Abstract
Sustainability in agriculture means the inclusion of several aspects, as sustainable agriculture systems must not compromise not only their ability to satisfy future needs by undermining soil fertility and the natural resource base but also sustainable agriculture has had to address a range of other issues including energy use, efficient use, and recycling of nutrients, the effects on adjacent ecosystems including the effects on water bodies and climate change. Organic manures are an important factor to keep the soil fertility level of soils. However, their management is often related to large emissions. In this context, anaerobic digestion is—similarly to composting—a treatment option for stabilization of biogenic wastes leading to a residual product called digestates, enabling the sanitation and the recycling and use as fertilizer. It is also a means to obtain energy from wastes as well as from dedicated energy crops. Therefore, anaerobic digestion potentially addresses several aspects of agricultural sustainability. This review discusses the current state of knowledge on the effects of anaerobic digestion on organic compounds in digestates and the most important processes influencing N emissions in the field, as well as the possible long-term effects on soil microbial biomass and soil fertility. The main findings are that (1) the direct effects of anaerobic digestion on long-term sustainability in terms of soil fertility and environmental impact at the field level are of minor relevance. (2) The most relevant effects of anaerobic digestion on soil fertility as well as on N emissions will be expected from indirect effects related to cropping system changes such as changes in crop rotation, crop acreage, cover cropping, and total amounts of organic manures including digestates. Furthermore, (3) the remaining organic fraction after anaerobic digestion is much more recalcitrant than the input feedstocks leading to a stabilization of the organic matter and a lower organic matter degradation rate after field application, enabling a similar reproduction of the soil organic matter as obtained by direct application of the feedstock or by composting of the feedstock. (4) Regarding emissions, the main direct effect of anaerobic digestion on a farm level is the influence on gaseous emissions during manure or digestate treatment and handling, whereas the direct effects of anaerobic digestion on a field level on emissions (NH3 − and N2O− emissions, NO3 - leaching) are negligible or at least ambiguous. (5) The main direct effects of anaerobic digestion on the field level are short-term effects on soil microbial activity and changes in the soil microbial community. Therefore, in terms of the effects on agricultural sustainability, potential cropping system-based changes induced by introduction of biogas plants are probably much more relevant for the overall performance and sustainability of the cropping system than the direct effects triggered by application of digestates in comparison to the undigested feedstocks. Furthermore, to get the full potential advances from implementation of biogas plants in terms of improvement of the nutrient use efficiency and reduction of greenhouse gas emissions, there is the need to introduce more sophisticated techniques to avoid counteracting effects by pollution swapping, e.g., by gas-tight closure of the digestate stores and direct soil incorporation of the field-applied digestates.
Similar content being viewed by others
Contents
8 References
1 Introduction
During anaerobic digestion, bacteria consume part of the organic matter and produce “biogas,” primarily composed of methane and carbon dioxide. The residues, called digestates, are a complex mixture of water and a multitude of particulate, suspended, and dissolved organic and inorganic substances, including nutrients, not decomposed organic matter, and pollutants. There is strong evidence that AD may help alleviate some of the environmental concerns associated with animal husbandry, e.g., N and C emissions during storage, odor emissions, etc. (Amon et al. 2006; Battini et al. 2014; Massé et al. 2011; Michel et al. 2010), while simultaneously affecting the composition and fertilizer properties of the remaining digestates (Möller and Müller 2012).
Anaerobic digestion means a transformation of about 20–95 % of the carbon (C) in the feedstock into gaseous C compounds, depending on kind and recalcitrance of the feedstocks. The digestates are used as a fertilizer. Digestion of manures and crop residues reduces the amounts of organic carbon introduced into the soil in comparison to direct soil incorporation of the undigested feedstocks (e.g., Reinhold et al. 1991). However, digestion can also mean an increase of organic matter inputs, when dedicated energy crops are introduced as new crops in a crop rotation instead of cash crops, as well as a complete redesign of crop rotations, crop acreage, and cropping systems (Möller et al. 2011). It was often hypothesized that on a long-term, soil organic matter content will decrease and the soil biological activity may be hampered when a systematic removal of carbon fixed in manure and crop residues for energy purposes are implemented, leading to decreased quantities of organic materials with less easily available C applied to soils after implementation of a biogas plant (Lampkin 1990; Heilmann 1992; Haas 2000; Scheller 2006). Therefore, especially organic farmers are concerned that fertilization with the digestates may impact the soil microbiota and soil fertility because they contain more mineral nitrogen and less organic matter than the non-digested input materials (e.g., untreated animal slurry, plant residues, or green manure biomass) (Johansen et al. 2013). Simultaneously, anaerobic digestion leads to an increase of manure pH and also to transformation of nitrogen (N) compounds (e.g., EI-Shinnawi et al. 1989; Möller and Müller 2012). This potentially affects N loss processes during manure handling and soil N turnover after field application (e.g., Petersen and Sommer 2011). Therefore, there is much interest in understanding the effects of manure treatment by anaerobic digestion on N losses (ammonia, nitrous oxides, nitrate leaching) during manure handling (e.g., storage and field application) and after field application. In this paper, the available literature on the effects of anaerobic digestion on composition and recalcitrance of the organic C components in digestates is summarized and evaluated, and the latest information and understanding of how anaerobic digestion influences the soil C and N turnover after field application, including the gaseous N emissions (N2O and NH3) and the losses via nitrate leaching during manure handling and after field application is reviewed. Furthermore, effects on soil physical properties and soil microbial activity will be discussed and further research needs are defined. This review complements a former review published by Möller and Müller (2012) which focused on effects of anaerobic digestion on nutrient cycles and nutrient availability.
2 Effects of anaerobic digestion on digestate organic matter composition
The total carbon content of digestates varies between 28 and 47 % of the dry matter (Tambone et al. 2010; Fouda 2011; Möller and Schultheiss 2014). Approximately 80–95 % of that C was organically bound with carbonate as the remaining inorganic C (Chantigny et al. 2004; Fouda et al. 2013). During anaerobic digestion, lignin is not degraded, whereas volatile fatty acids (>90 %), cellulose (>50 %), hemicellulose (>80 %), and raw protein are partially degraded (Asmus et al. 1988; Molinuevo-Salces et al. 2013). Using Fictitious Atomic-Group Separation (FAS) techniques, Marcato et al. (2009) reported that the degree of carbon oxidation was lower in undigested pig slurry (0.829) than in digested pig slurry (0.216). The higher oxidation degree of digestate is surprising since they came from a reduced environment (−300 mV). The reason is that the most reduced C is converted to biogas, leading to a relative increase in the C oxidation level of the remaining organic carbon.
Several authors evaluated the relative stabilization processes due to anaerobic digestion using biochemical characterization (van Soest Analyses) or spectral analysis (e.g., Fourier-transform infrared (FTIR) spectra, cross polarization magic angle spinning combined with nuclear magnetic resonance (13C CPMAS NMR), etc.). These methods provide information about molecule structure and dynamics on the atomic level. As compared to undigested manures or residues, spectral and thermogravimetrical profiles of digestates generally show lower lipide, amide, and polysaccharide content (approx. −15 % absolute) and an enrichment in thermostable compounds in the range of 32 to 625 %, an increase of the degree of aromaticity (e.g., aromatic lignin by approx. 30–60 %), and an accumulation of long-chain aliphatic components due to the inability of the organisms involved in the digestion process to degrade these kind of materials under anoxic conditions (Table 1), indicating a relative increase of the biological recalcitrance in the digestates compared to the input materials (Cuetos et al. 2009;Gómez et al. 2005, 2007a, b, 2011; Marcato et al. 2009; Pognani et al. 2009; Tambone et al. 2009, 2013) by unilateral decomposition of the easier decomposable C compounds. Similar conclusions have been drawn in many other reports by application of soil incubation approaches (e.g., Reinhold et al. 1991; Sánchez et al. 2008; Thomsen et al. 2013). The higher the obtained degradation of the feedstocks, the higher the relative increase of recalcitrance, as a consequence of the preferential degradation of easy degradable compounds in anaerobic digesters. The degree of increase of stable compounds corresponds to the degree of degradation obtained via anaerobic digestion (Asmus et al. 1988; Gómez et al. 2007a; Thomsen et al. 2013) and with the decrease of the biological oxygen demand after field application of the manures (Orzi et al. 2010; Tambone et al. 2009; Alburquerque et al. 2012a). Characterizations of the Corg compounds of digestates by FTIR spectra indicated that anaerobic stabilization of organic matter is mainly due to the buildup of more stable compounds in the dry matter rather than humification processes (Marcato et al. 2009). From the available references, it can be concluded that anaerobic digestion has only a minor influence on the total amounts of highly recalcitrant compounds in the organic manures, which basically influences long-term soil organic matter contents and long-term soil fertility.
3 Effects of anaerobic digestion on overall farm greenhouse gas emissions
The implementation of biogas plants in farming systems has the potential for a reduction of the overall greenhouse gas emissions of mixed farming systems with animal husbandry by reducing net emissions and after applying credits for the produced renewable energy (Michel et al. 2010; Battini et al. 2014). However, the effects on single treatment and handling steps differentiate (e.g., manure handling, manure storage, field spreading) and are strongly dependent on the design of the manure stores (e.g., Battini et al. 2014). Results published by Wang et al. (2014) indicate for the storage phase in open stores considerably higher emissions of CO2 (+22.0 %) and N2O (+463 %) from digestates in comparison to the undigested animal manure, as well as much lower emissions of CH4 (−98.9 %), resulting in similar total GHG emissions (1.05 for undigested and 1.12 g CO2-eq L−1 day−1 for digested animal manure). In the digested manures, N2O emissions accounted for the major part of the CO2-equivalent GHG emissions, whereas CH4 emissions accounted for the major part of the CO2-equivalent GHG emissions for undigested manures. In conclusion, the performance of the entire farming system regarding the effects of a biogas plant on GHG emissions is largely dependent on the design of the digestate stores of the farm.
4 Effects of anaerobic digestion on N losses
4.1 Impact of anaerobic digestion on soil nitrate leaching
Derived from results in greenhouse or growth chamber pot experiments (Goberna et al. 2011; Sänger et al. 2010, 2011; Walsh et al. 2012a) or from theoretical approaches (Ørtenblad 2002; Dalgaard et al. 2004), it is often hypothesized that the nitrate leaching risk after field spreading of digestates is lower than after spreading of the undigested manure. The statements are deducted from the assumed better match of N supply and crop N demand, as a consequence of the higher ammonium-N/total N share in digestates compared to the input feedstocks. However, several field studies have demonstrated that anaerobic digestion of animal manures do not affect soil mineral N content in autumn at the beginning of the main leaching period, meaning no differences in the nitrate leaching risk of digested in comparison to undigested animal slurries (Chantigny et al. 2008; Merz and Trösch 1989; Möller and Stinner 2009; Pötsch 2005). However, implementation of biogas plants can be accompanied by several changes of the cropping system (crop acreage, cover cropping, etc.) which potentially can indirectly affect nitrate leaching risk, for example:
-
(i)
Changes in the farm infrastructure, e.g., increase of the storage capacity for liquid manures, leading to less manure applications in autumn before the leaching period.
-
(ii)
Alternative use of the organic materials already available in the farm system (e.g., green manure crops, crop residues, etc.) for energy purposes instead of direct incorporation into the soil. Harvest of green manure crops and crop residues in autumn for anaerobic digestion with subsequent reallocation of nutrients in winter and spring removes substantial amounts of N in late summer and autumn (Gunnarsson et al. 2008, 2011; Möller et al. 2008; Brozyna et al. 2013; Erhart et al. 2014; Frøseth et al. 2014) substantially affecting the soil mineral N content in autumn (e.g., Möller and Stinner 2009).
