Abstract
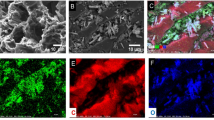
Nanocrystalline metal oxides including TiO2, Fe2O3, and ZnO and their combinations were impregnated on activated carbon (AC) and characterized by XRD, FTIR, and FESEM analyses. The results showed the size of most Fe2O3/AC, TiO2/AC, TiO2/Fe2O3/AC, ZnO/AC, and ZnO/Fe2O3/AC particles are in the range of 25–60 nm. BET analysis verified the high surface area of the six adsorbents (201–448 m2/g). The adsorption results confirmed that the modification could improve the adsorption capacity and removal efficiency as the maximum monolayer adsorption capacity and cyanide removal efficiency were observed for ZnO/Fe2O3/AC (101.0 mg/g, 82.5%), TiO2/Fe2O3/AC (96.2 mg/g, 75.1%), ZnO/AC (91.7 mg/g, 73.5%), TiO2/AC (90.9 mg/g, 72.4%), Fe2O3/AC (86.2 mg/g, 69.2%,), and AC (78.1 mg/g, 66.3%), respectively. Moreover, the study of different isotherm models including Langmuir, Freundlich, and Redlich–Peterson indicated that the Langmuir model was the most suitable one for the six adsorbents with 0.56 < RL < 0.64. The kinetic modeling of experimental data revealed the cyanide adsorption on all adsorbents followed the pseudo second-order model confirming chemisorption can be a main mechanism of adsorption. The regeneration and reusability results showed modified AC adsorbents have more reusable and stable structure than AC to be used as adsorbents in industrial wastewaters. The performance of adsorption process was compared with different methods of cyanide removal. The results approved that adsorption process as a cost-effective and simple design method using bioadsorbents can be highly effective in full-scale applications for the removal of high concentration of cyanide.
















Similar content being viewed by others
References
Abussaud B, Asmaly HA, Saleh TA, Gupta VK, Atieh MA (2016) Sorption of phenol from waters on activated carbon impregnated with iron oxide, aluminum oxide and titanium oxide. J Mol Liq 213:351–359
Adhoum N, Monser L (2002) Removal of cyanide from aqueous solution using impregnated activated carbon. Chem Eng Process 41:17–21
Agarwal B, Balomajumder C (2015) Removal of phenol and cyanide in multi-substrate system using copper impregnated activated carbon (Cu-GAC). Environ Prog Sustain Energy 34:1714–1723
Agarwal B, Balomajumder C, Thakur PK (2013) Simultaneous co-adsorptive removal of phenol and cyanide from binary solution using granular activated carbon. Chem Eng 228:655–664
Ahn CK, Park D, Woo SH, Park JM (2009) Removal of cationic heavy metal from aqueous solution by activated carbon impregnated with anionic surfactants. J Hazard Mater 164:1130–1136
Ali A, Mannan A, Hussain I, Hussain I, Zia M (2018) Effective removal of metal ions from aquous solution by silver and zinc nanoparticles functionalized cellulose: isotherm, kinetics and statistical supposition of process Environmental Nanotechnology. Monit Manage 9:1–11
Aram M, Farhadian M, Nazar ARS, Tangestaninejad S, Eskandari P, Jeon B-H (2020) Metronidazole and Cephalexin degradation by using of Urea/TiO2/ZnFe2O4/Clinoptiloite catalyst under visible-light irradiation and ozone injection. J Mole Liq 112764
Arimi A, Farhadian M, Nazar ARS, Homayoonfal M (2016) Assessment of operating parameters for photocatalytic degradation of a textile dye by Fe2O3/TiO2/clinoptilolite nanocatalyst using Taguchi experimental design. Res Chem Intermed 42:4021–4040
Asuquo ED, Martin AD (2016) Sorption of cadmium (II) ion from aqueous solution onto sweet potato (Ipomoea batatas L.) peel adsorbent: characterisation, kinetic and isotherm studies. J Environ Chem Eng 4:4207–4228
Behnamfard A, Salarirad MM (2009) Equilibrium and kinetic studies on free cyanide adsorption from aqueous solution by activated carbon. J Hazard Mater 170:127–133
