin. Pathol. 68, 6–11 (2015)
S. Siffroi-Fernandez, A. Cinaroglu, V. Fuhrmann-Panfalone, G. Normand, K. Bugra, J. Sahel, D. Hicks, Acidic fibroblast growth factor (FGF-1) and FGF receptor 1 signaling in human Y79 retinoblastoma. Arch. Ophthalmol. 123, 368–376 (2005)
N. Sethi, Y. Kang, Unravelling the complexity of metastasis—molecular understanding and targeted therapies. Nat. Rev. Cancer 11, 735–748 (2011)
A. Geurts van Kessel, The cancer genome: from structure to function. Cell. Oncol. 37, 155–165 (2014)
A.C. Chiang, J. Massagué, Molecular basis of metastasis. N. Engl. J. Med. 359, 2814–2823 (2008)
J.H. Tsai, J. Yang, Epithelial–mesenchymal plasticity in carcinoma metastasis. Genes Dev. 27, 2192–2206 (2013)
E.Y. Lee, W.J. Muller, Oncogenes and tumor suppressor genes. Cold Spring Harb. Perspect. Biol. 2, a003236 (2010)
C.G.A. Network, Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012)
C.Q. Lin, J. Singh, K. Murata, Y. Itahana, S. Parrinello, S.H. Liang, C.E. Gillett, J. Campisi, P.-Y. Desprez, A role for Id-1 in the aggressive phenotype and steroid hormone response of human breast cancer cells. Cancer Res. 60, 1332–1340 (2000)
Z. Shengbing, L. Jing Feng, W. Bin, G. Lingyun, H. Aimin, Expression of KiSS‐1 gene and its role in invasion and metastasis of human hepatocellular carcinoma. Anat. Rec. (Hoboken). 292, 1128–1134 (2009)
D. Nie, J. Nemeth, Y. Qiao, A. Zacharek, L. Li, K. Hanna, K. Tang, G.G. Hillman, M.L. Cher, D.J. Grignon, Increased metastatic potential in human prostate carcinoma cells by overexpression of arachidonate 12-lipoxygenase. Clin. Exp. Metastasis 20, 657–663 (2003)
J. Liu, W. Wei, W. Tang, Y. Jiang, H. Yang, J. Li, Mechanism of lysyl oxidase (LOX) in breast cancer invasion and metastasis. Zhonghua Yi Xue Za Zhi 92, 1379–1383 (2012)
D.X. Nguyen, P.D. Bos, J. Massagué, Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9, 274–284 (2009)
D. Maret, E. Gruzglin, M.S. Sadr, V. Siu, W. Shan, A.W. Koch, N.G. Seidah, R.F. Del Maestro, D.R. Colman, Surface expression of precursor N-cadherin promotes tumor cell invasion. Neoplasia 12, 1066–1038 (2010)
E. Haÿ, E. Laplantine, V. Geoffroy, M. Frain, T. Kohler, R. Müller, P.J. Marie, N-cadherin interacts with axin and LRP5 to negatively regulate Wnt/β-catenin signaling, osteoblast function, and bone formation. Mol. Cell. Biol. 29, 953–964 (2009)
M.E. Diamond, L. Sun, A.J. Ottaviano, M.J. Joseph, H.G. Munshi, Differential growth factor regulation of N-cadherin expression and motility in normal and malignant oral epithelium. J. Cell Sci. 121, 2197–2207 (2008)
H. Zhuo, K. Jiang, L. Dong, Y. Zhu, L. Lü, Y. Lü, Y. Zhang, H. Zhang, Y. Ye, S. Wang, Overexpression of N-cadherin is correlated with metastasis and worse survival in colorectal cancer patients. Chin. Sci. Bull. 58, 3529–3534 (2013)
E.H. Van Aken, P. Papeleu, P. De Potter, E. Bruyneel, J. Philippé, S. Seregard, A. Kvanta, J.-J. De Laey, M.M. Mareel, Structure and function of the N-cadherin/catenin complex in retinoblastoma. Invest. Ophthalmol. Vis. Sci. 43, 595–602 (2002)