-
(iii)
Changes of the performed crop rotation and crop acreage (e.g., changes in cover cropping, implementation of energy crops like silage maize at the cost of cash crops, etc.) (Table 2). They include a higher share of silage maize in the crop rotation (e.g., Kruska and Emmerling 2008). NO3 −-loss risk is usually highest after maize cropping in comparison to many other crops (Möller et al. 2011, and references therein). Furthermore, the increase of silage maize acreage reduces simultaneously the scope for cover cropping, as an effective measure to reduce NO3 −-leaching risk, due to the late harvesting time of silage maize. Another factor indirectly affecting nitrate leaching risk is often an increase of the total amounts of available organic manures after implementation of biogas plants due to digestion of dedicated energy crops (Möller et al. 2011), resulting very often in higher overall organic manure applications at inconvenient periods. The significantly lower N efficiencies of organic manures, including unseparated digestates, in comparison to mineral N fertilizers (e.g., Gutser et al. 2005) are a further reason for higher N loss risks after setup of such biogas plants as higher amounts of total N are often applied to secure the N demand of crops.
Table 2 Potential direct and indirect system change effects of implementation of anaerobic digestion plants on nitrate leaching risk at different system boundaries
It can be concluded that anaerobic digestion itself does not influence directly the nitrate leaching risk after field application. The described cropping system change-related effects have a much stronger effect on nitrate leaching risk than the chemical changes of the feedstocks induced by the anaerobic digestion process in the fermenter. An increased nitrate leaching risk could result from digestion of dedicated energy crops and due to digestion of off-farm feedstocks as consequence of the increase in the total amounts of organic manures and the increase in silage maize cropping. A reduced nitrate leaching risk is reported when crop residues or green manure crops are removed from the field in autumn before beginning of the main leaching period, instead of leaving these materials on the site.
4.2 Impact of anaerobic digestion on total nitrogen losses and ammonia volatilization
4.2.1 Impact of anaerobic digestion on total N losses during manure storage
Nitrogen is the nutrient that is most susceptible to transformations affecting the risk of unproductive losses. The transformations include mineralization to ammonium, immobilization, oxidation (nitrification), and denitrification. It has often been reported that total N is mostly conserved during anaerobic digestion process (e.g., Tietjen 1957; Field et al. 1984; Plaixats et al. 1988). However, Neuner et al. (2011) balanced the nutrient inputs and outputs of biogas digesters and found net N losses of 18 %. Schievano et al. (2011) reported about net N losses of 5–10 %. The NH4 +-N flux in the biogas stream, which is composed by CH4, CO2, H2O(g) and trace amounts of NH4 +, H2S, etc., explained only approx. 10 % of these losses; the rest of the losses should be attributed to other reasons, for example partial organic/inorganic matter sedimentation as well as struvite formation and precipitation and subsequent retention in the digesters (Massé et al. 2007; Möller and Müller 2012; Schievano et al. 2011).
In the manure stores after anaerobic digestion, digested slurry from animal manures does not commonly build up a “natural” surface crust by suspended fibrous material, as is often found in undigested slurry stores. Sommer (1997) reported N losses from digested slurry by volatilization in open stores of about 30 % of the total N. Clemens et al. (2006) reported that in winter, NH3 losses from digested slurry were similar to those from untreated slurry. In summer, NH3 emissions from biogas slurry were twice as high as those from untreated slurry. These emissions can be avoided if the store is covered by a protective gas-tight layer (Clemens et al. 2006; Battini et al. 2014).
Novel technologies of manure handling often include the separation of the digestates in a liquid fraction and a fibrous solid fraction (Hjorth et al. 2010). Both fractions have, immediately after the separation process, a high NH4 + to total N share (e.g., Möller et al. 2010; Fouda et al. 2013). The liquid fraction is stored in tanks (either uncovered or covered) and the solid fractions in open manure heaps. There are only few data about the total gaseous N losses after digestate separation. No data were found concerning N emissions from the liquid fraction stored in open tanks. Probably, losses will be similar to that from unseparated digestates. Concerning N losses from the solid fibrous fraction, this fraction has still 70–80 % water, meaning that also a high NH4 + to total N share at the beginning of the storage period. Petersen and Sørensen (2008) reported that the losses of NH4 +-N and total N during storage of the fibrous fraction accounted for 30–90 and 10–55 % of the initial amounts, respectively. A higher total N at the start of the storage phase means a disproportionately high increase of N losses via ammonia volatilization and denitrification (Petersen et al. 1998). Furthermore, the low availability of easily degradable organic C compounds reduces the potential for N immobilization, increasing the risk of gaseous N losses (Chadwick 2005; Larney et al. 2006; Maurer and Müller (2012); McCrory and Hobbs 2001; Paillat et al. 2005; Petersen et al. 1998; Petersen and Sørensen 2008). Therefore, particularly manures including digestates with a high initial N fertilizer value loose a great part of this advantage through gaseous N losses during storage (Külling et al. 2003; Paul et al. 1998). Furthermore, anaerobic digestion affects manure pH, and the transformation and loss of ammonia are very sensitive to the pH value: Relatively low loss occurs below a pH of 6, and very high loss occurs when the pH exceeds 8 (Muck and Steenhuis 1982), pH values often also found in digestates.
In conclusion, the gaseous N losses from solid digestate heaps represent the main challenge regarding management of digestate stores after anaerobic digestion. Techniques dedicated to prevent N losses when storing manures are equally valid when storing digestates. Therefore, in accordance with the recommendations for solid farmyard manures, solid digestates should be, whenever possible, applied directly to land, thus bypassing the storage phase (Petersen and Sørensen 2008; Thorman et al. 2007). Further strategies to reduce gaseous N losses are covering and compaction of the solid manure heaps (e.g., Chadwick 2005; Hansen et al. 2006) as well as storage in high manure heaps (Dong et al. 2011). However, probably the most efficient strategies to reduce the N losses during storage are technical approaches, like drying of the solid fraction under controlled conditions with subsequent N recovery, or ammonia removal before or after solid-liquid separation. One approach is, e.g., the vacuum application and heating before manure separation for NH3 and CO2 volatilization with subsequent N recovery by stripping. There is a lack of systematic data on the efficiency of the different approaches to reduce N emissions, including the effects of supplementation of additives such as acids, gypsum, calcium, or magnesium chlorides to solid digestate stores.
4.2.2 Impact of anaerobic digestion on ammonia volatilization from field-applied manures
Several factors that potentially influence N losses by NH3 volatilization during and after field spreading are affected by anaerobic digestion: Reduction of dry matter content (e.g., Asmus et al. 1988) and viscosity (Senbayram et al. 2009; Baudez et al. 2011) can increase the infiltration of liquid manure into the soil, thereby decreasing the exchange surface of slurry with the atmosphere thus lowering NH3 volatilization (Rubæk et al. 1996; Sommer and Hutchings 2001). However, digestion leads simultaneously to an increase in manure pH (by 0.5–2.0 U) and of the ammonium concentration (relative increase of >20 %) (Möller and Müller 2012); both factors promote N losses via NH3 volatilization (e.g., Gericke et al. 2012; Ni et al. 2012; Sommer and Hutchings 2001). The pH value affects the ammonia dissociation rate (NH4 + + OH− ↔ H2O + NH3(aq) ↔ NH3(g)), the NH4 + concentration of the manure and the substrate availability. Furthermore, NH3 is a weak base that increases the pH value of the manure. Therefore, the increased NH4 + concentration in digestates has per se a double effect promoting NH3 volatilization.
In concordance with the described potential effects, contradictory results regarding the effects of anaerobic digestion on NH3 volatilization after field application of digestates have been reported in literature: Some researchers report a decrease of NH3 losses after soil application of digested animal manures, others report an increase of losses, and others did not found any or ambiguous effects (Table 3). Other research groups compared volatilization after spreading of undigested slurry and a digestate derived from slurry plus other feedstocks (e.g., Wulf et al. 2002a; Ni et al. 2012), an approach which do not allow for the assessment of the effect of anaerobic digestion itself on NH3 volatilization, requesting for a careful interpretation of the results. In some reports, digestates were used which lost considerable amounts of N during the digestate storage, a situation which does not match with the current state of the art (Table 3), and probably reduced the NH3 losses after digestate field spreading.
Reliable NH3 emission estimates could potentially be derived from mathematical models based on the physicochemical processes controlling NH3 volatilization from manures and their interactions with soil, canopy, and atmospheric variables. Gericke et al. (2012) modeled ammonia volatilization after digestate field application using a linear model. According to their model, a change of the temperature by +1 K or of the pH by +0.1 pH units of ammonia volatilization will increase by about 1.0 or 1.6 % of the total applied NH4 +-N, respectively. However, the pH range considered in the measurements was between 6.9 and 7.7, and total ammonium-N ranged between 1.75 and 2.72 kg NH4 +-N Mg−1 (Gericke et al. 2012). Digestates can have considerable higher NH4 +-N concentrations of up to 6.8 kg Mg−1 and pH values up to 9 (Möller and Müller 2012; Möller and Schultheiss 2014). And, increasing dissociation of NH4 + to NH3 (H3O+ + NH3 ↔ H2O + NH4 +) with increasing pH is an exponential function, and the acid dissociation constant (pKa) of NH4 +/NH3 is 9.25. Therefore, the model of Gericke et al. (2012) is an approach to assess ammonia losses in a pH range of most digestates available in practice (e.g., with cattle slurry or silage maize as feedstocks). It is probably not able to assess NH3 losses from digestates with very high NH4 +-N concentrations, which are simultaneously characterized by very high pH values (e.g., digestates from poultry or/and pig manures, cereal grains, kitchen wastes, and other digestates derived from N-rich feedstocks with a high bio-degradability). The combination of both characteristics strongly increases the potential for NH3 losses. There are only very few publications available about the combination of anaerobic digestion with other treatments (separation, acidification, flocculation, etc.) on ammonia volatilization after field application (Table 3), indicating the need for further research work about the direct and indirect effects of liquid manure treatment technologies on field N emissions after digestate spreading.
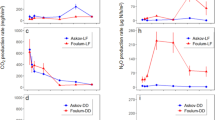
As conclusion, the higher pH and ammonia content in digestates counteracts the effects of the decreased viscosity. Therefore, application of digestates requires the implementation of more sophisticated field application techniques (Fig. 1a, b). Available models to assess these gaseous N losses were calibrated with digestates matching the characteristics of the most common digestates available in agriculture; however, they are probably less able to assess N losses of digestates derived from N-rich feedstocks with a high degradability.
4.2.3 Impact of anaerobic digestion on ammonia volatilization on the entire farm level
A holistic approach for assessment of the potential effects of anaerobic digestion on N losses via NH3 volatilization should include at least two relevant cropping system change-related effects: (i) Reduction of gaseous N losses during anaerobic digestion due to the closed storage of the feedstocks such as animal manures in the digestion facilities keeps higher N amounts within the nutrient cycle. Presumably, this will increase the risk of N losses in the subsequent manure handling steps, e.g., during and after field spreading, potentially resulting in a kind of pollution swapping. Furthermore, (ii) the use of crop residues, green manures, feedstocks from dedicated energy crops, and external feedstocks increases the amounts of total N and the amounts of NH4 +-N spread via organic manures (Möller 2009; Möller et al. 2011). Therefore, anaerobic digestion of crop residues or/and dedicated energy crops strongly increased the overall NH4 + losses due to an increase of the total amounts of liquid manures that are applied to fields and the related N losses via NH3 volatilization (Michel et al. 2010; Möller 2009), simultaneously increasing the eutrophication potential of the entire cropping system (Michel et al. 2010). In conclusion, implementation of an anaerobic digestion plant reduces the N losses during the first step of manure handling; however, it increases the risk of N losses during the later steps of manure handling and spreading, potentially resulting in pollution swapping. Therefore, a substantial reduction of ammoniacal N emissions during the manure management steps after the passage through the biogas digester is even more important than in undigested manures and required a holistic strategy which must include all the steps of manure handling (adequate digestate treatment including NH3 recovery, gas-tight stores for liquid fractions, immediate disposal or drying of the solid fraction, sophisticated digestate spreading techniques, use of additives like sulfuric acid, etc.) and direct soil incorporation after field spreading to avoid field N losses by ammonia volatilization.