Botz M, Mudder T, Akcil A (2005) Cyanide treatment: physical, chemical and biological processes. Adv Gold Ore Process 4528:672–702
Botz M, Mudder T, Akcil A (2016) Cyanide treatment: physical, chemical, and biological processes. In: Gold ore processing. Elsevier, pp 619–645
Dash RR, Balomajumder C, Kumar A (2008) Treatment of metal cyanide bearing wastewater by simultaneous adsorption and biodegradation (SAB). J Hazard Mater 152:387–396. https://doi.org/10.1016/j.jhazmat.2007.07.009
Dash RR, Balomajumder C, Kumar A (2009a) Removal of cyanide from water and wastewater using granular activated carbon. Chem Eng J 146:408–413
Dash RR, Gaur A, Balomajumder C (2009b) Cyanide in industrial wastewaters and its removal: a review on biotreatment. J Hazard Mater 163:1–11
Davari N, Farhadian M, Nazar ARS, Homayoonfal M (2017) Degradation of diphenhydramine by the photocatalysts of ZnO/Fe2O3 and TiO2/Fe2O3 based on clinoptilolite: Structural and operational comparison. J Environ Chem Eng 5:5707–5720
Deegan A, Shaik B, Nolan K, Urell K, Oelgemöller M, Tobin J, Morrissey A (2011) Treatment options for wastewater effluents from pharmaceutical companies. Int J Environ Sci Technol 8:649–666
Depci T (2012) Comparison of activated carbon and iron impregnated activated carbon derived from Gölbaşı lignite to remove cyanide from water. Chem Eng J 181–182:467–478. https://doi.org/10.1016/j.cej.2011.12.003
Do S-H, Jo Y-H, Park H-D, Kong S-H (2012) Synthesis of iron composites on nano-pore substrates: identification and its application to removal of cyanide. Chemosphere 89:1450–1456
Dwivedi N, Balomajumder C, Mondal P (2016) Comparative investigation on the removal of cyanide from aqueous solution using two different bioadsorbents. Water Resour Ind 15:28–40
Eskandari P, Farhadian M, SolaimanyNazar AR (2017) Performance enhancement and optimization of photocatalytic cyanide degradation in aqueous solution using Zn (II) and Fe(III) oxides as nanostructure supported on activated carbon. J Chem Technol Biotechnol 92:2360–2368
Eskandari P, Farhadian M, Solaimany Nazar AR, Jeon B-H (2019) Adsorption and photodegradation efficiency of TiO2/Fe2O3/PAC and TiO2/Fe2O3/zeolite nanophotocatalysts for the removal of cyanide. Ind Eng Chem Res 58:2099–2112
Federation WE, Association APH (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington
Ghaedi M, Biyareh MN, Kokhdan SN, Shamsaldini S, Sahraei R, Daneshfar A, Shahriyar S (2012) Comparison of the efficiency of palladium and silver nanoparticles loaded on activated carbon and zinc oxide nanorods loaded on activated carbon as new adsorbents for removal of Congo red from aqueous solution: kinetic and isotherm study. Mater Sci Eng, C 32:725–734
Ghasemi R, Sayahi T, Tourani S, Kavianimehr M (2016) Modified magnetite nanoparticles for hexavalent chromium removal from water. J Dispersion Sci Technol 37:1303–1314
Gicheva G, Yordanov G (2013) Removal of citrate-coated silver nanoparticles from aqueous dispersions by using activated carbon. Colloids Surf A 431:51–59
Green-Pedersen H, Jensen B, Pind N (1997) Nickel adsorption on MnO2, Fe (OH) 3, montmorillonite, humic acid and calcite: a comparative study. Environ Technol 18:807–815
Guo Y, Zhao C, Li C (2015) CO2 Adsorption Kinetics of K2CO3/Activated Carbon for Low-Concentration CO2 removal from confined spaces. Chem Eng Technol 38:891–899
Halet F, Yeddou AR, Chergui A, Chergui S, Nadjemi B, Ould-Dris A (2015) Removal of cyanide from aqueous solutions by adsorption on activated carbon prepared from lignocellulosic by-products. J Dispersion Sci Technol 36:1736–1741
Hernández A, Maya L, Sánchez-Mora E, Sánchez EM (2007) Sol-gel synthesis, characterization and photocatalytic activity of mixed oxide ZnO-Fe2O3. J Sol-Gel Sci Technol 42:71–78
