Abstract
Introduction
Palmoplantar pustulosis (PPP) is a chronic, inflammatory skin disease, with high disease burden, that is often refractory to treatment. There is a high unmet clinical need for the treatment of patients with PPP. The objectives of this study were to evaluate the safety and efficacy of spesolimab, a novel anti-interleukin-36 receptor antibody, in patients with PPP.
Methods
This was a phase IIa, multicenter, double-blind, randomized, placebo-controlled pilot study comparing 900 mg spesolimab (n = 19), 300 mg spesolimab (n = 19), and placebo (n = 21) administered intravenously every 4 weeks until week 12 in patients with PPP. The primary efficacy endpoint was the achievement of Palmoplantar Pustulosis Area and Severity Index 50 (PPP ASI50) at week 16, defined as achieving an ≥ 50% decrease from baseline PPP ASI.
Results
At week 16, 31.6% of patients in both spesolimab dose groups achieved PPP ASI50 versus 23.8% receiving placebo (risk difference 0.078; 95% confidence interval –0.190, 0.338). Thus, the primary endpoint was not met. Spesolimab was well tolerated with no clinically relevant treatment-emergent safety signals observed.
Conclusions
PPP severity declined over time in all treatment groups after the start of treatment, with a faster decline in the spesolimab arms than in the placebo arm, indicating a potential treatment effect for spesolimab. Limitations to the study included a small sample size and lower overall disease severity than expected at baseline. It is possible that the primary efficacy endpoint may have coincided with natural disease resolution in some patients. Further effects of the efficacy of spesolimab in PPP are being explored in a phase IIb trial.
Similar content being viewed by others
Video Abstract (MP4 54474 kb)
Why carry out this study? |
Palmoplantar pustulosis (PPP) is a chronic, inflammatory skin disease that is often refractory to treatment and has a high unmet clinical need. |
The interleukin (IL)-36 pathway has been shown to play a role in the pathogenesis of PPP in some patients. |
Spesolimab, an anti-IL36 receptor inhibitor, may offer a novel, targeted treatment for those with PPP. |
What was learned from this study? |
The primary endpoint of the study (the proportion of patients achieving Palmoplantar Pustulosis Area and Severity Index 50 [PPP ASI50] at week 16) was not met, with 31.6% of patients in both spesolimab dose groups achieving PPP ASI50 versus 23.8% in the placebo group. |
Although the primary endpoint was not met, post hoc analyses showed that PPP symptoms showed greater improvement with spesolimab compared with placebo in patients with more severe disease, indicating that spesolimab may be an effective treatment for those with severe PPP. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13912919.
Introduction
Palmoplantar pustulosis (PPP) is a chronic, inflammatory, debilitating, relapsing disease characterized by neutrophil-filled sterile pustules involving the palms of the hands and/or soles of the feet [1,2,3]. Patients with PPP experience periods of active disease followed by periods of partial remission [4, 5].
Historically, PPP has been part of a group of rare inflammatory skin conditions collectively known as pustular psoriasis, with PPP the most common localized variant [2, 6]. However, PPP is now recognized as a distinct disease, with a different genetic profile than non-pustular psoriasis [7,8,9].
Therapeutic intervention is a major challenge in patients with PPP because the condition is difficult to treat, resulting in a low response rate to treatment [10]. Commonly used treatments, including topical corticosteroids, systemic retinoids, methotrexate, cyclosporine, and psoralen plus ultraviolet light A, have limited efficacy and are also associated with adverse events (AE), limiting their long-term use [5, 10,11,12,13,14,15,16]. To date, clinical trials have been conducted on biologics, including ustekinumab, secukinumab, and guselkumab [11, 17,18,19]; however, the efficacy of these biologics in PPP is considerably lower than in plaque psoriasis.
The immunopathogenesis of PPP is not well characterized; comparisons of gene and protein expression between PPP and plaque psoriasis lesions found increased expression of genes, and their proteins, associated with the interleukin (IL)-1 and IL-36 pathways in PPP lesions [20]. In generalized pustular psoriasis (GPP), overexpression of IL-36 or loss-of-function mutations in the IL36RN gene, which encodes the IL-36 receptor antagonist (IL-36Ra) protein, lead to dysregulated IL-36R signaling and are linked to disease onset [21]. While IL36RN mutations are rare in PPP (though greater than in the general population), upregulation of the IL-36 pathway is reported in PPP [9, 20,21,22,23,24,25].