4.3 Nitrous oxide emissions
4.3.1 Impact of anaerobic digestion on N2O emissions during manure storage
Greenhouse gas emissions, expressed as CO2 equivalents, from liquid slurry or solid manure stores are very often higher than emissions after field application (Clemens et al. 2006; Michel et al. 2010). In manure stores, substantial amounts of N can be emitted as N2O (Hansen et al. 2006; Külling et al. 2003; Massé et al. 2011; Wang et al. 2014). Due to the low oxygen partial pressure in liquid slurry stores with a gas-tight cover, N2O emissions are negligible since oxygen is a prerequisite for N2O formation. Another situation arises when digestates are stored in open stores before field application: During the winter, emissions from digested and undigested manures are similar, while in the summer, the amounts emitted as N2O are twice as high from digested than from undigested liquid manure stores (Amon et al. 2006). After a solid-liquid separation, the solids are generally stored in open heaps (Fig. 1c), and during storage and management, these heaps are often turned/reallocated many times before field application (Möller et al. 2010). Stores of solid manure as well as digestates provide oxic and anoxic conditions within close proximity (Hansen et al. 2006), and they can be a considerable source of N2O. Currently, only few data are available about N2O emissions from separated solid digestates; most data are collected from solid animal manure heaps. Hansen et al. (2006) found that 4.8 % of the initial N content was lost as N2O during the storage of the fibrous solid fraction from separated digestates of pig manure. The relatively narrow C/N ratio ranging between 11.2 and 19.3 and the high share of NH4 +-N to total N (ranging between 26.0 and 49.4 %) measured in solid separated digestates directly after the separation process (Möller and Müller 2012) can explain the very high emission rates determined in such manure heaps. However, our understanding of the relevance of the composition of the solid manure and the different processes leading to N2O emissions in a solid digestate manure heap is low:
-
It was stated that N2O emissions from stored manures with high concentrations of NH4 + are produced during nitrification (Hao et al. 2005; Hao 2007; Yamulki 2006). Other authors stated that increased N2O emissions resulted as an intermediate product of denitrification (Lipschultz et al. 1981; Petersen et al. 1998). In the presence of O2, the NH4 +-N is nitrified to nitrate (NO3 -), which is highly mobile and therefore moves, by means of diffusion, within the solid manure or solid digestate heap to anoxic sections within the manure heap. In anoxic sections, nitrate is denitrified to N2O and N2.
-
The high NH4 +-N contents and the resulting NO3 −-N contents provide high substrate availability for the different processes leading to N2O formation. A high nitrate formation within the manure heap may also lead to the inhibition of the N2O reductase and therefore the reduction from N2O to N2, hence favoring the N2O release from denitrification (Swerts et al. 1996; Yamulki et al. 1995).
The pH value of manures is affected by the anaerobic digestion process (Möller and Müller 2012) and can also be influenced by CO2 removal or the addition of additives like sulfuric acids. No data were found about the effects of the pH value on the microbial activity in manure heaps and possible direct and indirect effects on N2O formation. According to Suzuki et al. (1974), the NH3 concentration as substrate for the ammonia monooxygenase enzyme decreases exponentially compared to NH4 + as pH declines. In contrast to NH3, uptake of NH4 + by microorganisms requires an active, energy-consuming mechanism of membrane transport, and therefore an energy source which may be limited at lower pH value (Burton and Prosser 2001). Baggs et al. (2010) found a pH-induced change in the source strength of N2O production in soils. A higher pH value of the soil solution can increase total N2O emissions or shift the predominance away from denitrification to ammonia oxidation. Probably, similar effects drive the processes in manure heaps.
It can be concluded that gas-tight storage of digestates is the most effective measure to reduce N2O emissions from liquid as well as solid digestates. Especially, solid digestate heaps are prone to emit huge amounts of N species, as the available techniques to reduce these N emissions are less efficient than for liquid digestates and difficult to implement under practical farming conditions. However, there is still a lack of data improving our understanding of the processes influencing the N2O and N2 formation in solid manure heaps depending on the several variables influenced by anaerobic digestion (NH4 +-N to total N ratio, Corg/N ratio, pH value, C availability, H2S content, etc.). Furthermore, no comparative studies were found about the influence of specific feedstocks on digestate composition and the effect of differences in digestate composition on subsequent N2O losses during storage.
4.3.2 Impact of anaerobic digestion on N2O emissions from field-applied manures
Due to the decomposition of the easily degradable C compounds during anaerobic digestion, the viscosity of manures becomes lower and, as stated above, the amounts of easily degradable C added to the soil decreased considerably. Consequently, less anoxic microsites, favorable for denitrifying activities, might emerge, and it can be assumed that anaerobic digestion will reduce N2O emissions after manure field spreading (e.g., Möller and Stinner 2009). Another hypothesis is that treatment technologies reducing the viscosity (e.g., due to degradation of organic matter) have the potential to reduce N2O emissions, as dissolved C and N are dispersed into a larger soil volume, changing the balance between aerobic and anaerobic decomposition (Petersen and Sommer 2011). Most of the available studies confirmed lower N2O emissions after digestate application in comparison to undigested feedstocks (Table 4). However, there are also some contradictory results. Miller et al. (2009) found a negative relationship between soil respiration and the N2O molar ratio, demonstrating that C availability in soil promotes the reduction of N2O to N2. This is in line with a conceptual model from Thomsen et al. (2010) which considers the ratio between O2 supply and O2 consumption as the main driving variable for changing N2/N2O ratios. Therefore, effects of a manure treatment affecting its biological and chemical oxygen demand will depend on soil conditions. In a relatively dry or inactive soil, an increase of the N2O fluxes can be expected by a slurry treatment such as anaerobic digestion, whereas a net decrease would result if the treated manure is applied to a soil where conditions are already conducive to denitrification, leading to an enhanced N2O reduction to N2, and thus to a higher N2/N2O ratio. Furthermore, some of the results published indicate an interaction of the effects of anaerobic digestion with other soil properties (Chantigny et al. 2007; Eickenscheidt et al. 2014). The soil type seems to influence the effects of manure treatments, as in a loamy soil, the reduction was significantly stronger than in a sandy soil (Chantigny et al. 2007), probably due to differences in soil water and aeration status and the related effects on the redox potential in the soil. The soil organic matter content influences also the effects of manure treatments, N2O emissions increase with increasing soil Corg content probably due to more favorable conditions for denitrification (Chantigny et al. 2010; Pelster et al. 2012; Eickenscheidt et al. 2014). Chantigny et al. (2010) as well as Pelster et al. (2012) argued that in soils low in Corg, N2O production responds to the manure C inputs, whereas in soils higher in C, N2O production is stronger related to the NO3 availability.
The feedstocks used for anaerobic digestion and the degradability of the remaining organic matter in the digestates influence the N2O emissions after field application (Johansen et al. 2013). Consequently, digestates with a high degradability of the organic matter such as grass-clover caused a significantly stronger increase of the N2O emissions than, e.g., digestates derived from maize digestion with a lower short-term biodegradability. Also, the treatment of digestates after anaerobic digestion by solid-liquid separation has the potential to influence the manure-induced field N2O emissions, as the Corg and the N compounds are partially segregated. However, no systematic studies about the specific effects of the different treatment steps on potential emissions were found. Bertora et al. (2008) compared the combined effects of anaerobic digestion and separation on N2O emissions; they measured lower N2O emissions from a loamy soil treated with either liquid or solid portions of treated pig slurry compared to the raw pig slurry. Chantigny et al. (2007, 2010) used the liquid fraction of treated pig slurry and measured similar or lower field N2O emissions from the treated slurry fractions compared with the raw pig slurry. The results of these studies do not allow an assessment of the effects of each of the single treatment steps on soil N2O emissions, as the difference to the control treatment comprises two sub-treatments, anaerobic digestion, and subsequent separation. As indicated previously, available data on N2O emissions after soil application of separated digestates indicated not only lower emissions after field application of the liquid fraction but also after field incorporation of the organic matter-rich solid residues (Schauss et al. 2006; Bertora et al. 2008; Möller and Stinner 2009; Collins et al. 2011). Currently, there is no explanation available for lower N2O emissions from each of the fractions after separation in comparison to an unseparated digestate. One possible reason for the lower N2O emissions after soil incorporation of the solid digestates can be the composting processes in the store before field spreading induced due to aeration after digestate separation.
It can be concluded that most findings indicate a reduction of the soilborne N2O emissions after application of digestates in comparison to the undigested feedstocks; however, the effects are influenced by several environmental conditions including soil water content, soil type, and soil organic matter content.
4.3.3 Cropping system change-based impacts on field N2O emissions
The implementation of anaerobic digestion plants often leads to broad changes in crop acreage and crop rotation. In Germany, mainly acreage of silage maize increased at the cost of cereals, rapeseeds, and other crops, when comparing acreage of crops before and after implementation of a biogas plant (e.g., Kruska and Emmerling 2008; Möller et al. 2011). Results of whole-year inventories on the effects of implementation of cropping feedstocks for digestion in biogas plants on the N2O emissions from soils at the level of the entire cropping system are contradictory. Silage maize cropping is related to large N2O emissions per unit area in comparison, for example, to cereal cropping. Therefore, implementation of biogas plants with silage maize as main feedstock is potentially linked to a large increase of total N2O emissions (e.g., Dittert et al. 2009). However, the implementation of anaerobic digestion plants also enables a use of the residues otherwise incorporated directly into the soil (crop residues, green manure crops like cover crops or clover-grass-leys) leading to a substantial decrease of soilborne N2O emissions as indicated by few studies presented in Table 4 (Möller and Stinner 2009; Nadeem et al. 2012). The harvest and anaerobic digestion of clover/grass leys, crop residues, and cover crops in autumn results in the removal of large amounts of N and its storage in “closed” stores during winter (Stinner et al. 2008; Gunnarsson et al. 2011; Nadeem et al. 2012), a period with substantial N2O losses which might account for 50 % or even more of the annual N2O flux (Flessa et al. 1995; Kaiser and Ruser 2000).
Whole-year inventories indicated that most soil N2O fluxes occurred within 20–40 days after fertilization (Schauss et al. 2006; Möller and Stinner 2009; Chantigny et al. 2010). However, for an assessment of cropping system change-related effects, whole-year inventories are mandatory, as very often, the entire effects are composed by several partial effects. For example, Schauss (2006) as well as Möller and Stinner (2009) reported a reduction of soilborne N2O fluxes after green manure removal in autumn and, simultaneously in spring, an increase of N2O fluxes after field application of the digestates obtained from those green manure feedstocks. However, the reduction in autumn was stronger than the increase of at the beginning of the following growing season, leading to a total reduction of field emissions.
