Abstract
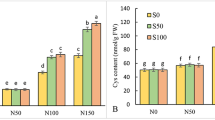
Although silicon (Si) is not considered as an essential element, it is beneficial to the plant growth. Its effect is more evident under abiotic and biotic stress conditions. The objective of this study is to investigate the role of Si on the in vitro growth and resistance to salt stress of Dianthus caryophyllus ‘Tula’. The experiment was designed as a factorial design with 0, 50, or 100 mg·L−1 of potassium silicate (K2SiO3) in combination with 0, 50, or 100 mM sodium chloride (NaCl). The treatment of 50 mg·L−1 Si improved the growth of plant. However, the treatment of Si at 100 mg·L−1 reduced the growth. Although NaCl retarded the growth, addition of Si along with NaCl to the culture medium mitigated the effect of NaCl. A primary defense line by Si to overcome the photosynthetic depression was apparent from the increased chlorophyll content in the Si + NaCl treatment as compared to the treatment of NaCl alone. Enhancement of growth and resistance to salinity by Si was thought to be due to the modulation in activity of antioxidant enzymes, such as superoxide dismutase, ascorbate peroxidase, guaiacol peroxidase, and catalase. Therefore, our results suggested that 50 mg·L−1 Si supplementation could be optimal for improved growth in vitro and enhanced resistance against salinity in D. caryophyllus ‘Tula’.
Similar content being viewed by others
Literature Cited
Abogadallah, G.M. 2010. Antioxidative defense under salt stress. Plant Signal. Behav. 5:369–374.
Agarie, S., W. Agata, H. Kubota, and P.B. Kaufmann. 1992. Physiological role of silicon in photosynthetic and dry matter production in rice plants. Crop Sci. 61:200–206.
Ahmad, R., S.H. Zaheer, and S. Ismail. 1992. Role of silicon in salt tolerance of wheat (Triticum aestivum L.). Plant Sci. 85:43–50.
Al-Aghabary, K., Z.J. Zhu, and Q.H. Shi. 2005. Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J. Plant Nutr. 27:2101–2115
Arnon, D.I. 1949. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24:1–15.
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254.
Cakmak, I. and H. Marschner. 1992. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 98:1222–1227.
Choudhury, S., P. Panda, L. Sahoo, and S.K. Panda. 2013. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 8:e23681.
Davies, K.J. 1995. Oxidative stress: The paradox of aerobic life. Biochem. Soc. Symp. 61:1–31.
Elliott, C.L. and G.H. Snyder. 1991. Autoclave-induced digestion for the colorimetric determination of silicon in rice straw. J. Agric. Food Chem. 39:1118–1119.
Epstein, E. 1999. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50:641–664.
Fauteux, F., W. Remus-Borel, J.G. Menzies, and R.R. Belanger. 2005. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett. 249:1–6.
Frantz, J.M., J.C. Locke, L. Datnoff, M. Omer, A. Widrig, D. Sturtz, L. Horst, and C.R. Krause. 2008. Detection, distribution, and quantification of silicon in floricultural crops utilizing three distinct analytical methods. Commun. Soil Sci. Plant Anal. 39:2734–2751.
Giannopolitis, C.N. and S.K. Ries. 1977. Superoxide dismutases. Plant Physiol. 59:309–314.
Gunes, A., A. Inal, E.G. Bagci, and D.J. Pilbeam. 2007. Silicon-mediated changes of some physiological and enzymatic parameters symptomatic for oxidative stress in spinach and tomato grown in sodic-B toxic soil. Plant Soil 290:103–114.
Kaya, C., H. Kirnak, and D. Higgs. 2001. Enhancement of growth and normal growth parameters by foliar application of potassium and phosphorus in tomato cultivars grown at high (NaCl) salinity. J. Plant Nutr. 24:357–367.
Kim, S.Y., J.H. Lim, M.R. Park, Y.J. Kim, T.I. Park, Y.W. Seo, K.G. Choi, and S.J. Yun. 2005. Enhanced antioxidant enzymes are associated with reduced hydrogen peroxide in barley roots under saline stress. J. Biochem. Mol. Biol. 38:218–224.
Kim, Y.H., A.L. Khan, M. Waqas, J.K. Shim, D.H. Kim, K.Y. Lee, and I.J Lee. 2014. Silicon application to rice root zone influenced the phytohormonal and antioxidant responses under salinity stress. J. Plant Growth Reg. 33:137–149.