Spesolimab (BI 655130) is a humanized antagonistic monoclonal immunoglobulin G1 anti-interleukin-36 receptor (anti-IL-36R) antibody that blocks human IL-36R signaling [26]. Spesolimab was previously investigated in seven patients with GPP who presented with an acute flare of moderate-to-severe intensity (ClinicalTrials.gov identifier NCT02978690) [27]; a single dose of spesolimab (10 mg per kilogram of body weight) demonstrated rapid pustule clearance, with marked improvements in other disease measures for all patients, irrespective of IL36RN mutation status. These findings raise the possibility that blockade of the IL-36 pathway may also be a therapeutic strategy in patients with PPP.
Here, we report the safety and efficacy of spesolimab in patients with PPP in a phase IIa, multicenter, double-blind, randomized, placebo-controlled pilot study. We hypothesized that spesolimab, an anti-IL-36-receptor antibody, is efficacious in PPP, a disease that has previously been associated with dysregulation of the IL-36 pathway.
Methods
Study Design and Participants
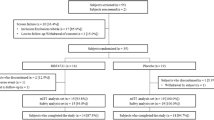
This 32-week trial (ClinicalTrials.gov identifier NCT03135548) investigated the safety and efficacy of spesolimab in patients with PPP and was conducted at 18 sites across Canada, Denmark, Germany, Italy, Spain, and Sweden. The trial consisted of three consecutive study periods: screening (from day-28 to day-7), treatment (16 weeks), and follow-up (16 weeks). Eligible patients identified during screening were randomized (1:1:1, blinded, using interactive response technology) to one of two dose arms of spesolimab (900 mg or 300 mg) or placebo intravenously every 4 weeks, corresponding to day 1 and weeks 4, 8, and 12. Patients were free to use any rescue medication after week 16.
Patients aged 18–65 years were eligible if they had PPP, defined as the presence of primary, persistent (> 3 months duration), sterile, macroscopically visible pustules on the palms of the hands and/or soles of the feet [1]. Eligible patients were permitted to have plaque psoriasis if it was on less than 10% of their body surface area. Pustulation had to be active (yellow pustules), and patients were required to have a minimum Palmoplantar Pustular Psoriasis Area and Severity Index (PPP ASI) of 12 (severity was assessed on a scale of 0–72, where 72 is the most severe), and a Palmoplantar Pustulosis Physician Global Assessment (PPP PGA) of at least moderate severity (≥ 3) at baseline; overall skin lesion status is graded on a scale of 0–4, with 0 indicating clear skin and 4 indicating very severe lesions. See the electronic supplementary material for full inclusion/exclusion criteria, including restricted medications, and full descriptions of further measures and PPP ASI.
The clinical trial protocol, patient information leaflet, informed consent form, and other locally required documents were reviewed and approved by the Independent Ethics Committees and/or Institutional Review Boards of participating centers (master committee: Ethikkommission der Medizinischen Fakultät der Christian-Albrechts-Universität zu Kiel, Germany). The study was conducted in compliance with the clinical trial protocol, in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and other applicable regulatory requirements and Boehringer Ingelheim’s standard operating procedures. Written informed consent was obtained from all patients prior to study initiation.
Efficacy and Safety Assessments
As there are currently no established or well-validated endpoints available to assess clinician- or patient-reported outcomes in PPP, several endpoints were explored in this pilot study. The primary endpoints were achievement of a 50% improvement from baseline in PPP ASI (PPP ASI50) at week 16 following treatment with spesolimab, and the number of patients with drug-related AEs over the 32-week trial. Evaluations were also performed between week 16 and week 32, with main evaluations taking place up to week 16; however, as patients were free to use any rescue medication between week 16 and week 32, these results are not included in the overall results of this trial.