The experiments reported by Schauss (2006) were carried out with frost-sensitive cover crops; however, the implementation of biogas plants enables farmers to use winter cover crops as a further feedstock for anaerobic digestion. Winter cover cropping alters the soil water and the soil nitrate N content during the winter period (McCracken et al. 1994; Parkin et al. 2006) due to plant uptake, which are two main drivers and regulators of the processes in soils leading to denitrification (e.g., Ciarlo et al. 2007; Dobbie and Smith 2003; Miller et al. 2009; Ruser et al. 2001; Skiba et al. 1998). The NO3 − concentration in soil solution influences N2O emissions not only by the substrate availability but also by the molar ratio of N2O and N2 during denitrification: The higher the NO3 − concentration, the lower the N2/N2O ratio (Blackmer and Bremner 1978; Miller et al. 2009). A (winter) cover crop also influences the soil water household and soil mineral N content during establishment of the following main crop, possibly also influencing emissions during the main crop growth, due to its indirect influence on soil conditions. No studies have been found with respect to the influence of winter cover cropping, followed by a spring-sown main crop, on soil total N2O emissions in whole-year inventories.
It can be concluded that there are only few publications available assessing the effects based on changes of cropping system effects on N2O emissions. These cropping system-based effects have the potential to be of much higher relevance for the overall emission inventories than the direct effects of digestate application.
5 Effects of anaerobic digestion on soil properties and soil biological activity
There are only few studies about the effect of digestates on soil physical properties. Application of digestates improved soil properties by reducing the bulk density, increasing saturated hydraulic conductivity, moisture retention capacity of soils (Garg et al. 2005; Beni et al. 2012), and aggregate stability (Beck and Brandhuber 2012; Beni et al. 2012; Erhart et al. 2014; Frøseth et al. 2014), compared to an untreated control. However, an assessment on the long-term direct effects of digestate application on soil physical properties in comparison to the undigested feedstock is not available. Simultaneously, indirect effects due to changes in the cropping system (e.g., overall crop acreage) are expected. In conclusion, a minor direct effect of digestion on the soil physical properties is expected; the potentially major impact is expected for indirect effects related to changes in the cropping system.
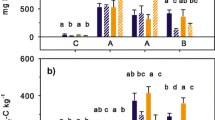
Many reports indicate an enhanced soil microbial activity after field applications of digestates in comparison to inorganic fertilizers or untreated controls (Alburquerque et al. 2012b; Bachmann et al. 2011, 2014; Galvez et al. 2012; Kautz and Rauber 2007; Lošák et al. 2011; Odlare et al. 2008, 2011; Ross et al. 1989; Schröder et al. 1996; Walsh et al. 2012a, b; Clements 2013). Elste et al. (2010) reported that soil application of digestates enhanced the abundance and biomass of earthworms. From all these reports, it can be concluded that digestate application per se enhanced the soil biological activity. However, comparisons of parameters describing the soil microbial activity such as basal respiration, substrate-induced respiration, specific growth rate, metabolic quotient, or N mineralization capacity indicate that the effects of digestate application on promotion of the soil microbial activity are in the short-term view lower than the effects of application of the undigested feedstock (Merz 1988; Reinhold et al. 1991; Schröder et al. 1996). The available data show an interaction with the specific experimental setup. In cropped fields, very often, no or minor differences in soil biological activity were measured in comparison to application of undigested feedstocks (Allan et al. 2003; Schauss et al. 2006; Terhoeven-Urselmans et al. 2009; Clements et al. 2012; Walsh et al. 2012b; Bachmann et al. 2014), whereas in an experimental setup carried out on fallow land, the differences between undigested and digested manures were very often significant (Friedel et al. 1996; Ernst et al. 2008; Terhoeven-Urselmans et al. 2009). Simultaneously, the influence of anaerobic digestion on soil microbial activity is also mediated by the used feedstock (Johansen et al. 2013). Digestates with a high degradability of the organic matter such as clover-grass have a stronger effect on the short-term soil microbial activity than digestates with a low degradability such as silage maize. However, there are some indications that in a medium-term view of several months or even years, the differences in the effects of application of digestates in comparison to the undigested feedstocks are minor or even negligible (Schröder et al. 1996; Schauss 2006). Schauss et al. (2006) reported for example that after approx. 4 years of conversion, soil in situ potential denitrification and nitrification activities did not differ significantly between the fertilization treatments with and without application of digestates obtained from several kinds of by-products originated from the same crop rotation. Moreover, investigations of soil respiration, microbial biomass C, water-extractable C, total C content, and the carbon-source utilization assay were similar in all treatments, despite very large differences in total C inputs due to anaerobic digestion of crop residues and cover crops in some of the biogas treatments (Fig. 2). The substrate-induced respiration was strongly affected by total N inputs than by the C inputs, whereas basal respiration and the soil microbial biomass were not affected by the overall C and N inputs. However, there are some indications that application of digestates instead of the undigested feedstock indirectly changes the soil microbial community by a differentiated effect of digestate application on soil microbial activity. For example, there are some indications in a number of trials that earthworm populations react differently to slurry or digestate applications, with Lumbricus terrestris preferring slurry and Eisenia fetida preferring digestate (Clements 2013). Another study indicates that digestates did not influence earthworm populations, whereas earthworms were positively influenced by the green manure crop otherwise used as feedstock for anaerobic digestion (Frøseth et al. 2014).
Influence of the mean annual C and N inputs via organic amendments and digestates on soil N and C parameters (left) and on parameters describing soil biological activity (right) after a period of 4 years of differentiated manure management (data soil N and C inputs, soil N and C content, soil C/N ratio: Möller 2009; data soil water extractable C content: Schauss et al. 2006; Schauss 2006).
Alburquerque et al. (2012b) found a significant increase in alkaline phosphatase activity (linked to the P cycle) in soils treated with digestates; however, no changes in β-glucosidase (linked to the carbon cycle), urease, and protease (both linked to the N cycle) activities were measured. Also, Bachmann et al. (2014) reported that the activities of dehydrogenase and alkaline phosphatase were 50 % lower in the soils that were amended with digestate compared with input not digested feedstock. These effects were probably mediated by the lower inputs of easily available Corg and Norg sources added with the digestates. In line with these findings, Kautz and Rauber (2007) reported an increase of the dehydrogenase activity, whereas the β-glucosidase activity did not respond to digestate application, indicating that digestate application has no effects to parameters linked to the C inputs, but large influence on parameters linked to nutrient supply. Also, Chen et al. (2012) found an increase in soil microbial biomass and a significant promotion of chitinase and leucine amino peptidase activities (related to N-cycle) but no effects of digestate application on three tested enzymes b-glucosidase, cellobiohydrolase (involved in cellulose decomposition), and xylanase (involved in hemicellulose decomposition). The activity of chitinase and leucine amino peptidase is promoted by N-enriched organic components, e.g., peptidoglucan accumulated as microbial residues during the biogas fermentation (Chen et al. 2012). Furthermore, a clear shift in the structure of the microbial community in response to digestate application in comparison to an undigested feedstock was reported (Chen et al. 2012; Abubaker et al. 2013). Due to digestate application, the slowly growing microorganism became dominant and a transition of r to K strategists took place. This shift seems to be related to changes in the fungi-to-bacteria ratio (Chen et al. 2012). Abubaker (2012) also concluded that his findings indicate that two different types of microbial shifts occur in residue-amended soils, one faster shift directly correlated with functional properties and one slower shift that may be attributed to altered microbial community composition. Simultaneously, the effects of adding digestates and undigested cattle slurry on bacterial community structure were greatest in the sandy soil (Abubaker et al. 2013).
From a methodological point of view, any assessment of the potential effects of implementation of biogas plants on soil properties and soil biological activity in comparison to a similar reference cropping system without anaerobic digestion depends on the definition of the reference system. The available results are sometimes difficult to interpret because of the fact that the comparisons were often performed based on equivalent amounts of total N or total fresh matter applied. Such comparisons do not take into consideration the differences in mass losses (mainly C and N losses) which occur during the entire cycle started with the generation of the original feedstocks or wastes, until the final field application. For example, based on application of equivalent amounts of fresh matter manure, Singh et al. (2007) reported that the improvement of the soil aggregate mean weight diameter tended to be higher after the application of biogas slurry than after application of solid farmyard manures. This approach did not take into account that much less feedstocks are commonly needed to get the same amounts of fresh matter digestates in comparison to solid farmyard manures. Odlare et al. (2008) compared the effects of composted and digested household wastes by the application of fertilizer rates equivalent to 100 kg N ha−1 year−1, without any consideration of the fact that to obtain 100 kg N of composted fertilizer, much more original feedstocks are required than for 100 kg N of digestates from the same feedstock. However, a holistic assessment of the effects of different treatment methods must include the whole chain effects and not only the final products.
From the presented results, it became obvious that the selective degradation of the easily degradable compounds during anaerobic digestion affects mainly the soil microbial activity shortly after manure application and, on a longer perspective, potentially the composition of the soil microbial community. In a system with anaerobic digestion of residues and wastes in a biogas plant, the ecological function of some soil organisms is no longer fully necessary to decompose the supplied organic matter and mineralize the nutrients, as the respective decomposition step was formerly performed by the microorganisms in the digester under more controlled conditions. From the available literature, it remains unclear, whether the anaerobic digestion itself or changes in the cropping system such as changes in the crop acreage and crop diversity triggered by the implementation of the biogas plants are the main driving factors for any changes in soil microbial or soil physical properties. All these factors will potentially affect soil microbial population and activity, as well as the soil fauna composition (Zak et al. 2003; Vepsäläinen et al. 2004). From a methodological point of view, any assessment of potential effects of the implementation of anaerobic digestion should take into consideration the system boundaries as well as the overall mass flows affected by the entire treatment chain.
6 Effects of anaerobic digestion on soil organic matter
6.1 Effects of anaerobic digestion of farm wastes
Little information is available about the long-term field effects of anaerobic digestion on the soil organic matter level. Adequate soil inputs of organic matter are important for maintaining the fertility of arable soils, since arable soils under intensive management tend to lose C (e.g., Schjønning et al. 2009). There are congruent reports about a lower short-term carbon mineralization and consequently a higher recalcitrance of digested manure in comparison to undigested slurry (Table 5). In field experiments, Möller (2009) did not found any difference in the total N and total C content of soils after 4 years of treatment either with digested or undigested slurry (Fig. 2). Similar results regarding soil C were recorded by Bachmann et al. (2014) after 3 years of maize cropping and by Erhart et al. (2014) regarding the soil humus balance of different management options of green manures with and without anaerobic digestion. In an incubation experiment, Reinhold et al. (1991) reported that the amounts of organic matter remaining in the soil after degradation of the easily degradable compounds were similar for pig slurry and the digestate obtained from this manure. A recently published study of Thomsen et al. (2013) confirmed these findings and suggests that on a longer-term perspective, the retention of plant-derived C in soil is little affected by pretreatments such as passage through a ruminant and/or anaerobic digestion. Fouda (2011) carried out a pot experiment with different digestates and with undigested cattle slurry as a control treatment. Regardless of anaerobic digestion, soil C accumulation was similar, although the Corg input in the undigested treatment was nearly twice as high as in the anaerobic digestion treatment. These results are in agreement with results reported by Marcato et al. (2009) and Sánchez et al. (2008). Therefore, it can be concluded that C losses during the anaerobic digestion process are compensated by the lower C degradation after field application of the fertilizer.
