Abstract
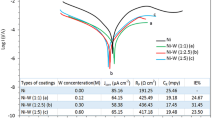
The nickel–copper alloy (70:30) prepared by metallurgical route is currently employed in marine environments because of its good resistance to corrosion. This alloy forms a thin protective surface layer when exposed to marine atmosphere and thus provides its corrosion resistance. Electrodeposition of nickel–copper alloy from sulphamate acetate-based electrolyte is a new and novel approach and was experimented. The detailed study was performed on the effect of electrolyte composition, current density and pH on the preparation of alloy deposit; the prepared alloy deposit particle size is of 78 nm, and the surface morphology of the alloy deposit was characterized with X-ray diffraction, scanning electron microscopy, EDAX, and atomic force microscope. Nickel–copper alloy deposited from the sulphamate acetate-based electrolyte operated at a temperature of 30 °C, with a pH of 6.6 and at 3 A/dm2, produces nickel–copper (70:30) alloy deposit. The corrosion behaviour of this alloy deposit was studied by potentiodynamic polarization method; the corrosion current of nickel is 8.67 μA cm−2 and the nickel–copper alloy is 2.65 μA cm−2.






Similar content being viewed by others
References
Abd El Rehim SS, Abd El Wahab S, Rashwan SM, Anwar ZM (1999) Electrodeposition of copper–nickel alloys from a citrate bath containing boric acid. Trans IMF 77(6):242–245
Alper M, Baykul MC, Peter L, Toth J, Bakonyi I (2004) Preparation and characterisation of electrodeposited Ni–Cu/Cu multilayers. J Appl Electrochem 34(8):841–848
Baskaran I, Sankaranarayanan TSN, Stephen A (2006) Pulsed electrodeposition of nano crystalline copper nickel alloy and evaluation of their characteristics. Mater Lett 60:1990–1995
Bonou L, Massiani Y, Crousier J (1994) Electrodeposition and corrosion behavior of copper–nickel alloy. Br Corros J 29:201–206
Bradley P, Roy S, Landolt D (1996) Pulse plating of copper–nickel alloys from sulfamate solution. J Chem Soc Faraday Trans 92:4015–4019
Chassaing E, Vu Quang K (1987) Mechanism of copper–nickel alloy electrodeposition. J Appl Electrochem 17:1267–1280
Cherkaoui M, Chassaing E, Vu Quang K (1988) Pulse plating of copper nickel alloy. Surf Coat Technol 34:243–252
Dube CE, Workie B, Kounaves SP, Robbat A Jr, Aksu ML (1995) Electrodeposition of metal alloy and mixed oxide films using a single-precursor tetranuclear copper–nickel complex. J Electrochem Soc 142(10):3357–3365
El-sayed sherif M, Almajid AA, Bairamov AK, Al-Zahrani E (2011) Corroison of Monal-400 in aerated stagnant Arabin gulf seawater after different exposure intervals. Int J Electrochem Sci 6:5430–5444
Falke WL, Agnes Lee R, Schwaneke AE (1979). Electroplating with Ni-Cu alloy US patent., US 4,167,459 A
Ghosh SK, Grover AK, Totlani MK (1999) Nickel–copper alloy electroplating by pulse deposition. Bull Electrochem 15(5-6):174–178
Green TA, Russell AE, Roy S (1998) The development of a stable citrate electrolyte for the electrodeposition of copper–nickel alloys. J Electrochem Soc 145(3):875–881
Horkans J, Chang ICH, Andricacos PC, Podlaha EJ (1991) Determination of partial currents for CuNi and CuCo electrodeposition using rotating ring-disk electrodes. J Electrochem Soc 138(2):411–416
Ishikawa M, Enomoto H, Matsuoaka M, Iwakura C (1995) Effect of some factors on electrodeposition of nickel copper alloy from pyrophosphate tetra borate baths. Electrochemica Acta 40(11):1663–1668
Ismail KM, Fathi AM, Badawy WA (2004) The influence of Ni content on the stability of copper–nickel alloys in alkaline sulphate solutions. J Appl Electrochem 34(8):823–831
Mizushima L, Chikazawa M, Watanabe T (1996) Microstructure of electrodeposited Cu–Ni binary alloy films. J Electrochem Soc 143(6):1978–1983
Mohan S, Rajasekaran N (2011) Influence of electrolyte pH on composition of corrosion properties and surface morphology of electrodeposited Cu–Ni alloy. Surf Eng 27(7):519–523
Ogden C (1986) High-strength, composite copper–nickel electrodeposits. Plat Surf Finish 73(5):130–134
Podlaha EJ, Bonhote Ch, Landolt D (1994) A Mathematical model and experimental study of the electrodeposition of Ni–Cu alloys from complexing electrolytes. Electrochemica Acta 39(18):2649–2657
Priscott BH (1958) Electrodeposition of copper–nickel alloys from citrate solutions. Trans Inst Metal Finish 36:93–96
Roy S, Landolt D (1995) Effect of off-time on the composition of pulse-plated Cu–Ni alloys. J Electrochem Soc 142(9):3021–3027
Roy S, Matlosz M, Landolt D (1994) Effect of corrosion on the composition of pulse-plated Cu–Ni alloys. J Electrochem Soc 141(6):1509–1517
Sobha J, Krishna N, Sujatha KP, Krishnan RM, Sriveeraraghavan S, Natarajan SR (1996) Electroplating of nickel copper alloys. Bull Electrochem 12:259–265
Toth-Kadar E, Peter L, Becsei T, Toth J, Pogany L, Tarnoczi T, Kamasa P, Bakonyi I, Lang G, Cziraki A, Schwarzacherd W (2000) Preparation and magnetoresistance characteristics of electrodeposited Ni–Cu alloys and Ni–Cu/Cu multilayers. J Electrochem Soc 147(9):3311–3318
Vu Quang K, Chassaing E, Le Viet B, Celis JP, Roos JR (1985) Co-deposition of nickel and copper. Met Finish 83(10):25–26
Ying RY, Patrick KNg, Mao Z, White RE (1988) Electrodeposition of copper–nickel alloys from citrate solution on rotating disc electrode. J Electrochem Soc 135(12):2964–2971
Acknowledgments
Authors are expressing earnest thanks and acknowledge for the funding support of this study to INTELCOAT–CSC-0114-Council of Scientific and Industrial Research, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silaimani, S.M., Vivekanandan, G. & Veeramani, P. Nano-nickel–copper alloy deposit for improved corrosion resistance in marine environment. Int. J. Environ. Sci. Technol. 12, 2299–2306 (2015). https://doi.org/10.1007/s13762-014-0591-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-014-0591-2