Safety assessments included evaluation of AEs (coded using the Medical Dictionary for Drug Regulatory Activities [MedDRA] version 21.1; AE intensity was assessed using the Rheumatology Common Toxicity Criteria [RCTC] version 2.0), serious AEs, laboratory assessments, physical examinations of vital signs, and 12-lead electrocardiograms over the duration of the trial (32 weeks). The main secondary endpoints were the achievement of PPP ASI75 and percent change from baseline in PPP ASI at week 16.
Statistical Analyses
This was an exploratory trial, and formal confirmatory statistical testing was not performed. The efficacy analyses were performed on the full analysis set (FAS), comprising all patients who were randomized, received at least one dose of study drug during the trial, and had a baseline measurement for the primary endpoint. Safety was assessed in all patients who were randomized and received at least one dose during the trial.
Primary analysis of the unadjusted absolute risk difference versus placebo was calculated as the difference in the observed proportion of patients with PPP ASI50 at week 16 for each treatment scenario for the FAS. A 95% Wilson confidence interval (CI) around this difference was provided. Additionally, a parametric bootstrap 95% CI was generated by sampling from the binomial distribution for each treatment, with the number of patients and observed proportion of responders per treatment representing the sampling parameters.
Exploratory post hoc analyses were conducted based on the database snapshot taken for the primary analysis to dissect the results. Post hoc analyses included percent change in PPP ASI from baseline to week 16 versus percent change in PPP ASI from screening to baseline; percent change in PPP ASI from baseline to week 16 in patients with/without improvement in PPP ASI from screening to baseline; and percent change in PPP ASI from baseline to week 16 in patients with baseline PPP ASI above/below the median baseline PPP ASI. Further secondary endpoints and post hoc analyses are listed in the supplementary material.
Secondary and exploratory binary endpoints were analyzed using the same methodology described for the primary endpoint. For each continuous endpoint, the mean change from baseline was analyzed using a restricted maximum likelihood (REML)-based repeated measures approach. The safety analysis was conducted descriptively and focused on treatment-emergent AEs.
For all binary endpoints, no response imputation was used to enter missing data. If the data were available both before and after the visit with a missing outcome, and indicated a response, the missing outcome was also imputed as achieving a response; otherwise, no response was imputed. For patients receiving rescue medication for PPP prior to the primary endpoint, all subsequent data post-rescue medication was considered to represent no response.
Skin Samples
After obtaining informed consent, biopsies were taken from 23 patients from either the palm of the hand or the sole of the foot, depending on where the patient exhibited lesions; it was not required for biopsies to be taken from both palms and soles. Pustules were not permitted to be directly biopsied but areas within the lesion in close proximity to a pustule were permitted. At day 1, a single 5 mm punch biopsy was taken from each patient and immediately placed in RNAlater™ solution (Ambion Inc.) for RNA extraction. For each patient, biopsy samples were taken from the same target lesion or in close proximity to the target lesion (same anatomical area).
Gene Expression Analysis
Gene expression analysis of IL36A, IL36B, IL36G, and IL36RN from lesional skin biopsy samples was performed on a subset of 23 patients with PPP at baseline. Gene expression was analyzed by means of TaqMan polymerase chain reaction (PCR); all real-time PCRs were run on the 7900HT Sequence Detection System (SDS; Applied Biosystems, Foster City, CA, USA). All samples were run in triplicates, and raw cycle threshold (Ct) values were calculated using SDS v.2.4. Fold change of expression was calculated using the group means as a comparator sample for the comparative Ct method (2−ΔΔct).
Results
Patients
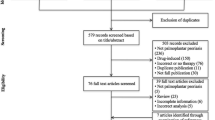
Of the 79 patients screened, 59 were randomly assigned to either 900 mg (n = 19) or 300 mg (n = 19) spesolimab, or placebo (n = 21) (Fig. 1). Baseline demographics and disease characteristics were generally well balanced between treatment arms. The mean (standard deviation [SD]) time since first diagnosis was 9.1 (11.3) years in the overall trial population (Table 1); this was slightly less in the placebo group (6.7 [7.0] years) than in the investigational treatment groups (10.4 [13.0] years) (Table 1).