6.2 Influence of feedstocks
The type of feedstock to biogas plants influences the properties of the by-product digestate and also the C and N dynamics after application of the digestates (Sänger et al. 2014; Fouda 2011). Fouda (2011) compared the effects of digestates from different mixtures of feedstocks on soil C accumulation based on the application of equivalent NH4 + amounts. In spite of much higher total C inputs in clover-grass digestates (factor 1.5–2), the soil C accumulation was not different in the treatments with feedstock mixtures rich in silage maize. The lowest C accumulation was measured with digestates derived from mixtures rich in concentrates, either directly introduced as grain grist or indirectly introduced as poultry manure, which in turn was derived from a concentrate-rich feed ration (Fouda et al. 2013). The soil C inputs were much lower for the digestates from concentrates than from digestates from feedstocks rich in fibers (Fouda et al. 2013). The high degradability of digestates from clover-grass was confirmed by findings reported by Johansen et al. (2013).
It can be concluded that available results indicate a significant effect of feedstock-depending composition of digestates on the soil organic matter. Digestates derived from concentrates seem to accumulate less amounts of C in soils. Digestates from clover/grass had a lower specific C accumulation rate in soils than digestates from silage maize, showing similar soil Corg accumulation in spite of large differences in total Corg inputs.
6.3 Impact of effects related on changes of the cropping system
Independently of the short- and long-term direct effects of anaerobic digestion of manures on soil C and N turnover, any approach to assess the long-term effects of the implementation of an anaerobic digester on soil fertility needs to take into account the entire cropping system. Implementation of biogas plants is often related to cropping of new crops or adoption of other crop management systems such as changed harvesting times, removal of crop residues as feedstock, implementation of new energy crops or semi-perennial arable crops, double-cropping systems, etc.. There are only single studies available describing these effects. In an experiment assessing the influence of only animal manure digestion, combined animal manure, and crop residue treatment and other treatments with the usual management (direct incorporation of animal manures, crop residues, and green manure crops) over a period of 4 years, the soil water extractable C content was not correlated, or even negatively correlated, to total C and N inputs, possibly indicating that C priming effects in the treatments without anaerobic digestion and consequently with large inputs of organic C have affected the soil water extractable C content (Fig. 2). Priming effects related to application of digestates and non-digested feedstocks were also reported by Bernal and Kirchmann (1992) (Table 5). Furthermore, no influence of total C and N inputs on changes of soil total N and soil Corg contents were measured in spite of large differences in total organic matter inputs.
It was concluded that the direct influence of anaerobic digestion on soil organic matter content are even in a long-term view probably negligible. Probably, indirect effects induced by changes in the entire cropping system related to anaerobic digestion implementation such as crop rotation or acreage of single crops are much more relevant for the effects on long-term soil fertility than direct effects of anaerobic digestion.
7 Conclusions and research needs
Anaerobic digestion affects a lot of characteristics of the treated feedstocks, which potentially also affects soil processes and N emissions after field spreading. Studies on the consequences of anaerobic digestion on the subsequent turnover of N and C in soil generally rest on different composition of the starting feedstocks. This is a crucial drawback because the differences between digested materials and their respective non-digested feedstocks in their effect on soil N and C turnover and in contributions to soil C storage is small compared to the amount of N and C already residing in the soil and therefore difficult to quantify over shorter time-span. Furthermore, any assessment of the effects of anaerobic digestion on emissions and soil properties of farming activities should take into account possible effects on different scales (e.g., field and farm scale), design of the manure store and manure treatment after digestion (e.g., open or closed digestate store, solid-liquid separation, and subsequent treatment of the solid fraction), as well as changes in the entire cropping system triggered by introduction of the anaerobic digestion plant such as changes in the crop acreage and crop diversity, and total amounts of available organic manures. Main direct effects of anaerobic digestion on a farm level are the influence on gaseous emissions during manure treatment and handling, whereas the direct effects of anaerobic digestion on a field level on NH3 emissions and on soil organic matter are negligible or at least ambiguous. The main direct effects of anaerobic digestion on the field level are short-term effects on soil microbial activity and changes in the soil microbial community, as the decomposition process of biomass is transferred from the soil to more controlled conditions in a biogas plant, reducing the activity of C decomposers in soils. Very often, direct effects of anaerobic digestion include a moderate decrease of the N2O emissions, as less amounts or readily available C is provided. Therefore, most of the direct effects of anaerobic digestion on soil properties and soil fertility are of short-term character; our current understanding regarding long-term effects on soil fertility indicate a minor direct influence of anaerobic digestion. Carbon losses during the anaerobic digestion process are mostly compensated for by lower organic matter decomposition after field application, attenuating any possibly negative effect of anaerobic digestion on soil organic matter content and humus reproduction. The most relevant effects on soil fertility as well as on N emissions will be expected from indirect effects related to cropping system changes such as changes in crop rotation, crop acreage, cover cropping, total amounts of organic manures, etc. The overall effects depend also from the specific setup of the biogas plant (e.g., design of the digestate stores), the composition of feedstocks, and the reference system.
Currently, a wide range of feedstocks of very different composition regarding plant nutrients and organic matter composition (degradability, nutrient concentration, water content) are used for anaerobic digestion (Weiland 2010). Therefore, more research is needed concerning the influence of single feedstocks and their characteristics on digestate composition (Corg and Norg content and fractions, SO4 −2 content, etc.) and on the effects triggered by digestate application to soils (nitrous oxide emissions, soil carbon household, soil biological activity, etc.). Another major challenge related to digestate treatment after anaerobic digestion is the improvement of the management of solid fraction of the digestates after a solid-liquid separation in terms of reduction of the overall emissions. These techniques could include drying combined with ammonia recovery by stripping in acids, or ammonia removal by heating and partial vacuum also combined with ammonia recovery, or the use of additives like gypsum, acids, or chlorides. These techniques possibly also influence the substrate availability for nitrifiers and denitrifiers, potentially also affecting the N2O emissions.
More research is also needed regarding cropping system-based changes and their interaction with the changes induced by the anaerobic digestion process on the different soil processes, meaning the interaction of direct and indirect effects induced by the implementation of biogas plants. These potential cropping system-based changes induced by introduction of biogas plants are probably much more relevant for the overall performance and sustainability of the cropping system than the direct effects triggered by application of digestates in comparison to the undigested feedstocks.
References
Abubaker J (2012) Effects of fertilisation with biogas residues on crop yield, soil microbiology and greenhouse gas emissions—recycling of plant nutrients from bioenergy production. PhD thesis Swedish University of Agricultural Sciences, Uppsala
Abubaker J, Cederlund H, Arthurson V, Pell M (2013) Bacterial community structure and microbial activity in different soils amended with biogas residues and cattle slurry. Appl Soil Ecol 72:171–180. doi:10.1016/j.apsoil.2013.07.002
Alburquerque JA, de la Fuente C, Bernal MP (2012a) Chemical properties of anaerobic digestates affecting C and N dynamics in amended soils. Agric Ecosyst Environ 160:15–22. doi:10.1016/j.agee.2011.03.007
Alburquerque JA, de la Fuente C, Campoy L, Nájera I, Baixauli C, Caravaca F, Roldán A, Cegarra J, Bernal MP (2012b) Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur J Agron 43:119–128. doi:10.1016/j.eja.2012.06.001
Allan D, Katovich E, Nelson C (2003) Fertilizer value and weed seed destruction potential of digested manure. Available at: http://agrienvarchive.ca/bioenergy/download/fert_weed_destruct_digestedmanure_MN.pdf. downloaded 12 Sept 2011
Ammann C, Spirig C, Leifeld J, Neftel A (2009) Assessment of the nitrogen and carbon budget of two managed temperate grassland fields. Agric Ecosyst Environ 133:150–162. doi:10.1016/j.agee.2009.05.006
Amon B, Kryvoruchko V, Amon T (2006) Influence of different methods of covering slurry stores on greenhouse gas and ammonia emissions. Int Congr Ser 1293:315–318
Asmus F, Linke B, Dunkel H (1988) Eigenschaften und Düngerwirkung von ausgefaulter Gülle aus der Biogasgewinnung. Arch Acker- Pflanzenbau Bodenkd Berl 32:527–532
Bachmann S, Wentzel S, Eichler-Löbermann B (2011) Co-digested dairy slurry as a phosphorus and nitrogen source for Zea mays L. and Amaranthus cruentus L. J Plant Nutr Soil Sci 174:908–915. doi:10.1002/jpln.201000383
Bachmann S, Gropp M, Eichler-Löberman B (2014) Phosphorus availability and soil microbial activity in a 3 year field experiment amended with digested dairy slurry. Biomass Bioenergy. doi:10.1016/j.biombioe.2014.08.004
Baggs EM, Smales CL, Bateman EJ (2010) Changing pH shifts the microbial source as well as the magnitude of N2O emission from soil. Biol Fertil Soils 46:793–805. doi:10.1007/s00374-010-0484-6
Battini F, Agostini A, Boulamanti AK, Giuntoli J, Amaducci S (2014) Mitigating the environmental impacts of milk production via anaerobic digestion of manure: case study of a dairy farm in the Po Valley. Sci Total Environ 481:196–208. doi:10.1016/j.scitotenv.2014.02.038