Hijosa-Valsero M, Molina R, Schikora H, Müller M, Bayona JM (2013) Removal of cyanide from water by means of plasma discharge technology. Water Res 47:1701–1707
Islam MA, Khan MM, Mozumder MS (2004) Adsorption equilibrium and adsorption kinetics: a unified approach. Chem Eng Technol Ind Chem Plant Equip Process Eng Biotechnol 27:1095–1098
Kim SH, Lee SW, Lee GM, Lee B-T, Yun S-T, Kim S-O (2016) Monitoring of TiO2-catalytic UV-LED photo-oxidation of cyanide contained in mine wastewater and leachate. Chemosphere 143:106–114
Kim T-K, Kim T, Jo A, Park S, Choi K, Zoh K-D (2018) Degradation mechanism of cyanide in water using a UV-LED/H2O2/Cu2+ system. Chemosphere 208:441–449
Komkiene J, Baltrenaite E (2016) Biochar as adsorbent for removal of heavy metal ions [Cadmium (II), Copper (II), Lead (II), Zinc (II)] from aqueous phase. Int J Environ Sci Technol 13:471–482
Kuyucak N, Akcil A (2013) Cyanide and removal options from effluents in gold mining and metallurgical processes. Miner Eng 50:13–29
Lazarević S, Janković-Častvan I, Potkonjak B, Janaćković D, Petrović R (2012) Removal of Co 2 + ions from aqueous solutions using iron-functionalized sepiolite. Chem Eng Process 55:40–47
Li K, Zheng Z, Huang X, Zhao G, Feng J, Zhang J (2009) Equilibrium, kinetic and thermodynamic studies on the adsorption of 2-nitroaniline onto activated carbon prepared from cotton stalk fibre. J Hazard Mater 166:213–220
Maulana I, Takahashi F (2018) Cyanide removal study by raw and iron-modified synthetic zeolites in batch adsorption experiments. J Water Process Eng 22:80–86
Maya-Treviño M, Guzmán-Mar J, Hinojosa-Reyes L, Ramos-Delgado N, Maldonado MI, Hernández-Ramírez A (2014) Activity of the ZnO–Fe2O3 catalyst on the degradation of Dicamba and 2, 4-D herbicides using simulated solar light. Ceram Int 40:8701–8708
Mishra J, Pattanayak DS, Das AA, Mishra DK, Rath D, Sahoo NK (2019) Enhanced photocatalytic degradation of cyanide employing Fe-porphyrin sensitizer with hydroxyapatite palladium doped TiO2nano-composite system. J Mol Liq 287:110821
Mondal P, Mohanty B, Balomajumder C (2010) Treatment of arsenic contaminated groundwater using calcium impregnated granular activated carbon in a batch reactor: optimization of process parameters CLEAN–Soil. Air, Water 38:129–139
Mor S, Chhoden K, Negi P, Ravindra K (2017) Utilization of nano-alumina and activated charcoal for phosphate removal from wastewater Environmental Nanotechnology. Monit Manage 7:15–23
Moussavi G, Khosravi R (2010) Removal of cyanide from wastewater by adsorption onto pistachio hull wastes: parametric experiments, kinetics and equilibrium analysis. J Hazard Mater 183:724–730
Ouzzine M, Romero-Anaya AJ, Lillo-Ródenas MA, Linares-Solano A (2014) Spherical activated carbon as an enhanced support for TiO2/AC photocatalysts. Carbon 67:104–118
Pal B, Kaur R, Grover IS (2016) Superior adsorption and photodegradation of eriochrome black-T dye by Fe 3 + and Pt 4 + impregnated TiO 2 nanostructures of different shapes. J Ind Eng Chem 33:178–184
Pan H, Tian M, Zhang H, Zhang Y, Lin Q (2013) Adsorption and desorption performance of dichloromethane over activated carbons modified by metal ions. J Chem Eng Data 58:2449–2454
Papandreou A, Stournaras C, Panias D, Paspaliaris I (2011) Adsorption of Pb(II), Zn (II) and Cr(III) on coal fly ash porous pellets. Miner Eng 24:1495–1501
Reed BE, Vaughan R, Jiang L (2000) As (III), As (V), Hg, and Pb removal by Fe-oxide impregnated activated carbon. J Environ Eng 126:869–873
Saadi R, Saadi Z, Fazaeli R, Fard NE (2015) Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J Chem Eng 32:787–799
Saleh TA, Alhooshani KR, Abdelbassit MS (2015) Evaluation of AC/ZnO composite for sorption of dichloromethane, trichloromethane and carbon tetrachloride: kinetics and isotherms. Journal of the Taiwan Institute of Chemical Engineers 55:159–169