In total, 43 patients (72.9%) completed trial medication administration; all patients, regardless of whether they completed administration of all medication as planned, were followed until week 32. At week 16, 53 patients (89.8%) completed the primary endpoint visit and 47 patients (79.7%) completed the trial observation period. The rate of premature discontinuation was similar in all treatment groups. The most frequent reasons for discontinuing treatment were AEs (10.2%) or withdrawal by the patient (10.2%). Six patients discontinued treatment due to worsening of disease (n = 3) or lack of improvement (n = 3) (three in the 900 mg spesolimab dose group and three in the placebo group). At baseline, patients reported considerable pain according to the pain visual analog scale (VAS) across all groups (Table 1).
Efficacy
At week 16, six of 19 patients (31.6%) in each of the 900 mg and 300 mg spesolimab dose groups achieved a PPP ASI50 response; in the placebo group, five of 21 patients (23.8%) achieved a PPP ASI50 response. The risk difference versus placebo was 0.078 (95% CI −0.190, 0.338) for both spesolimab dose groups (Table 2); thus, the primary endpoint (PPP ASI50 at week 16) was not met. Only two randomized patients (one in the placebo group and one in the 300 mg spesolimab group) were IL36RN mutation positive, and neither of these patients were PPP ASI50 responders at week 16. Four of 19 patients (21.1%) in the 900 mg spesolimab dose group, and no patients in the 300 mg spesolimab dose group, achieved a PPP ASI75 response at week 16; in the placebo group, two of 21 patients (9.5%) achieved a PPP ASI75 response at week 16 (Table 3). The risk difference versus placebo was 0.115 (95% CI −0.116, 0.348) for the 900 mg dose group and –0.095 (95% CI −0.289, 0.086) for the 300 mg dose group. The mean percent change over time in PPP ASI from baseline is shown in Fig. 2i. At week 16, the mean percent change in PPP ASI was greatest in the 900 mg spesolimab dose group (−45.80% [95% CI −60.75%, −30.85%]), followed by the placebo group (−39.97% [95% CI −58.22%, −21.73%]); it was lowest in the 300 mg spesolimab dose group (−32.74% [95% CI −54.98%, −10.50%]) (Table 2).
Mean (95% CI) percent change from baseline in PPP ASI over time in (i) all patients and (ii) patients with no improvement in PPP ASI from screening to baseline (screening < 1.2 × baseline). Panel (i) shows the mean (95% CI) percent change from baseline in PPP ASI over time in all patients, represented as negative percent change from baseline. The full analysis set was used. Observed cases were used without imputation of missing data. All values after intake of medication are excluded. Panel (ii) shows mean (95% CI) change from baseline in PPP ASI in patients with no improvement in PPP ASI from screening to baseline: four patients in the placebo group, one in the spesolimab 300 mg group, and three in the spesolimab 900 mg group were not included in this analysis. The full analysis set was used. The last observation carried forward method was used to impute missing data. CI confidence interval, PPP ASI Palmoplantar Pustular Psoriasis Area and Severity Index
In post hoc analyses, eight patients exhibited a natural improvement in PPP ASI from screening to baseline (screening ≥ 1.2 × baseline). Analyses excluding these patients demonstrated larger declines in mean total PPP ASI in the spesolimab treatment groups than in the placebo group at each time point up to week 16 (Fig. 2ii).
Because overall disease severity in the trial population was lower than anticipated [17,18,19], the population was divided into two subgroups: patients with disease severity at baseline lower than the median baseline PPP ASI (16.7), and patients with disease severity at baseline higher than the median baseline PPP ASI. In patients with greater baseline disease severity, the efficacy of spesolimab versus placebo resulted in a greater change from baseline in PPP ASI (Fig. 3) and pustular severity (Fig. 4) in patients receiving spesolimab until week 16.
