Abstract
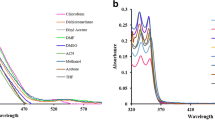
We report synthesis, structural and computational studies, and photophysical properties of a chiral (1R,2R)-N,N′-bis-(salicylidene)-1,2-diphenyl-1,2-ethanediamine Schiff base dye (1). The structure of 1 was found to be in the enol-imine tautomer, stabilized by two intramolecular O–H···N hydrogen bonds. Molecules are packed into a 1D supramolecular chain through intermolecular C–H···O interactions. 1D chains are interlinked through intermolecular C–H···π intercations. As a result of intermolecular interactions, molecules of 1 are packed into a 3D supramolecular framework, yielding a pcu alpha-Po primitive cubic; 6/4/c1; sqc1 topology defined by the point symbol of (412·63). Favoured intermolecular H···H, H···C and H···O contacts are responsible for the overall crystal packing of the dye. Energy frameworks have been calculated to additionally analyse the overall crystal packing of 1. The absorption spectra of 1 in THF, CH2Cl2 and CH3CN each exhibit three intense bands in the UV region. The absorption spectra of 1 in EtOH, nPrOH, iPrOH and nBuOH, besides the same three intense bands in the UV region, contain an additional band in the visible region centred at about 410 nm, corresponding to the cis-keto-enamine tautomer. The absorption spectrum of 1 in MeOH contains an additional intense shoulder at about 350 nm. The emission spectrum of 1 in MeOH contains a broad band at 438 nm, arising from the emission of the enol-imine* form. Theoretical calculations based on density functional theory (DFT) were performed to verify the structure of 1 as well as its electronic and optical properties. The global chemical reactivity descriptors were estimated from the energy of the HOMO and LUMO orbitals. Molecular docking studies were performed to evaluate the antifungal activity of 1 against cytochrome P450 14 alpha-sterol demethylase (CYP51).















Similar content being viewed by others
Availability of data and materials
Not applicable.
References
H. Schiff, Eur. J. Org. Chem. 131, 118–119 (1864)
IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A.D. McNaught, A. Wilkinson. Blackwell Scientific Publications, Oxford (1997)
S. Yamada, Coord. Chem. Rev. 190–192, 537–555 (1999)
P.G. Cozzi, Chem. Soc. Rev. 33, 410–421 (2004)
C. Baleizão, H. Garcia, Chem. Rev. 106, 3987–4043 (2006)
A. Decortes, A.M. Castilla, A.W. Kleij, Angew. Chem. Int. Ed. 49, 9822–9837 (2010)
S. Shaw, J.D. White, Chem. Rev. 119, 9381–9426 (2019)
W. Zhang, J.L. Loebach, S.R. Wilson, E.N. Jacobsen, J. Am. Chem. Soc. 112, 2801–2803 (1990)
E.N. Jacobsen, W. Zhang, A.R. Muci, J.R. Ecker, L. Deng, J. Am. Chem. Soc. 113, 7063–7064 (1991)
W. Dabelstein, A. Reglitzky, A. Schütze, K. Reders, A. Brunner, Ullmann’s Encyclopedia of Industrial Chemistry (Wiley, Weinheim, 2016).
H.C. Aspinall, Chem. Rev. 102, 1807–1850 (2002)
J. Crassous, Chem. Soc. Rev. 38, 830–845 (2009)
M. Liu, L. Zhang, T. Wang, Chem. Rev. 115, 7304–7397 (2015)
E. Hadjoudis, M. Vitterakis, I. Moustakali, I. Mavridis, Tetrahedron 43, 1345–1360 (1987)
E. Hadjoudis, I.M. Mavridis, Chem. Soc. Rev. 33, 579–588 (2004)
K. Amimoto, T. Kawato, J. Photochem. Photobiol. C 6, 207–226 (2005)
T. Haneda, M. Kawano, T. Kojima, M. Fujita, Angew. Chem. Int. Ed. 46, 6643–6645 (2007)
A. Filarowski, A. Koll, L. Sobczyk, Curr. Org. Chem. 13, 172–193 (2009)
V. Bertolasi, P. Gilli, G. Gilli, Curr. Org. Chem. 13, 250–268 (2009)
E. Hadjoudis, S.D. Chatziefthimiou, I.M. Mavridis, Curr. Org. Chem. 13, 269–286 (2009)
V.I. Minkin, A.V. Tsukanov, A.D. Dubonosov, V.A. Bren, J. Mol. Struct. 998, 179–191 (2011)
Y. Inokuma, M. Kawano, M. Fujita, Nat. Chem. 3, 349–358 (2011)
K.T. Mahmudov, A.J.L. Pombeiro, Chem. Eur. J. 22, 16356–16398 (2016)
D.A. Safin, K. Robeyns, Y. Garcia, CrystEngComm 14, 5523–5529 (2012)
D.A. Safin, K. Robeyns, Y. Garcia, RSC Adv. 2, 11379–11388 (2012)
D.A. Safin, Y. Garcia, RSC Adv. 14, 6466–6471 (2013)
D.A. Safin, M. Bolte, Y. Garcia, CrystEngComm 16, 5524–5526 (2014)
D.A. Safin, M.G. Babashkina, K. Robeyns, M. Bolte, Y. Garcia, CrystEngComm 16, 7053–7061 (2014)
D.A. Safin, M. Bolte, Y. Garcia, CrystEngComm 16, 8786–8793 (2014)