J. Schiffman, G. Grunwald, Differential cell adhesion and expression of N-cadherin among retinoblastoma cell lines. Invest. Ophthalmol. Vis. Sci. 33, 1568–1574 (1992)
A. Kvanta, B. Steen, S. Seregard, Expression of vascular endothelial growth factor (VEGF) in retinoblastoma but not in posterior uveal melanoma. Exp. Eye Res. 63, 511–518 (1996)
C. Gialeli, A.D. Theocharis, N.K. Karamanos, Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 278, 16–27 (2011)
M. Kandalam, M. Mitra, K. Subramanian, J. Biswas, Molecular pathology of retinoblastoma. Middle East Afr. J. Ophthalmol. 17, 217–223 (2010)
C. Martins, B. Fernandes, E. Antecka, S. Di Cesare, J. Mansure, J. Marshall, M. Burnier, Expression of the metastasis suppressor gene KISS1 in uveal melanoma. Eye (Lond). 22, 707–711 (2008)
F.-S. Wang, H. Chen, Z.-Y. Wu, J.-H. Lin, KISS1 expression in osteosarcoma: high in chinese clinical cases, but lower in cell lines. Asian Pac. J. Cancer Prev. 12, 3229–3234 (2011)
K.T. Nash, P.A. Phadke, J.-M. Navenot, D.R. Hurst, M.A. Accavitti-Loper, E. Sztul, K.S. Vaidya, A.R. Frost, J.C. Kappes, S.C. Peiper, Requirement of KISS1 secretion for multiple organ metastasis suppression and maintenance of tumor dormancy. J. Natl. Cancer Inst. 99, 309–321 (2007)
M. Sanchez-Carbayo, P. Capodieci, C. Cordon-Cardo, Tumor suppressor role of KiSS-1 in bladder cancer: loss of KiSS-1 expression is associated with bladder cancer progression and clinical outcome. Am. J. Pathol. 162, 609–617 (2003)
T.M. Goto, Y. Arima, O. Nagano, H. Saya, Lysyl oxidase is induced by cell density-mediated cell cycle suppression via RB-E2F1-HIF-1α axis. Cell Struct. Funct. 38, 9–14 (2013)
J.J. Park, S.J. Hwang, J.-H. Park, H.-J. Lee, Chlorogenic acid inhibits hypoxia-induced angiogenesis via down-regulation of the HIF-1α/AKT pathway. Cell. Oncol. 38, 1–8 (2015)
Z. Lu, F. Bauzon, H. Fu, J. Cui, H. Zhao, K. Nakayama, K.I. Nakayama and L. Zhu, Skp2 suppresses apoptosis in Rb1-deficient tumours by limiting E2F1 activity. Nat Commun. 5, 3463 (2014) doi: 10.1038/ncomms4463
H.-G. Kopp, S.T. Avecilla, A.T. Hooper, S. Rafii, The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 20, 349–356 (2005)
B. Furusato, A. Mohamed, M. Uhlén, J.S. Rhim, CXCR4 and cancer. Pathol. Int. 60, 497–505 (2010)
M.M. Balla, G.K. Vemuganti, C. Kannabiran, S.G. Honavar, R. Murthy, Phenotypic characterization of retinoblastoma for the presence of putative cancer stem-like cell markers by flow cytometry. Invest. Ophthalmol. Vis. Sci. 50, 1506–1514 (2009)
C. Salinas-Souza, R. De Oliveira, M.T.D.S. Alves, R.J. Garcia Filho, A.S. Petrilli, S.R.C. Toledo, The metastatic behavior of osteosarcoma by gene expression and cytogenetic analyses. Hum. Pathol. 44, 2188–2198 (2013)
G.D. Roodman, Genes associate with abnormal bone cell activity in bone metastasis. Cancer Metastasis Rev. 31, 569–578 (2012)