Safety
Overall, spesolimab was well tolerated, with an AE profile similar to that of placebo (Table 3). Through 32 weeks, 16 patients (42.1%) receiving spesolimab had an investigator-defined drug-related AE (Table 3); most AEs were graded as mild or moderate. Two patients had serious AEs requiring hospitalization: one patient (in the 300 mg spesolimab group) had idiopathic abducens paresis (non-drug related), the other (in the placebo group) had worsening PPP (drug related). In addition, six patients (two patients in each arm) had severe AEs (Table 3) (RCTC grade 3 or 4), including syncope, PPP, gastric ulcer, and sixth nerve paralysis. Of these, two were thought to be drug related: one patient in the 900 mg arm (syncope) and one patient in the placebo arm (PPP). The most frequently reported AEs were nasopharyngitis (900 mg: 42.1%; 300 mg: 26.3%; placebo: 38.1%), headache (31.6%; 21.1%; 33.3%), PPP (15.8%; 10.5%; 19.0%), arthralgia (10.5%; 15.8%; 4.8%), and cough (10.5%; 15.8%; 4.8%) (Table 3).
Biomarker Analysis
Using RNA sequencing, a substudy comparing gene expression levels in skin biopsies from the worst affected areas in patients with a PPP ASI above the median (16.7; by PPP ASI of the region of skin where the biopsy was taken; n = 23) revealed a distinct molecular profile, with significantly higher expression of IL36A (p = 0.0301), IL36B (p = 0.0140), and IL36RN (p = 0.0168) in patients with greater disease severity compared with those with lower disease severity (Fig. 5). Higher IL36G expression was also observed in patients with greater disease severity compared with those with lower disease severity, but this difference did not reach statistical significance at alpha = 0.05 (p = 0.0739).
Lesional biomarker analysis comparing gene expression levels in patients (n = 23) with a PPP ASI above/below the median (16.7) at baseline. Lesional biomarker analysis of IL-36 pathway genes was measured by RNA sequencing to compare differences in gene expression in patients with a PPP ASI above or below the median (16.7) at baseline prior to spesolimab treatment. Biomarker samples were collected prior to infusion at the infusion visit. Box plot denotes IQR, with bars representing the minimum and maximum values within 1.5 × IQR. Individual values are denoted by circles (green), and mean values are denoted by diamonds (gray). Baseline biomarker levels could not be determined for one patient with PPP ASI below the median at baseline for the analysis of IL36G or IL36RN; therefore, this patient was excluded from analysis for these genes. IL interleukin, IQR interquartile range, PPP ASI Palmoplantar Pustular Psoriasis Area and Severity Index
Discussion
Based on the apparent efficacy of spesolimab in GPP [27], spesolimab was tested in patients with PPP in this pilot study. The primary endpoint in this study, PPP ASI50 at week 16, was not met, and no significant differences between spesolimab and placebo were observed for the main secondary endpoints (achievement of PPP ASI75 and percent change from baseline in PPP ASI at week 16). Overall, PPP ASI declined in all treatment groups after the start of treatment, with a faster decline in the spesolimab arms than in the placebo arm. PPP ASI50 and PPP ASI75 response rate was numerically higher in the 900 mg spesolimab group compared with the placebo group in individuals with disease severity higher than the median at baseline; this suggested a potential treatment effect for spesolimab, which was further explored in post hoc analyses.
In post hoc analyses, patients with PPP ASI above the median at baseline (i.e., greater disease severity) had a rapid and discernible improvement in PPP ASI and pustule severity with both spesolimab doses compared with placebo. This was not seen in patients with disease severity below the median PPP ASI at baseline, suggesting a treatment effect for spesolimab in patients with more severe disease.
The safety profile of spesolimab was consistent with earlier data [27], with no new or unexpected safety signals observed. No clinically relevant treatment-emergent safety signals with spesolimab were identified, adding to previous safety data from 40 healthy volunteers (unpublished data) and seven patients with GPP [27]. All patients had AEs that were graded as mild or moderate, and no serious AEs were reported. This finding aligns with the recent characterization of individuals with IL36R knockout mutations, resulting in the complete absence of IL-36R, but without any evidence of an increased risk of superinfection, or a clinically relevant impact on the innate or adaptive immune responses [28].
Analysis of gene expression levels in skin biopsies from the worst affected areas revealed a distinct molecular profile, characterized by greater expression of IL-36 pathway markers in patients with greater disease severity compared with those with less severe disease. While these findings suggest stronger keratinocyte activation in patients with more severe disease, they also support the finding that PPP is an autoinflammatory-driven disease. Together with the post hoc analysis of spesolimab in patients with PPP ASI above the median at baseline, this study suggests that the IL-36 pathway may play an important role in the pathogenesis of PPP.