Baudez JC, Markis F, Eshtiaghi N, Slatter P (2011) The rheological behaviour of anaerobic digested sludge. Water Res 45:5675–5680. doi:10.1016/j.watres.2011.08.035
Beck R, Brandhuber R (2012) Effekte der Gärrestdüngung auf Humusbilanz und Bodenstruktur—Zwischenbilanz. In: Bayerische Landesanstalt für Landwirtschaft (ed) Schriftenreihe no. 11/2012: Düngung mit Biogasgärresten—effektiv-umweltfreundlich-bodenschonend. ISSN 1611-4159, pp 49–58
Beni C, Servadio P, Marconi S, Neri U, Aromolo R, Diana G (2012) Anaerobic digestate administration: effect on soil physical and mechanical behavior. Commun Soil Sci Plant Anal 43(5):821–834. doi:10.1080/00103624.2012.648359
Bernal PM, Kirchmann H (1992) Carbon and nitrogen mineralization and ammonia volatilization from fresh, aerobically and anaerobically treated pig manure during incubation with soil. Biol Fertil Soils 13:135–141. doi:10.1007/BF00336268
Bertora C, Alluvione F, Zavattaro L, van Groenigen JW, Velthof G, Grignani C (2008) Pig slurry treatment modifies slurry composition, N2O, and CO2 emissions after soil incorporation. Soil Biol Biochem 40:1999–2006. doi:10.1016/j.soilbio.2008.03.021
Blackmer AM, Bremner JM (1978) Inhibitory effect of nitrate on reduction of N2O to N2 by soil microorganisms. Soil Biol Biochem 10:187–191. doi:10.1016/0038-0717(78)90095-0
Brozyna MA, Petersen SO, Chirinda N, Olesen JE (2013) Effects of grass-clover management and cover crops on nitrogen cycling and nitrous oxide emissions in a stockless organic crop rotation. Agric Ecosyst Environ 181:115–126. doi:10.1016/j.agee.2013.09.013
Burton SAQ, Prosser JI (2001) Autotrophic ammonia oxidation at low pH through urea hydrolysis. Appl Environ Microbiol 67:2952–2957. doi:10.1128/AEM. 67.7.2952-2957
Chadwick DR (2005) Emissions of ammonia, nitrous oxide and methane from cattle manure heaps: effect of compaction and covering. Atmos Environ 39:787–799. doi:10.1016/j.atmosenv.2004.10.012
Chantigny MH, Rochette P, Angers DA, Massé D, Côte D (2004) Ammonia volatilization and selected soil characteristics following application of anaerobically digested pig slurry. Soil Sci Soc Am J 68:306–312. doi:10.2136/sssaj2004.3060
Chantigny MH, Angers DA, Rochette P, Belanger G, Massé DI, Côté D (2007) Gaseous nitrogen emissions and forage nitrogen uptake on soils fertilized with raw and treated swine manure. J Environ Qual 36:1864–1872. doi:10.2134/jeq2007.0083
Chantigny MH, Angers DA, Bélanger G, Rochette P, Eriksen-Hamel N, Bittman S, Buckley K, Massé D, Gasser MO (2008) Yield and nutrient export of grain corn fertilized with raw and treated liquid swine manure. Agron J 100:1303–1309. doi:10.2134/agronj2007.0361
Chantigny MH, MacDonald JD, Beaupré C, Rochette P, Angers DA, Massé D, Parent LE (2009) Ammonia volatilization following surface application of raw and treated liquid swine manure. Nutr Cycl Agroecosyst 85:275–286. doi:10.1007/s10705-009-9266-7
Chantigny MH, Rochette P, Angers DA, Bittman S, Buckley K, Massé D, Belanger G, Eriksen-Hamel N, Gasser MO (2010) Soil nitrous oxide emissions following band-incorporation of fertilizer nitrogen and swine manure. J Environ Qual 39:1545–1553. doi:10.2134/jeq2009.0482
Chen R, Blagodatskaya E, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Kuzyakov Y (2012) Decomposition of biogas residues in soil and their effects on microbial growth kinetics and enzyme activities. Biomass Bioenergy 45:221–229. doi:10.1016/j.biombioe.2012.06.014
Ciarlo E, Conti M, Bartoloni N, Rubio G (2007) The effect of moisture on nitrous oxide emissions from soil and the N2O/(N2O + N2) ratio under laboratory conditions. Biol Fertil Soils 43:675–681. doi:10.1007/s00374-006-0147-9
Clemens J, Huschka A (2001) The effect of biological oxygen demand of cattle slurry and soil moisture on nitrous oxide emissions. Nutr Cycl Agroecosyst 59:193–198. doi:10.1023/A:1017562603343
Clemens J, Trimborn M, Weiland P, Amon B (2006) Mitigation of greenhouse gas emissions by anaerobic digestion of cattle slurry. Agric Ecosyst Environ 112:171–177. doi:10.1016/j.agee.2005.08.016
Clements LJ (2013) The suitability of anaerobic digesters on organic farms. PhD thesis, University of Southhampton. Available at: http://eprints.soton.ac.uk
Clements LJ, Salter AM, Banks CJ, Poppy GM (2012) The usability of digestate in organic farming. Water Sci Technol 66:1864–1870. doi:10.2166/wst.2012.389
Collins HP, Alva AK, Streubel JD, Fransen SF, Frear C, Chen S, Kruger C, Granatstein D (2011) Greenhouse gas emissions from an irrigated silt loam soil amended with anaerobically digested dairy manure. Soil Sci Soc Am J 75:2206–2216. doi:10.2136/sssaj2010.0360
Cuetos MJ, Morán A, Otero M, Gómez X (2009) Anaerobic co-digestion of poultry blood with OFMSW: FTIR and TG-DTG study of process stabilization. Environ Technol 30:571–582. doi:10.1080/09593330902835730
Dalgaard R, Olesen JE, Halberg N, Berntsen J (2004) Miljøeffekter og energibalancer ved energiproduktion på økologiske planteavlsbedrifter. In: Jørgensen, U., Dalgaard, T. (Eds.) Energi i økologisk jordbrug. FØJO rapport nr. 19:103–123
Dittert K, Senbayram M, Wienforth B, Kage H, Muehling KH (2009) Greenhouse gas emissions in biogas production systems. The Proceedings of the International Plant Nutrition Colloquium XVI UC Davis. Permalink: http://escholarship.org/uc/item/18p5q83f
Dobbie KE, Smith KA (2003) Nitrous oxide emission factors for agricultural soils in Great Britain: the impact of soil water-filled pore space and other controlling variables. Global Change Biol 9:204–218. doi:10.1046/j.1365-2486.2003.00563.x
Dong H, Zhu Z, Zhou Z, Xin H, Chen Y (2011) Greenhouse gas emissions from swine manure stored at different stack heights. Anim Feed Sci Technol 166–167:557–561. doi:10.1016/j.anifeedsci.2011.04.039
Eickenscheidt T, Freibauer A, Heinichen J, Augustin J, Drösler M (2014) Short-term effects of biogas digestate and cattle slurry application on greenhouse gas emissions from high organic carbon grasslands. Biogeosci Discuss 11:5765–5809. doi:10.5194/bgd-11-5765-2014
EI-Shinnawi M, EI-Tahawy BS, El-Shimi SA, Fahmy SS (1989) Fractionation of organic substances during anaerobic digestion of farm wastes for biogas generation. MIRCEN J 5:27–42. doi:10.1007/BF01724956
Elste B, Tischer S, Christen O (2010) Einfluss von Biogasgärrückständen auf Abundanz und Biomasse von Lumbriciden. In: Berichte der DBG: Gemeinsame Sitzung Kommission III DBG und Fachgruppe 4 Bundesverband Boden mit dem Titel: Boden und Standortqualität-Bioindikation mit Regenwürmern, FH Osnabrück, 25. -26. Februar 2010. http://www.dbges.de
Erhart E, Siegl Th, Bonell M, Unterfrauner H, Peticzka R, Ableidinger Chr, Haas D, Hartl W (2014) Fertilization with liquid digestate in organic farming—effects on humusbalance, soil potassium contents and soil physical properties. In EGU General Assembly Conference Abstracts 16:4419
Ernst G, Müller A, Göhler H, Emmerling C (2008) C and N turnover of fermented residues from biogas plants in soil in the presence of three different earthworm species (Lumbricus terrestris, Aporrectodea longa, Aporrectodea caliginosa). Soil Biol Biochem 40:1413–1420. doi:10.1016/j.soilbio.2007.12.026
Field JA, Caldwell JS, Jeyanayagam S, Reneau RB, Kroontje W, Collins ER (1984) Fertilizer recovery from anaerobic digesters. Trans ASAE 27:1871–1876
Flessa H, Dörsch P, Beese F (1995) Seasonal variation of N2O and CH4 fluxes in differently managed arable soils in southern Germany. J Geophys Res: Atmos 100:23115–23124. doi:10.1029/95JD02270
Fouda S (2011) Nitrogen availability of biogas residues. PhD Technische Universität München
Fouda S, von Tucher S, Lichti F, Schmidhalter U (2013) Nitrogen availability from different biogas residues applied to ryegrass. J Plant Nutr Soil Sci 176:572–584. doi:10.1002/jpln.201100233
Friedel JK, Bezler A, Fischer WR (1996) Stickstoffmineralisierung von verflüssigtem, anaerob fermentiertem Rindermist im Boden. Agribiol Res 49:1–9
Frøseth RB, Bakken AK, Bleken MA, Riley H, Pommeresche R, Thorup-Kristensen K, Hansen S (2014) Effects of green manure herbage management and its digestate from biogas production on barley yield, N recovery, soil structure and earthworm populations. Eur J Agron 52:90–102. doi:10.1016/j.eja.2013.10.006
Galvez A, Sinicco T, Cayuela ML, Mingorance MD, Fornasier F, Mondini C (2012) Short term effects of bioenergy by-products on soil C and N dynamics, nutrient availability and biochemical properties. Agric Ecosyst Environ 160:3–14. doi:10.1016/j.agee.2011.06.015
Garg RN, Pathak H, Das DK, Tomar RK (2005) Use of flyash and biogas slurry for improving wheat yield and physical properties of soil. Environ Monit Assess 107:1–9. doi:10.1007/s10661-005-2021-x
Gericke D, Bornemann L, Kage H, Pacholski A (2012) Modelling ammonia losses after field application of biogas slurry in energy crop rotations. Water Air Soil Pollut 223:29–47. doi:10.1007/s11270-011-0835-4
Goberna M, Podmirseg SM, Waldhuber S, Knapp BA, García C, Insam H (2011) Pathogenic bacteria and mineral N in soils following the land spreading of biogas digestates and fresh manure. Appl Soil Ecol 49:18–25. doi:10.1016/j.apsoil.2011.07.007
Gómez X, Cuetos MJ, García AI, Morán A (2005) Evaluation of digestate stability from anaerobic process by thermogravimetric analysis. Thermochim Acta 426:179–184. doi:10.1016/j.tca.2004.07.019
Gómez X, Diaz MC, Cooper M, Blanco D, Morán A, Snape CE (2007a) Study of biological stabilization processes of cattle and poultry manure by thermogravimetric analysis and 13C NMR. Chemosphere 68:1889–1897. doi:10.1016/j.chemosphere.2007.02.065
Gómez X, Cuetos MJ, García AI, Morán A (2007b) An evaluation of stability by thermogravimetric analysis of digestate obtained from different biowastes. J Hazard Mater 149:97–105. doi:10.1016/j.jhazmat.2007.03.049
Gómez X, Blanco D, Lobato A, Calleja A, Martínez-Núñez F, Martin-Villacorta J (2011) Digestion of cattle manure under mesophilic and thermophilic conditions: characterization of organic matter applying thermal analysis and 1H NMR. Biodegradation 22:623–635. doi:10.1007/s10532-010-9436-y
Gunnarsson A, Lindén B, Gertsson U (2008) Residual nitrogen effects in organically cultivated beetroot following a harvested/green manured grass-clover ley. J Plant Nutr 31:1355–1381. doi:10.1080/01904160802206190
Gunnarsson A, Lindén B, Gertsson U (2011) Biodigestion of plant material can improve nitrogen use efficiency in a red beet crop sequence. Hort Sci 46:765–775
Gutser R, Ebertseder Th, Weber A, Schraml M, Schmidhalter U (2005) Short-term and residual availability of nitrogen after long-term application of organic fertilizers on arable land. J Plant Nutr Soil Sci 168:439–446. doi:10.1002/jpln.200520510