F. Navid, V.M. Santana, R.C. Barfield, Anti-GD2 antibody therapy for GD2-expressing tumors. Curr. Cancer Drug Targets 10, 200–209 (2010)
D. Dávila, Optimization of molecular detection of GD2 synthase mRNA in retinoblastoma. Mol. Med. Rep. 3, 253–259 (2010)
J. Di, T. Duiveman-de Boer, P.L. Zusterzeel, C.G. Figdor, L.F.G. Massuger, R. Torensma, The stem cell markers Oct4A, Nanog and c-Myc are expressed in ascites cells and tumor tissue of ovarian cancer patients. Cell. Oncol. 36, 363–374 (2013)
T. Hiraga, S. Ito, H. Nakamura, Cancer stem–like cell marker CD44 promotes bone metastases by enhancing tumorigenicity, cell motility, and hyaluronan production. Cancer Res. 73, 4112–4122 (2013)
F. Rahim, S. Hajizamani, E. Mortaz, A. Ahmadzadeh, M. Shahjahani, S. Shahrabi, N. Saki, Molecular regulation of bone marrow metastasis in prostate and breast cancer. Bone Marrow Res. 2014, 1–13 (2014)
P. Massoner, T. Thomm, B. Mack, G. Untergasser, A. Martowicz, K. Bobowski, H. Klocker, O. Gires, M. Puhr, EpCAM is overexpressed in local and metastatic prostate cancer, suppressed by chemotherapy and modulated by MET-associated miRNA-200c/205. Br. J. Cancer 111, 955–964 (2014). doi:10.1038/bjc.2014.366
S. Krishnakumar, A. Mohan, K. Mallikarjuna, N. Venkatesan, J. Biswas, M.P. Shanmugam, L. Ren-Heidenreich, EpCAM expression in retinoblastoma: a novel molecular target for therapy. Invest. Ophthalmol. Vis. Sci. 45, 4247–4250 (2004)
B. Ell and Y. Kang, MicroRNAs as regulators of bone homeostasis and bone metastasis . Bonekey Rep. 3, 1608 (2014) doi: 10.1038/bonekey.2014.44
C. Salazar, R. Nagadia, P. Pandit, J. Cooper-White, N. Banerjee, N. Dimitrova, W.B. Coman, C. Punyadeera, A novel saliva-based microRNA biomarker panel to detect head and neck cancers. Cell. Oncol. 37, 331–338 (2014)
C. Yu, M. Wang, Z. Li, J. Xiao, F. Peng, X. Guo, Y. Deng, J. Jiang, C. Sun, MicroRNA-138-5p regulates pancreatic cancer cell growth through targeting FOXC1. Cell. Oncol. 38, 1–9 (2015)
H. Zhang, Y. Li, M. Lai, The microRNA network and tumor metastasis. Oncogene 29, 937–948 (2009)
Y. Bai, X. Bai, Z. Wang, X. Zhang, C. Ruan, J. Miao, MicroRNA-126 inhibits ischemia-induced retinal neovascularization via regulating angiogenic growth factors. Exp. Mol. Pathol. 91, 471–477 (2011)
S.F. Tavazoie, C. Alarcón, T. Oskarsson, D. Padua, Q. Wang, P.D. Bos, W.L. Gerald, J. Massagué, Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451, 147–152 (2008)
N. Venkatesan, P. Deepa, M. Vasudevan, V. Khetan, A.M. Reddy, S. Krishnakumar, Integrated analysis of dysregulated miRNA-gene expression in HMGA2-silenced retinoblastoma cells. Bioinform. Biol. Insights. 8, 177–191 (2014)
M. Scarola, S. Schoeftner, C. Schneider, R. Benetti, miR-335 directly targets Rb1 (pRb/p105) in a proximal connection to p53-dependent stress response. Cancer Res. 70, 6925–6933 (2010)