Several factors may have contributed to the lack of a significant treatment effect with spesolimab in this trial. Undoubtedly, the relatively small study size is a limitation, as an effect in a small number of patients can have a major impact on overall proportional results, as revealed by post hoc analyses. It should also be noted that the majority of the patients enrolled in this study were obese, white, smoking females. While a similar demographic has been analyzed in other European populations, this may not be representative of the whole PPP population.
Additionally, the timing of the primary and secondary endpoints at week 16 may have coincided with a natural improvement in some patients with chronic PPP, a disease characterized by exacerbations and partial remissions. In clinical studies of guselkumab and secukinumab in patients with PPP, observed treatment responses at week 16 continued to improve to week 52 [17, 19]. The distinction between disease activity and treatment effects might have been hindered by the number of patients with low disease activity at baseline (compounded by improvements from screening to baseline), obscuring observable responses in the trial population compared with other clinical trials in PPP [17,18,19]. In some trials, patients are excluded if they exhibit improvement between screening and baseline; this could be addressed in future trials with spesolimab to stabilize the variability in disease severity, which could mask potential treatment effects. Efforts to validate clinical patient-reported outcomes are currently underway to combat the variability in disease severity at baseline.
Post hoc analyses identified several patients across all three study groups who displayed a spontaneous improvement in disease activity between screening and baseline. Thus, patients with a considerable decline in PPP ASI from screening to baseline showed higher responses at week 16, irrespective of the study group, including with placebo. These patients may have experienced a spontaneous fluctuation in their disease course, which is characteristic of PPP. This observation is important for the design of future clinical trials in PPP.
Thus, the limitations of the current study include the small sample size, and patient demographics at enrollment, which are not typically representative of the wider PPP population. In addition, the natural resolution of disease between screening and baseline could have masked a treatment effect for spesolimab in patients with PPP.
Although the primary endpoint was not met in this study, these results, together with the gene expression analysis, support the concept that IL-36 upregulation may be involved in the pathogenesis of PPP; however, further investigation of targeted inhibition of IL-36R with spesolimab is required. Indeed, observations from this study have informed the design of an ongoing, phase IIb, proof-of-concept, dose-finding study (ClinicalTrials.gov identifier NCT04015518) of spesolimab in patients with PPP.
Change history
21 June 2021
“A peer-reviewed video abstract was retrospectively added to this publication.”.
References
Navarini AA, Burden AD, Capon F, et al. European consensus statement on phenotypes of pustular psoriasis. J Eur Acad Dermatol Venereol. 2017;31(11):1792–9.
Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178(3):614–8.
Murakami M, Terui T. Palmoplantar pustulosis: current understanding of disease definition and pathomechanism. J Dermatol Sci. 2020;98(1):13–9.
Marsland AM, Chalmers RJ, Hollis S, et al. Interventions for chronic palmoplantar pustulosis. Cochrane Database Syst Rev. 2006;1:CD001433.
Raposo I, Torres T. Palmoplantar psoriasis and palmoplantar pustulosis: current treatment and future prospects. Am J Clin Dermatol. 2016;17(4):349–58.
Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131–44.
Asumalahti K, Ameen M, Suomela S, et al. Genetic analysis of PSORS1 distinguishes guttate psoriasis and palmoplantar pustulosis. J Invest Dermatol. 2003;120(4):627–32.
Bissonnette R, Suarez-Farinas M, Li X, et al. Based on molecular profiling of gene expression, palmoplantar pustulosis and palmoplantar pustular psoriasis are highly related diseases that appear to be distinct from psoriasis vulgaris. PLoS ONE. 2016;11(5):e0155215.
Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94(5):790–7.
Mrowietz U, van de Kerkhof PC. Management of palmoplantar pustulosis: do we need to change? Br J Dermatol. 2011;164(5):942–6.
Bertelsen T, Kragballe K, Johansen C, et al. Efficacy of ustekinumab in palmoplantar pustulosis and palmoplantar pustular psoriasis. Int J Dermatol. 2014;53(10):e464–6.
Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36(5):279–94.