Sánchez-Castillo MA, Carrillo-Pedroza FR, Fraga-Tovar F, Soria-Aguilar MdJ (2015) Ozonation of cyanide catalyzed by activated carbon. Ozone Sci Eng 37:240–251
Shah I, Adnan R, Ngah WSW, Mohamed N (2015) Iron impregnated activated carbon as an efficient adsorbent for the removal of methylene blue: regeneration and kinetics studies. PloS one 10
Shen H-X, Yao J-L, Gu R-A (2009) Fabrication and characteristics of spindle Fe2O3@ Au core/shell particles. Trans Nonferr Metals Soc China 19:652–656
Singh N, Balomajumder C (2016) Simultaneous removal of phenol and cyanide from aqueous solution by adsorption onto surface modified activated carbon prepared from coconut shell Journal of Water. Process Eng 9:233–245. https://doi.org/10.1016/j.jwpe.2016.01.008
Sirianuntapiboon S, Chairattanawan K, Rarunroeng M (2008) Biological removal of cyanide compounds from electroplating wastewater (EPWW) by sequencing batch reactor (SBR) system. J Hazard Mater 154:526–534
Solaimany Nazar AR, Jokar Baloochi S, Goshadrou A (2018) 2, 4-dichlorophenoxyacetic acid adsorption from contaminated water through activated carbon reclaimed with zero-valent iron and titanium dioxide. Scientiairanica 25:1395–1411
Stavropoulos G, Skodras G, Papadimitriou K (2015) Effect of solution chemistry on cyanide adsorption in activated carbon. Appl Therm Eng 74:182–185
Sun Q, Hu X, Zheng S, Sun Z, Liu S, Li H (2015) Influence of calcination temperature on the structural, adsorption and photocatalytic properties of TiO2 nanoparticles supported on natural zeolite. Powder Technol 274:88–97b
Tan IAW, Ahmad AL, Hameed BH (2009) Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J Hazard Mater 164:473–482. https://doi.org/10.1016/j.jhazmat.2008.08.025
Valizadeh S, Younesi H, Bahramifar N (2016) Highly mesoporous K2CO3 and KOH/activated carbon for SDBS removal from water samples: batch and fixed-bed column adsorption process Environmental Nanotechnology. Monit Manage 6:1–13
Vasques ÉDC, Carpiné D, Dagostin JLA, Canteli AMD, Igarashi-Mafra L, Mafra MR, Scheer AdP (2014) Modelling studies by adsorption for the removal of sunset yellow azo dye present in effluent from a soft drink plant. Environ Technol 35:1532–1540
Wang C, Shi H, Li Y (2011a) Synthesis and characteristics of natural zeolite supported Fe3 + -TiO2 photocatalysts. Appl Surf Sci 257:6873–6877
Wang L, Zhang J, Zhao R, Zhang C, Li C, Li Y (2011b) Adsorption of 2, 4-dichlorophenol on Mn-modified activated carbon prepared from Polygonum orientale Linn. Desalination 266:175–181
Yeddou AR, Nadjemi B, Halet F, Ould-Dris A, Capart R (2010) Removal of cyanide in aqueous solution by oxidation with hydrogen peroxide in presence of activated carbon prepared from olive stones. Miner Eng 23:32–39
Yeddou AR et al (2011) Removal of cyanide in aqueous solution by oxidation with hydrogen peroxide in presence of copper-impregnated activated carbon. Miner Eng 24:788–793
Yu X-Z (2015) Uptake, assimilation and toxicity of cyanogenic compounds in plants: facts and fiction. Int J Environ Sci Technol 12:763–774
Acknowledgment
The authors would like to thank the Environmental Research Institute—University of Isfahan and Iranian National Nanotechnology Initiative for scientific assistance with this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Fatih ŞEN.
Rights and permissions
About this article
Cite this article
Eskandari, P., Farhadian, M., Solaimany Nazar, A.R. et al. Cyanide adsorption on activated carbon impregnated with ZnO, Fe2O3, TiO2 nanometal oxides: a comparative study. Int. J. Environ. Sci. Technol. 18, 297–316 (2021). https://doi.org/10.1007/s13762-020-02791-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-02791-0