G. Gabriely, T. Wurdinger, S. Kesari, C.C. Esau, J. Burchard, P.S. Linsley, A.M. Krichevsky, MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol. Cell. Biol. 28, 5369–5380 (2008)
L.-X. Yan, X.-F. Huang, Q. Shao, M.-Y. Huang, L. Deng, Q.-L. Wu, Y.-X. Zeng, J.-Y. Shao, MicroRNA miR-21 overexpression in human breast cancer is associated with advaanced clinical stage, lymph node metastasis and patient poor prognosis. RNA 14, 2348–2360 (2008)
M. Bullock, K. Pickard, B.S. Nielsen, A.E. Sayan, V. Jenei, M. Mellone, J. Primrose, G. Thomas, G. Packham, A. Mirnezami, Deregulated stromal microRNA-21 and promotion of metastatic progression in colorectal cancer. Lancet 383, S30 (2014)
E.C. Connolly, K. Van Doorslaer, L.E. Rogler, C.E. Rogler, Overexpression of miR-21 promotes an in vitro metastatic phenotype by targeting the tumor suppressor RHOB. Mol. Cancer Res. 8, 691–700 (2010)
F. Shen, M.-H. Mo, L. Chen, S. An, X. Tan, Y. Fu, K. Rezaei, Z. Wang, L. Zhang, S.W. Fu, MicroRNA-21 down-regulates Rb1 expression by targeting PDCD4 in retinoblastoma. J. Cancer. 5, 804–812 (2014)
B. Psaila, D. Lyden, The metastatic niche: adapting the foreign soil. Nat. Rev. Cancer 9, 285–293 (2009)
S. Wan, Y. Liu, Y. Weng, W. Wang, W. Ren, C. Fei, Y. Chen, Z. Zhang, T. Wang, J. Wang, BMP9 regulates cross-talk between breast cancer cells and bone marrow-derived mesenchymal stem cells. Cell. Oncol. 37, 363–375 (2014)
S. Shahrabi, S. Azizidoost, M. Shahjahani, F. Rahim, A. Ahmadzadeh, N. Saki, New insights in cellular and molecular aspects of BM niche in chronic myelogenous leukemia. Tumour Biol. 35, 10627–10633 (2014)
C.F. Chantrain, O. Feron, E. Marbaix, Y.A. DeClerck, Bone marrow microenvironment and tumor progression. Cancer Microenviron. 1, 23–35 (2008)
A. Ganguly, C.L. Shields, Differential gene expression profile of retinoblastoma compared to normal retina. Mol. Vis. 16, 1292–1303 (2010)
A.M. Roccaro, A. Sacco, W.G. Purschke, M. Moschetta, K. Buchner, C. Maasch, D. Zboralski, S. Zöllner, S. Vonhoff, Y. Mishima, SDF-1 inhibition targets the bone marrow niche for cancer therapy. Cell Rep. 9, 118–128 (2014)
P. Bianco, M. Riminucci, S. Gronthos, P.G. Robey, Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 19, 180–192 (2001)
S.-P. Lin, F.-Y. Chiu, Y. Wang, M.-L. Yen, S.-Y. Kao and S.-C. Hung, RB Maintains Quiescence and Prevents Premature Senescence through Upregulation of DNMT1 in Mesenchymal Stromal Cells. Stem Cell Rep. 3, 975–986 (2014) doi: 10.1016/j.stemcr.2014.10.002
C.R. Walkley, J.M. Shea, N.A. Sims, L.E. Purton, S.H. Orkin, Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell 129, 1081–1095 (2007)
G.B. Adams, D.T. Scadden, The hematopoietic stem cell in its place. Nat. Immunol. 7, 333–337 (2006)
S. Flowers, G.R. Beck, E. Moran, Transcriptional activation by pRB and its coordination with SWI/SNF recruitment. Cancer Res. 70, 8282–8287 (2010)