Browne H, Mason G, Tang T. Retinoids and pregnancy: an update. Obstet Gynaecol. 2014;16:7–11.
Abraham A, Roga G. Topical steroid-damaged skin. Indian J Dermatol. 2014;59(5):456–9.
Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol. 1998;134(5):595–8.
Kromer C, Wilsmann-Theis D, Gerdes S, et al. Drug survival and reasons for drug discontinuation in palmoplantar pustulosis: a retrospective multicenter study. J Dtsch Dermatol Ges. 2019;17(5):503–16.
Mrowietz U, Bachelez H, Burden AD, et al. Secukinumab for moderate-to-severe palmoplantar pustular psoriasis: results of the 2PRECISE study. J Am Acad Dermatol. 2019;80(5):1344–52.
Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154(3):309–16.
Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab in Japanese patients with palmoplantar pustulosis: a phase 3 randomized clinical trial. JAMA Dermatol. 2019;155(10):1153–61.
Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are the dominant cytokines in palmar plantar pustulosis. J Dermatol Sci. 2016;84(1):e99.
Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365(7):620–8.
Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89(3):432–7.
Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133(5):1366–9.
Wang TS, Chiu HY, Hong JB, et al. Correlation of IL36RN mutation with different clinical features of pustular psoriasis in Chinese patients. Arch Dermatol Res. 2016;308(1):55–63.
Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143(3):1021–6.
Ganesan R, Raymond EL, Mennerich D, et al. Generation and functional characterization of anti-human and anti-mouse IL-36R antagonist monoclonal antibodies. MAbs. 2017;9(7):1143–54.
Bachelez H, Choon SE, Marrakchi S, et al. Inhibition of the interleukin-36 pathway for the treatment of generalized pustular psoriasis. N Engl J Med. 2019;380(10):981–3.
Mahil SK, Catapano M, Di Meglio P, et al. An analysis of IL-36 signature genes and individuals with IL1RL2 knockout mutations validates IL-36 as a psoriasis therapeutic target. Sci Transl Med. 2017;9(411):eaan2514.
Acknowledgements
Funding
Boehringer Ingelheim, Ingelheim, Germany, sponsored this study and provided funding for the conduct, data analysis, and medical writing assistance for the study’s publication, and for the journal’s Rapid Service fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
David Hall, PhD, contributed to analysis of the data. The authors would like to thank the following Principal Investigators involved with this trial: Dr Afsaneh Alavi, Dr Asunción Ballester, Prof Luca Bianchi, Dr Pablo de la Cueva, Dr Sascha Gerdes, Prof Lars Iversen, Dr Natalia Kuzmina, Dr Amra Osmancevic, Dr Sandra Philipp, Dr Luis Puig, Prof Franco Rongioletti, Prof Lone Skov, Prof Simon Francis Thomsen, and Dr Irina Turchin. Agreements between Boehringer Ingelheim and the authors included confidentiality of the study data. All authors collaborated on the writing of the manuscript. We thank all patients who participated in this clinical study.
Medical Writing and Editorial Assistance
Editorial assistance in the preparation of this article was provided by Amy Pashler, PhD, and Leigh Church, PhD, of OPEN Health Communications (London, UK) and funded by Boehringer Ingelheim.
Prior presentation
This manuscript is based on work that was previously presented at the 119th Annual Meeting of the Japanese Dermatological Association, June 4–7, 2020, Kyoto, Japan.