Haas G (2000) Was gut ist, kann noch besser werden. Bioland, Heft 5, 6–7
Hansen MN, Henriksen K, Sommer SG (2006) Observations of production and emission of greenhouse gases and ammonia during storage of solids separated from pig slurry: effects of covering. Atmos Environ 40:4172–4181. doi:10.1016/j.atmosenv.2006.02.013
Hao X (2007) Nitrate accumulation and greenhouse gas emissions during compost storage. Nutr Cycl Agroecosyst 78:189–195. doi:10.1007/s10705-006-9084-0
Hao X, Larney FJ, Chang C, Travis GR, Nichol CK, Bremer E (2005) The effect of phosphogypsum on greenhouse gas emissions during cattle manure composting. J Environ Qual 34:774–781. doi:10.2134/jeq2004.0388
Heilmann H (1992) Offene Fragen zur Biogastechnologie. Ökologie und Landbau, Heft 83, 25–26
Hjorth M, Christensen KV, Christensen ML, Sommer SG (2010) Solid–liquid separation of animal slurry in theory and practice. A review. Agron Sustain Dev 30:153–180. doi:10.1007/978-94-007-0394-0_43
Johansen A, Carter MS, Jensen ES, Hauggard-Nielsen H, Ambus P (2013) Effects of digestate from anaerobically digested cattle slurry and plant materials on soil microbial community and emission of CO2 and N2O. Appl Soil Ecol 63:36–44. doi:10.1016/j.apsoil.2012.09.003
Kaiser EA, Ruser R (2000) N2O emissions from arable soils in Germany—an evaluation of six long-term experiments. J Plant Nutr Soil Sci 163:249–260. doi:10.1002/1522-2624(200006)163:3<249::AID-JPLN249>3.0.CO;2-Z
Kautz T, Rauber R (2007) Einfluss der Düngung mit Gärrückständen aus der Biogaserzeugung auf den Ertrag von Silomais und auf die bodenmikrobielle Aktivität. http://orgprints.org/9854/01/9854_Kautz_Vortrag.pdf 12 Dec 2008
Kirchmann H, Lundvall A (1993) Relationship between N immobilization and volatile fatty acids in soil after application of pig and cattle slurry. Biol Fertil Soils 15:161–164. doi:10.1007/BF00361605
Kruska V, Emmerling C (2008) Flächennutzungswandel durch Biogaserzeugung—Regionale und lokale Erhebungen in Rheinland-Pfalz. Naturschutz Landschaftsplanung 40:69–72
Külling DR, Menzi H, Sutter F, Lischer P, Kreuzer M (2003) Ammonia, nitrous oxide and methane emissions from differently stored dairy manure derived from grass- and hay-based rations. Nutr Cycl Agroecosyst 65:13–22. doi:10.1023/A:1021857122265
Lampkin N (1990) Organic farming. Farming Press Books, Ipswich
Larney FJ, Buckley KE, Hao X, McCaughey WP (2006) Fresh, stockpiled, and composted beef cattle feedlot manure. J Environ Qual 35:1844–1854. doi:10.2134/jeq2005.0440
Lemke RL, Malhi SS, Selles F, Stumborg M (2012) Relative effects of anaerobically-digested and conventional liquid swine manure, and N fertilizer on crop yield and greenhouse gas emissions. Agric Sci 3:799–805. doi:10.4236/as.2012.36097
Lipschultz F, Zafiriou OC, Wofsy SC, Mcelroy MB, Valois FW, Watson SW (1981) Production of NO and N2O by soil nitrifying bacteria. Nature 294:641–643. doi:10.1038/294641a0
Lošák T, Hlušek J, Zatloukalová A, Tůmová D, Tůma I, Szostková M, Pospíšilová L, Martensson A (2011) Effect of mineral fertilizer and digestate application on soil properties and yield and chemical composition of kohlrabi. Acta Univ Agric Silviculturae Mendelianea Brunensis 59:117–122, ISSN 1211-8516
Marcato C-E, Mohtar R, Revel J-C, Pouech P, Hafidi M, Guiresse M (2009) Impact of anaerobic digestion on organic matter quality in pig slurry. Int Biodeterior Biodegrad 63:260–266. doi:10.1016/j.ibiod.2008.10.001
Massé DI, Croteau F, Masse L (2007) The fate of crop nutrients during digestion of swine manure in psychrophilic anaerobic sequencing batch reactors. Bioresour Technol 98:2819–2823. doi:10.1016/j.biortech.2006.07.040
Massé DI, Talbot G, Gilbert Y (2011) On farm biogas production: a method to reduce GHG emissions and develop more sustainable livestock operations. Anim Feed Sci Technol 166–167:436–445. doi:10.1016/j.anifeedsci.2011.04.075
Maurer C, Müller J (2012) Ammonia (NH3) emissions during drying of untreated and dewatered biogas digestate in a hybrid waste-heat/ solar dryer. Eng Life Sci 12:321–326. doi:10.1002/elsc.201100113
McCracken DV, Smith MS, Grove JH, MacKown CT, Blevins RL (1994) Nitrate leaching as influenced by cover cropping and nitrogen source. Soil Sci Soc Am J 58:1476–1483. doi:10.2136/sssaj1994.03615995005800050029x
McCrory DF, Hobbs, PJ (2001) Additives to reduce ammonia and odor emissions from livestock wastes. J Environ Qual 30:345–355. doi:10.2134/jeq2001.302345x
Merz HU (1988) Untersuchungen zur Wirkung von unbehandelter und methanvergorener Rindergülle auf den N-Umsatz unter Dactylis glomerata L. sowie auf das Keimverhalten verschiedener Pflanzenarten. Diss. Univ. Hohenheim
Merz H-U, Trösch W (1989) Vergleichende Untersuchungen zur N-Bilanz unter Dactylis glomerata L. nach Gülle-, Biogas-Gülle- und Mineraldüngung. 2. Mitteilung: Nitratauswaschung und Nährstoffgehalte im Boden. D. wirtschaftseig. Futter 35:226–237
Michel J, Weiske A, Möller K (2010) The effect of biogas digestion on the environmental impact and energy balances in organic cropping systems using the life-cycle assessment methodology. Renew Agric Food Syst 25:204–218. doi:10.1017/S1742170510000062
Miller MN, Zebarth BJ, Dandie CE, Burton DL, Goyer C, Trevors JT (2009) Influence of liquid manure on soil denitrifier abundance, denitrification, and nitrous oxide emissions. Soil Sci Soc Am J 73:760–768. doi:10.2136/sssaj2008.0059
Molinuevo-Salces B, Gómez X, Morán A, García-González MC (2013) Anaerobic co-digestion of livestock and vegetable processing wastes: fibre degradation and digestate stability. Waste Manag 33:1332–1338. doi:10.1016/j.wasman.2013.02.021
Möller K (2009) Effects of biogas digestion on soil organic matter and nitrogen inputs, flows and budgets in organic cropping systems. Nutr Cycl Agroecosyst 84:179–202. doi:10.1007/s10705-008-9236-5
Möller K, Müller T (2012) Effects of anaerobic digestion on digestate nutrient availability and crop growth: a review. Eng Life Sci 12:1–16. doi:10.1002/elsc.201100085
Möller K, Schultheiss U (2014) Organische Handelsdüngemittel im ökologischen Landbau. KTBL Schrift 499, Darmstadt/Germany, p 392
Möller K, Stinner W (2009) Effects of different manuring systems with and without biogas digestion on soil mineral nitrogen content and on gaseous nitrogen losses (ammonia, nitrous oxides). Eur J Agron 30:1–16. doi:10.1016/j.eja.2008.06.003
Möller K, Stinner W, Leithold G (2008) Growth, composition, biological N2 fixation and nutrient uptake of a leguminous cover crop mixture and the effect of their removal on field nitrogen balances and nitrate leaching risk. Nutr Cycl Agroecosyst 82:233–249. doi:10.1007/s10705-008-9182-2
Möller K, Schulz R, Müller T (2010) Substrate inputs, nutrient flows and nitrogen loss of two centralized biogas plants in southern Germany. Nutr Cycl Agroecosyst 87:307–325. doi:10.1007/s10705-009-9340-1
Möller K, Schulz R, Müller T (2011) Effects of setup of centralized biogas plants on crop acreage and balances of nutrients and soil humus. Nutr Cycl Agroecosyst 89:303–312. doi:10.1007/s10705-010-9395-z
Muck RE, Steenhuis TS (1982) Nitrogen losses from manure storages. Agric Wastes 4:41–54. doi:10.1016/0141-4607(82)90053-1
Nadeem S, Hansen S, Azzaroli Bleken M, Dörsch P (2012) N2O emission from organic barley cultivation as affected by green manure management. Biogeosciences 9:2747–2759. doi:10.5194/bg-9-2747-2012
Neuner K-H, Raba C, Ahrens W (2011) Mineralstoff-Gehalte in Gärresten von Biogas-Anlagen. In: Elsäßer M, Diepolder M, Huguenin-Eile O, Pötsch E, Nußbaum H, Messner J (eds) Proc. of the conference, Gülle 11 – Gülle und Gärrestdüngung auf Grünland“ in Kloster Reute, pp 75–77
Ni K, Pacholski A, Kage H (2012) Analysis of ammonia losses after field application of biogas slurries by an empirical model. J Plant Nutr Soil Sci 175:253–264. doi:10.1002/jpln.201000358
Odlare M, Pell M, Svensson K (2008) Changes in soil chemical and microbiological properties during 4 years of application of various organic residues. Waste Manag 28:1246–1253. doi:10.1016/j.wasman.2007.06.005
Odlare M, Arthurson V, Pell M, Svensson K, Nehrenheim E, Abubaker J (2011) Land application of organic waste—effects on the soil ecosystem. Appl Energy 88:2210–2218. doi:10.1016/j.apenergy.2010.12.043
Ørtenblad H. (2002) The use of digested slurry within agriculture. Downloaded from http://homepage2.nifty.com/biogas/cnt/refdoc/whrefdoc/d9manu.pdf. Accessed 30 Jan 2007
Orzi V, Cadena E, D’Imporzano G, Artola A, Davoli E, Crivelli M, Adani F (2010) Potential odour emission measurement in organic fraction of municipal solid waste during anaerobic digestion: relationship with process and biological stability parameters. Bioresour Technol 101:7330–7337. doi:10.1016/j.biortech.2010.04.098
Paillat JM, Robin P, Hassouna M, Leterme P (2005) Predicting ammonia and carbon dioxide emissions from carbon and nitrogen biodegradability during animal waste composting. Atmos Environ 39:6833–6842. doi:10.1016/j.atmosenv.2005.07.045
Pain BF, Misselbrook TH, Clarkson CR, Rees YJ (1990) Odour and ammonia emissions following the spreading of anaerobically-digested pig slurry on grassland. Agric Wastes 34:259–267. doi:10.1016/0269-7483(90)90027-P
Parkin TB, Kaspar TC, Singer JW (2006) Cover crop effects on the fate of N following soil application of swine manure. Plant Soil 289:141–152. doi:10.1007/s11104-006-9114-3
Paul JW, Dinn NE, Kannangara T, Fisher LJ (1998) Protein content in dairy cattle diets affects ammonia losses and fertilizer nitrogen value. J Environ Qual 27:528–534. doi:10.2134/jeq1998.00472425002700030008x
Pelster DE, Chantigny MH, Rochette C, Angers DA, Rieux C, Vanasse A (2012) Nitrous oxide emissions respond differently to mineral and organic N sources in contrasting soil types. J Environ Qual 41:427–435. doi:10.2134/jeq2011.0261
Petersen SO (1999) Nitrous oxide emissions from manure and inorganic fertilizers applied to spring barley. J Environ Qual 28:1610–1618. doi:10.2134/jeq1999.00472425002800050027x
Petersen SO, Sommer SG (2011) Ammonia and nitrous oxide interactions: roles of manure organic matter management. Anim Feed Sci Technol 166–167:503–513. doi:10.1016/j.anifeedsci.2011.04.077
Petersen J, Sørensen P (2008) Loss of nitrogen and carbon during storage of the fibrous fraction of separated pig slurry and influence on nitrogen availability. J Agric Sci (Camb) 146:403–413
Petersen SO, Lind A-M, Sommer SG (1998) Nitrogen and organic matter losses during storage of cattle and pig manure. J Agric Sci 130:69–79
Plaixats J, Barcelo J, Garcia-Moreno J (1988) Characterization of the effluent residue from anaerobic digestion of pig excreta for its utilization as fertilizer. Agrochemica 32:236–239