G. Kapatai, M.A. Brundler, H. Jenkinson, P. Kearns, M. Parulekar, A.C. Peet, C.M. McConville, Gene expression profiling identifies different sub-types of retinoblastoma. Br. J. Cancer 109, 512–525 (2013). doi:10.1038/bjc.2013.283
N. Erez, Cancer: opening LOX to metastasis. Nature (2015). doi:10.1038/nature14529
F. Dillmann, M.R. Veldwijk, S. Laufs, M. Sperandio, G. Calandra, F. Wenz, W.J. Zeller, S. Fruehauf, F. Dillmann, M.R. Veldwijk, Plerixafor inhibits chemotaxis toward SDF-1 and CXCR4-mediated stroma contact in a dose-dependent manner resulting in increased susceptibility of BCR-ABL+ cell to Imatinib and Nilotinib. Leuk. Lymphoma 50, 1676–1686 (2009)
S. Stefanovic, F. Schuetz, C. Sohn, P. Beckhove, C. Domschke, Bone marrow microenvironment in cancer patients: immunological aspects and clinical implications. Cancer Metastasis Rev. 32, 163–178 (2013)
K. Grymula, M. Tarnowski, M. Wysoczynski, J. Drukala, F.G. Barr, J. Ratajczak, M. Kucia, M.Z. Ratajczak, Overlapping and distinct role of CXCR7-SDF-1/ITAC and CXCR4-SDF-1 axes in regulating metastatic behavior of human rhabdomyosarcomas. Int. J. Cancer 127, 2554–2568 (2010)
J.-J. Zhao, J. Yang, J. Lin, N. Yao, Y. Zhu, J. Zheng, J. Xu, J.Q. Cheng, J.-Y. Lin, X. Ma, Identification of miRNAs associated with tumorigenesis of retinoblastoma by miRNA microarray analysis. Childs Nerv. Syst. 25, 13–20 (2009)
W. Tang, Y. Zhu, J. Gao, J. Fu, C. Liu, Y. Liu, C. Song, S. Zhu, Y. Leng, G. Wang, MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. Br. J. Cancer 110, 450–458 (2013)
K. Conkrite, M. Sundby, S. Mukai, J.M. Thomson, D. Mu, S.M. Hammond, D. MacPherson, miR-17 ∼ 92 cooperates with RB pathway mutations to promote retinoblastoma. Genes Dev. 25, 1734–1745 (2011)
Y. Fan, S. Yin, Y. Hao, J. Yang, H. Zhang, C. Sun, M. Ma, Q. Chang, J.J. Xi, miR-19b promotes tumor growth and metastasis via targeting TP53. RNA 20, 765–772 (2014)
S. Lu, Q. Zhu, Y. Zhang, W. Song, M.J. Wilson, P. Liu, Dual‐functions of miR‐373 and miR‐520c by differently regulating the activities of MMP2 and MMP9. J. Cell. Physiol. 230, 1862–1870 (2015). doi:10.1002/jcp.24914
M.Y. Shah, G.A. Calin, MicroRNAs miR-221 and miR-222: a new level of regulation in aggressive breast cancer. Genome. Med. 3, 56 (2011)
Acknowledgments
We appreciate the contributions of all our colleagues in the Health Research Institute, Research Center of Thalassemia & Hemoglobinopathy. We thank Dr. Courtney Schaal for her help in the writing and the proofreading of the manuscript.
Authors’ contributions
Najmaldin Saki conceived the manuscript and revised it. Abbas Khosravi, Saeid Shahrabi and Mohammad Shahjahani wrote the manuscript and prepared the figures and the table.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khosravi, A., Shahrabi, S., Shahjahani, M. et al. The bone marrow metastasis niche in retinoblastoma. Cell Oncol. 38, 253–263 (2015). https://doi.org/10.1007/s13402-015-0232-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-015-0232-x