Kwon, O.K., Y.A. Kim, K.S. Kim, and H.K. Shin. 2005. Growth and ion balance of carnation under salt stress. J. Kor. Soc. Hort. Sci. 46:380–384.
Liang, Y.C. 1998. Effect of silicon on leaf ultrastructure, chlorophyll content and photosynthetic activity of barley under salt stress. Pedosphere 8:289–296.
Liang, Y.C., Q. Chen, Q. Liu, W.H. Zhang, and R.X. Ding. 2003. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 160:1157–1164.
Liang, Y.C., Q.R. Shen, Z.G. Shen, and T.S. Ma. 1996. Effects of silicon on salinity tolerance of two barley cultivars. J. Plant Nutr. 19:173–183.
Lin, C.C. and C.H. Kao. 2000. Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul. 30:151–155.
Lu, Z. and P.M. Neumann. 1999. Low cell-wall extensibility can limit maximum leaf growth rates in rice. Crop Sci. 36:126–130.
Ma, J.F. 2004. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nut. 50:11–18.
Ma, J.F. and E. Takahashi. 2002a. Silicon uptake and accumulation in plants, p. 73–106. In: J.F. Ma and E. Takahashi (eds.). Soil, fertilizer, and plant silicon research in Japan. Elsevier Science, Amsterdam, The Netherlands.
Ma, J.F. and E. Takahashi. 2002b. Effect of silicate fertilizer application on paddy rice, p. 49–62. In: J.F. Ma and E. Takahashi (eds.). Soil, fertilizer, and plant silicon research in Japan. Elsevier Science, Amsterdam, The Netherlands.
Ma, J.F. and N. Yamaji. 2006. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 11:392–397.
Mattson, N.S. and W.R. Leatherwood. 2010. Potassium silicate drenches increased leaf silicon content and affect morphological traits of several floricultural crops grown in a peat-based substrate. Hortscience 45:43–47.
Mitani, N. and J.F. Ma. 2005. Uptake system of silicon in different plant species. J. Expt. Bot. 1255-1261.
Montoliu A., M.F. Lopez-Climent, V. Arbona, R.M. Perez-Clemente, and A. Gomez-Cadenas. 2009. A novel in vitro tissue culture approach to study salt stress responses in citrus. Plant Growth Regul. 59:179–187.
Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497.
Nakano, Y. and K. Asada. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22:867–880.
Porra, R.J. 2002. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosyn. Res. 73:149–156.
Potts, W.C., J.B. Reid, and I.C. Murfet. 1985. Internode length in Pisum. Gibberellins and the slender phenotype. Physiol. Plant. 63: 357–364.
Prabhakaran, S., I. Sivanesan, E.H. Jo, and B.R. Jeong. 2013. Silicon promotes shoot proliferation and shoot growth of Salvia splendens under salt stress in vitro. Hort. Environ. Biotechnol. 54:311–318.
Prabhakaran, S., I. Sivanesan, S. Jana, and B.R. Jeong. 2014. Influence of silicon on growth and tolerance to high temperature in Salvia splendens. Hort. Environ. Biotechnol. 55:271–279.
Richmond, K.E. and M. Sussman. 2003. Got silicon? The non-essential beneficial plant nutrient. Current Opin. Plant Biol. 6:268–272.
Rouhier, N. and J.P. Jacquot. 2008. Getting sick may help plants overcome abiotic stress. New Phytol. 180:738–741.
Sangster, A.G. 1978. Silicon in the roots of higher plants. Am. J. Bot. 65:929–935.
Takahashi, E., J.F. Ma, and Y. Miyake. 1990. The possibility of silicon as an essential element for higher plants. Comments Agri. Food Chem. 2:99–122.
Tester, M. and R. Davenport. 2003. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 91:503–527.
Yeo, A.R., S.A. Flowers, G. Rao, K. Welfare, N. Senanayake, and T.J. Flowers. 1999. Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for by a reduction in the transpirational bypass flow. Plant Cell Environ. 22:559–565.
Zhu, Z., G. Wei, J. Li, Q. Qian, and J. Yu. 2004. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 167:527–533.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Soundararajan, P., Manivannan, A., Park, Y.G. et al. Silicon alleviates salt stress by modulating antioxidant enzyme activities in Dianthus caryophyllus ‘Tula’. Hortic. Environ. Biotechnol. 56, 233–239 (2015). https://doi.org/10.1007/s13580-015-0111-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-015-0111-4