D.A. Safin, M.G. Babashkina, K. Robeyns, Y. Garcia, RSC Adv. 16, 53669–53678 (2016)
D.A. Safin, K. Robeyns, M.G. Babashkina, Y. Filinchuk, A. Rotaru, C. Jureschi, M.P. Mitoraj, J. Hooper, M. Brela, Y. Garcia, CrystEngComm 16, 7249–7259 (2016)
D.A. Safin, K. Robeyns, Y. Garcia, CrystEngComm 18, 7284–7296 (2016)
A.A. Shiryaev, T.M. Burkhanova, G. Mahmoudi, M.G. Babashkina, D.A. Safin, J. Lumin. 226, 117454 (2020)
D.S. Shapenova, A.A. Shiryaev, M. Bolte, M. Kukułka, D.W. Szczepanik, J. Hooper, M.G. Babashkina, G. Mahmoudi, M.P. Mitoraj, D.A. Safin, Chem. Eur. J. 26, 12987–12995 (2020)
R. Dennington, T.A. Keith, J.M. Millam, GaussView, Version 6.0, Semichem Inc., Shawnee Mission, KS, 2016
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery Jr., J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, O. Farkas, J.B. Foresman, J.V. Ortiz, J. Cioslowski, D.J. Fox, Gaussian 09, Revision D.01 (2013)
R. Krishnan, J.S. Binkley, R. Seeger, J.A. Pople, J. Chem. Phys. 72, 650–654 (1980)
A.D. Becke, J. Chem. Phys. 98, 5648–5652 (1993)
M.J. Frisch, J.A. Pople, J.S. Binkley, J. Chem. Phys. 80, 3265–3269 (1984)
C.R. Groom, I.J. Bruno, M.P. Lightfoot, S.C. Ward, Acta Crystallogr. B 72, 171–179 (2016)
T. Wu, CSD Communication (2017)
T.M. Krygowski, K. Woźniak, R. Anulewicz, D. Pawlak, W. Kolodziejski, E. Grech, A. Szady, J. Phys. Chem. A 101, 9399–9404 (1997)
P.M. Dominiak, E. Grech, G. Barr, S. Teat, P. Mallinson, K. Woźniak, Chem. Eur. J. 9, 963–970 (2003)
G.M. Mercier, K. Robeyns, T. Leyssens, Cryst. Growth Des. 16, 3198–3205 (2016)
R.F. Martínez, E. Matamoros, P. Cintas, J.C. Palacios, J. Org. Chem. 85, 5838–5862 (2020)
V.A. Blatov, A.P. Shevchenko, D.M. Proserpio, Cryst. Growth Des. 14, 3576–3586 (2014)
M.A. Spackman, D. Jayatilaka, CrystEngComm 11, 19–32 (2009)
M.A. Spackman, J.J. McKinnon, CrystEngComm 4, 378–392 (2002)
M.J. Turner, J.J. McKinnon, S.K. Wolff, D.J. Grimwood, P.R. Spackman, D. Jayatilaka, M.A. Spackman, CrystalExplorer17, University of Western Australia, http://hirshfeldsurface.net (2017)
C. Jelsch, K. Ejsmont, L. Huder, IUCrJ 1, 119–128 (2014)
C.F. Mackenzie, P.R. Spackman, D. Jayatilaka, M.A. Spackman, IUCrJ 4, 575–587 (2017)
S.H. Alarcón, A.C. Olivieri, D. Sanz, R.M. Claramunt, J. Elguero, J. Mol. Struct. 705, 1–9 (2004)
P.I. Nagy, W.M.F. Fabian, J. Phys. Chem. B 110, 25026–25032 (2006)
M.J. Kamlet, J.-L.M. Abboud, M.H. Abraham, R.W. Taft, J. Org. Chem. 48, 2877–2887 (1983)
Y. Marcus, J. Solut. Chem. 20, 929–944 (1991)
P. Geerlings, F. De Proft, W. Langenaeker, Chem. Rev. 103, 1793–1873 (2003)
G.M. Morris, R. Huey, W. Lindstrom, M.F. Sanner, R.K. Belew, D.S. Goodsell, A.J. Olson, J. Comp. Chem. 16, 2785–2791 (2009)
O. Trott, A.J. Olson, J. Comput. Chem. 31, 455–461 (2010)
Discovery Studio 2015: Dassault Systemes BIOVIA, Discovery Studio Modelling Environment, Release 4.5, San Diego: Dassault Systemes
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
A. A. S. involved in conducting research and partial analysis of results; A. N. G. involved in conducting research and partial analysis of results; T. M. B. involved in planning research, analysis results, and preparing a manuscript; L. E. A. involved in conducting research and partial analysis of results; M. G. B. involved in planning research, analysis results, and preparing a manuscript; R. C. involved in conducting molecular docking and analysis of results; D. A. S. involved in planning research, analysis results, and preparing a manuscript.
Corresponding author
Ethics declarations
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest in this work.
Ethical approval
Not applicable.
Rights and permissions
About this article
Cite this article
Shiryaev, A.A., Goncharenko, A.N., Burkhanova, T.M. et al. A chiral (1R,2R)-N,N′-bis-(salicylidene)-1,2-diphenyl-1,2-ethanediamine Schiff base dye: synthesis, crystal structure, Hirshfeld surface analysis, computational study, photophysical properties and in silico antifungal activity. J IRAN CHEM SOC 18, 2897–2911 (2021). https://doi.org/10.1007/s13738-021-02237-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02237-5