Pognani M, D’Imporzano G, Scaglia B, Adani F (2009) Substituting energy crops with organic fraction of municipal solid waste for biogas production at farm level: a full-scale plant study. Process Biochem 44:817–821. doi:10.1016/j.procbio.2009.03.014
Pötsch EM (2005) Nährstoffgehalt von Gärrückständen aus landwirtschaftlichen Biogasanlagen und deren Einsatz im Dauergrünland. Abschlussbericht Projektnummer 2941:32
Reinhold G, Klimanek EM, Breitschuh G (1991) Zum Einfluss der Biogaserzeugung auf Veränderungen in der Kohlenstoffdynamik von Gülle. Arch Acker- u Pflanzenbau u Bodenkd 35:129–137
Ross DJ, Tate KR, Speir TW, Stewart DJ, Hewitt AE (1989) Influence of biogas-digester effluents on crop growth and soil biochemical properties under rotational cropping. N Z J Crop Hortic Sci 17:77–87. doi:10.1080/01140671.1989.10428013
Rubæk GH, Henriksen K, Petersen J, Rasmussen B, Sommer S (1996) Effects of application technique and anaerobic digestion on gaseous loss from animal slurry applied to ryegrass (Lolium perenne). J Agric Sci (Camb) 126:481–492. doi:10.1017/S0021859600075572
Ruser R, Flessa H, Schilling R, Beese F, Munch JC (2001) Effect of crop-specific field management and N fertilization on N2O emissions from a fine-loamy soil. Nutr Cycl Agroecosyst 59:177–191. doi:10.1023/A:1017512205888
Sánchez M, Gomez X, Barriocanal G, Cuetos MJ, Morán A (2008) Assessment of the stability of livestock farm wastes treated by anaerobic digestion. Int Biodeterior Biodegrad 62:421–426. doi:10.1016/j.ibiod.2008.04.002
Sänger A, Geisseler D, Ludwig B (2010) Effects of rainfall pattern on carbon and nitrogen dynamics in soil amended with biogas slurry and composted cattle manure. J Plant Nutr Soil Sci 173:692–698. doi:10.1002/jpln.200900254
Sänger A, Geisseler D, Ludwig B (2011) Effects of moisture and temperature on greenhouse gas emissions and C and N leaching losses in soil treated with biogas slurry. Biol Fertil Soils 47:249–259. doi:10.1007/s00374-010-0528-y
Sänger A, Geisseler D, Ludwig B (2014) C and N dynamics of a range of biogasslurries as a function of applicationrate and soil texture: a laboratory experiment. Arch Agron Soil Sci 60:1779–1794. doi:10.1080/03650340.2014.907491
Saunders OE, Fortuna A-M, Harrison JH, Cogger CG, Whitefield E, Green T (2012) Gaseous nitrogen and bacterial responses to raw and digested dairy manure applications in incubated soil. Environ Sci Technol 46:11684–11692. doi:10.1021/es301754s
Schauss K (2006) Impact of fermented organic fertilizers on in-situ trace gas fluxes and on soil bacterial denitrifying communities in organic agriculture. PhD thesis, Universität Gießen, available at: http://geb.uni-giessen.de/geb/volltexte/2007/3894/pdf/SchaussKristina-2006-10-26.pdf
Schauss K, Ratering S, Stinner W, Deuker A, Möller K, Schnell S (2006) Auswirkungen auf die bodenbürtigen Distickstoffoxid- und Methanemissionen. In: Möller K et al (eds) Auswirkung der Fermentation biogener Rückstände in Biogasanlagen auf Flächenproduktivität und Umweltverträglichkeit im Ökologischen Landbau—Pflanzenbauliche, ökonomische und ökologische Gesamtbewertung im Rahmen typischer Fruchtfolgen viehhaltender und viehloser ökologisch wirtschaftender Betriebe. Final report, available at: http://orgprints.org/10970/, pp 169–240
Scheller E (2006) Thesen zu den Auswirkungen des Biogasgärsubstrats auf den Boden. KTBL-Fachgespräch “Biogas im ökologischen Landbau” April 4–5, 2006 in Braunschweig/Germany. ISBN 978-3-939371-32-8
Schievano A, D’Imporzano G, Salati S, Adani F (2011) On-field study of anaerobic digestion full-scale plants. Part I. An on-field methodology to determine mass, carbon and nutrients balance. Bioresour Technol 102:7737–7744. doi:10.1016/j.biortech.2011.06.006
Schjønning P, Heckrath G, Christensen BT (2009) Threats to Soil quality in denmark e a review of existing knowledge in the context of the EU soil thematic strategy. DJF Report Plant Science No. 143. Faculty of Agricultural Sciences, Aarhus University, Tjele, Denmark, pp 1–121
Schröder D, Schumacher B, Wallerath S (1996) Wirkung aerob und anaerob behandelter organischer Reststoffe auf bodenchemische und bodenmikrobiologische Eigenschaften, Ertrag und Nitrataustrag. VDLUFA-Schriftenreihe 44:643–646
Senbayram M, Chen R, Mühling KH, Dittert K (2009) Contribution of nitrification and denitrification to nitrous oxide emissions from soils after application of biogas waste and other fertilizers. Rapid Commun Mass Spectrom 23:2489–2498. doi:10.1002/rcm.4067
Singh KP, Suman A, Singh PN, Srivastava TK (2007) Improving quality of sugarcane-growing soils by organic amendments under subtropical climatic conditions of India. Biol Fertil Soils 44:367–376. doi:10.1007/s00374-007-0216-8
Skiba UM, Sheppard LJ, MacDonald J, Fowler D (1998) Some key environmental variables controlling nitrous oxide emissions from agricultural and semi-natural soils in Scotland. Atmos Environ 32:3311–3320. doi:10.1016/S1352-2310(97)00364-6
Sommer SG (1997) Ammonia volatilization from farm tanks containing anaerobically digested animal slurry. Atmos Environ 31:863–868. doi:10.1016/S1352-2310(96)00250-6
Sommer SG, Hutchings NJ (2001) Ammonia emissions from field applied manure and its reduction - invited paper. Eur J Agron 15:1–15. doi:10.1016/S1161-0301(01)00112-5
Stinner W, Möller K, Leithold G (2008) Effects of biogas digestion of clover/grass-leys, cover crops and crop residues on nitrogen cycle and crop yield in organic stockless farming systems. Eur J Agron 29:125–134. doi:10.1016/j.eja.2008.04.006
Suzuki I, Dular U, Kwok SC (1974) Ammonia or ammonium ion as substrate for oxidation by Nitrosomonas europea cells and extracts. J Bacteriol 120:556–558
Swerts M, Merckx R, Vlassak K (1996) Denitrification, N2-fixation and fermentation during anaerobic incubation of soils amended with glucose and nitrate. Biol Fertil Soils 23:229–235. doi:10.1007/BF00335949
Tambone F, Genevini P, D’Imporzano G, Adani F (2009) Assessing amendment properties of digestate by studying the organic matter composition and the degree of biological stability during the anaerobic digestion of the organic fraction of MSW. Bioresour Technol 100:3140–3142. doi:10.1016/j.biortech.2009.02.012
Tambone F, Scaglia B, D’Imporzano G, Schievano A, Orzi V, Salati S, Adani F (2010) Assessing amendment and fertilizing properties of digestates from anaerobic digestion through a comparative study with digested sludge and compost. Chemosphere 81:577–583. doi:10.1016/j.chemosphere.2010.08.034
Tambone F, Adani F, Gigliotti G, Volpe D, Fabbri C, Provenzano MR (2013) Organic matter characterization during the anaerobic digestion of different biomasses by means of CPMAS 13C NMR spectroscopy. Biomass Bioenergy 48:111–120. doi:10.1016/j.biombioe.2012.11.006
Terhoeven-Urselmans T, Scheller E, Raubuch M, Ludwig B, Joergensen RG (2009) CO2 evolution and N mineralization after biogas slurry application in the field and its yield effects on spring barley. Appl Soil Ecol 42:297–302. doi:10.1016/j.apsoil.2009.05.012
Thomsen IK, Pedersen AR, Nyord T, Petersen SO (2010) Effects of slurry pre-treatment and application technique on short-term N2O emissions as determined by a new non-linear approach. Agric Ecosyst Environ 136:227–235. doi:10.1016/j.agee.2009.12.001
Thomsen IK, Olesen JE, Møller HB, Sørensen P, Christensen BT (2013) Carbon dynamics and retention in soil after anaerobic digestion of dairy cattle feed and faeces. Soil Biol Biochem 58:82–87. doi:10.1016/j.soilbio.2012.11.006
Thorman RE, Chadwick DR, Harrison R, Boyles LO, Matthews R (2007) The effect on N2O emissions of storage conditions and rapid incorporation of pig and cattle farmyard manure into tillage land. Biosyst Eng 97:501–511. doi:10.1016/j.biosystemseng.2007.03.039
Tietjen, C. (1957). Bilanzuntersuchungen bei Stallmistaufbereitung in Biogasanlagen. Zeitschrift für Pflanzenernährung, Düngung, Bodenkunde 77:198–212. doi:10.1002/jpln.19570770303
Vallejo A, Skiba U, García-Torres L, Arce A, López-Fernández S, Sánchez-Martín L (2006) Nitrogen oxides emission from soils bearing a potato crop as influenced by fertilization with treated pig slurries and composts. Soil Biol Biochem 38:2782–2793. doi:10.1016/j.soilbio.2006.04.040
Vepsäläinen M, Erkomaa K, Kukkonen S, Vestberg M, Wallenius K, Niemi RM (2004) The impact of crop plant cultivation and peat amendment on soil microbial activity and structure. Plant Soil 264:273–286. doi:10.1023/B:PLSO.0000047763.46795.cb
Walsh JJ, Rousk J, Edwards-Jones G, Jones DL, Williams AP (2012a) Replacing inorganic fertilizer with anaerobic digestate may maintain agricultural productivity at less environmental cost. J Plant Nutr Soil Sci 175:840–845. doi:10.1002/jpln.201200214
Walsh JJ, Rousk J, Edwards-Jones G, Jones DL, Williams AP (2012b) Fungal and bacterial growth following the application of slurry and anaerobic digestate of livestock manure to temperate pasture soils. Biol Fertil Soils 48:889–897. doi:10.1007/s00374-012-0681-6
Wang Y, Dong H, Zhu Z, Liu C, Xin H (2014) Comparison of air emissions from raw liquid pig manure and biogas digester effluent storages. Trans ASABE 5:635–645. doi:10.13031/trans.57.10292
Weiland P (2010) Biogas production: current state and perspectives. Appl Microbiol Biotechnol 85:849–860. doi:10.1007/s00253-009-2246-7
Wulf S, Maeting M, Clemens J (2002a) Application technique and slurry co-fermentation effects on ammonia, nitrous oxide, and methane emissions after spreading: I. Ammonia volatilization. J Environ Qual 31:1789–1794. doi:10.2134/jeq2002.1789
Wulf S, Maeting M, Clemens J (2002b) Application technique and slurry co-fermentation effects on ammonia, nitrous oxide, and methane emissions after spreading: II. Greenhouse gas emissions. J Environ Qual 31:1795–1801. doi:10.2134/jeq2002.1795
Yamulki S (2006) Effect of straw addition on nitrous oxide and methane emissions from stored farmyard manures. Agric Ecosyst Environ 112:140–145. doi:10.1016/j.agee.2005.08.013
Yamulki S, Harrison RM, Goulding KWT, Webster CP (1995) Effect of fertilizer application on NO and N2O fluxes from agricultural fields. J Geophys Res 100:923–925. doi:10.1029/95JD02461
Zak DR, Holmes WE, White DC, Peacock AD, Tilman D (2003) Plant diversity, soil microbial communities, and ecosystem function: are there any links? Ecology 2042. doi:10.1890/02-0433
Acknowledgments
This publication was prepared in the frame of the research projects “GärWert” and “Gärresteigenschaften.” We acknowledge the supporting of both projects by Fachagentur Nachwachsende Rohstoffe e.V. (FNR) on behalf of the German Federal Ministry of Food and Agriculture based on a decision of the German Bundestag.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Möller, K. Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity. A review. Agron. Sustain. Dev. 35, 1021–1041 (2015). https://doi.org/10.1007/s13593-015-0284-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13593-015-0284-3