Disclosures
Ulrich Mrowietz has served as an advisor/received speaker’s honoraria/received grants from/investigator for AbbVie, Almirall, Aristea, Boehringer Ingelheim (BI), Celgene, Dr. Reddy’s Laboratories, Eli Lilly, Foamix, Formycon, Forward Pharma, Janssen, LEO Pharma, medac, Novartis, Union Chimique Belge (UCB), and XenoPort. A. David Burden has served as an advisor/received speaker’s honoraria/grants/investigator for AbbVie, Almirall, BI, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, and UCB. Robert Bissonnette is an advisory board member, consultant, speaker, investigator for/received honoraria/grants from, AbbVie, Almirall, AnaptysBio, Arcutis, Aristea, Bausch Health/Valeant, BI, Boston Pharma, Bristol Myers Squibb (BMS), Dermavant, Eli Lilly, Escalier, Janssen, Kineta, Kyowa Kirin, LEO Pharma, Pfizer, Regeneron, Sienna, and UCB. Robert Bissonnette is also an employee and shareholder of Innovaderm Research. Kristian Reich has served as an advisor/speaker for/investigator for AbbVie, Affibody, Almirall, Amgen, Avillion, Biogen, BI, BMS, Celgene, Janssen Biotech Covagen, Forward Pharma, Fresenius Medical Care, Galapagos, GlaxoSmithKline (GSK), Janssen-Cilag, Kyowa Kirin, LEO Pharma, Eli Lilly, medac, Merck Sharp & Dohme (MSD), Novartis, Miltenyi Biotec, Ocean Pharma, Pfizer, Regeneron, Samsung Bioepis, Sanofi, Sun Pharma, Takeda, UCB, Valeant, and XenoPort. Andreas Pinter has served as an advisor/speaker/received grants from/investigator for AbbVie, Almirall Hermal, Amgen, Biogen Idec, BI, Celgene, GSK, Eli Lilly, Galderma, Hexal AG, Janssen, LEO Pharma, MC2, medac, Merck Serono, Mitsubishi, MSD, Novartis, Pascoe, Pfizer, Tigercat Pharma, Regeneron, Roche, Sandoz Biopharmaceuticals, Sanofi Genzyme, Schering-Plough, and UCB. Knut Schäkel has served as an advisor/speaker/received grants from/investigator for AbbVie, Amgen, Almirall, Biogen, BI, BMS, Celgene, Chugai, Galderma, Janssen-Cilag, LEO Pharma, Lilly, MSD, Miltenyi Biotec, MorphoSys, Novartis, Pfizer, Polichem, Regeneron, and UCB. Yakov Datsenko, Hongjie Deng, Patrick Baum, Steven J Padula, and Christian Thoma are employed by BI.
Compliance with Ethics Guidelines
The clinical trial protocol, patient information leaflet, informed consent form, and other locally required documents were reviewed and approved by the Independent Ethics Committees and/or Institutional Review Boards of participating centers (master committee: Ethikkommission der Medizinischen Fakultät der Christian-Albrechts-Universität zu Kiel, Germany). The study was conducted in compliance with the clinical trial protocol, in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and other applicable regulatory requirements and Boehringer Ingelheim’s standard operating procedures. Written informed consent was obtained from all patients prior to study initiation.
Data Availability
To ensure independent interpretation of clinical study results, Boehringer Ingelheim grants all external authors access to all relevant material, including participant-level clinical study data, and relevant material as needed by them to fulfill their role and obligations as authors under the ICMJE criteria. Furthermore, clinical study documents (e.g., study report, study protocol, statistical analysis plan) and participant clinical study data are available to be shared after publication of the primary manuscript in a peer-reviewed journal and if regulatory activities are complete and other criteria met per the BI Policy on Transparency and Publication of Clinical Study Data: https://trials.boehringer-ingelheim.com/transparency_policy.html. Prior to providing access, documents will be examined, and, if necessary, redacted with data deidentified, to protect the personal data of study patients and personnel, and to respect the boundaries of the informed consent of the study patients. Clinical study reports and related clinical documents can be requested via this link: https://trials.boehringer-ingelheim.com/trial_results/clinical_submission_documents.html.All such requests will be governed by a document sharing agreement. Bona fide, qualified scientific and medical researchers may request access to deidentified, analyzable participant clinical study data with corresponding documentation describing the structure and content of the datasets. Upon approval, and governed by a data sharing agreement, data are shared in a secured data access system for a limited period of 1 year, which may be extended upon request. Researchers should use https://clinicalstudydatarequest.com to request access to study data.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mrowietz, U., Burden, A.D., Pinter, A. et al. Spesolimab, an Anti-Interleukin-36 Receptor Antibody, in Patients with Palmoplantar Pustulosis: Results of a Phase IIa, Multicenter, Double-Blind, Randomized, Placebo-Controlled Pilot Study. Dermatol Ther (Heidelb) 11, 571–585 (2021). https://doi.org/10.1007/s13555-021-00504-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-021-00504-0