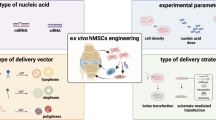
Abstract
Background:
Viral vector-based therapeutic gene therapy is a potent strategy to enhance the intrinsic reparative abilities of human orthopaedic tissues. However, clinical application of viral gene transfer remains hindered by detrimental responses in the host against such vectors (immunogenic responses, vector dissemination to nontarget locations). Combining viral gene therapy techniques with tissue engineering procedures may offer strong tools to improve the current systems for applications in vivo.
Methods:
The goal of this work is to provide an overview of the most recent systems exploiting biomaterial technologies and therapeutic viral gene transfer in human orthopaedic regenerative medicine.
Results:
Integration of tissue engineering platforms with viral gene vectors is an active area of research in orthopaedics as a means to overcome the obstacles precluding effective viral gene therapy.
Conclusions:
In light of promising preclinical data that may rapidly expand in a close future, biomaterial-guided viral gene therapy has a strong potential for translation in the field of human orthopaedic regenerative medicine.

Similar content being viewed by others
References
Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34.
Madry H, Grün UW, Knutsen G. Cartilage repair and joint preservation: medical and surgical treatment options. Dtsch Arztebl Int. 2011;108:669–77.
Tuan RS, Chen AF, Klatt BA. Cartilage regeneration. J Am Acad Orthop Surg. 2013;21:303–11.
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95.
Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A:455–64.
Einhorn TA. Enhancement of fracture-healing. J Bone Joint Surg Am. 1995;77:940–56.
Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11:45–54.
Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–5.
McDermott I. Meniscal tears, repairs and replacement: their relevance to osteoarthritis of the knee. Br J Sports Med. 2011;45:292–7.
Verdonk R, Madry H, Shabshin N, Dirisamer F, Peretti GM, Pujol N, et al. The role of meniscal tissue in joint protection in early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24:1763–74.
Hildebrand KA, Frank CB. Scar formation and ligament healing. Can J Surg. 1998;41:425–9.
Woo SL, Vogrin TM, Abramowitch SD. Healing and repair of ligament injuries in the knee. J Am Acad Orthop Surg. 2000;8:364–72.
Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science. 2018. https://doi.org/10.1126/science.aan4672.
Lieberman JR, Ghivizzani SC, Evans CH. Gene transfer approaches to the healing of bone and cartilage. Mol Ther. 2002;6:141–7.
Wu D, Razzano P, Grande DA. Gene therapy and tissue engineering in repair of the musculoskeletal system. J Cell Biochem. 2003;88:467–81.
Nixon AJ, Watts AE, Schnabel LV. Cell- and gene-based approaches to tendon regeneration. J Shoulder Elbow Surg. 2012;21:278–94.
Evans CH, Huard J. Gene therapy approaches to regenerating the musculoskeletal system. Nat Rev Rheumatol. 2015;11:234–42.
Cucchiarini M, McNulty AL, Mauck RL, Setton LA, Guilak F, Madry H. Advances in combining gene therapy with cell and tissue engineering-based approaches to enhance healing of the meniscus. Osteoarthritis Cartilage. 2016;24:1330–9.
Im GI. Gene transfer strategies to promote chondrogenesis and cartilage regeneration. Tissue Eng Part B Rev. 2016;22:136–48.
Grol MW, Lee BH. Gene therapy for repair and regeneration of bone and cartilage. Curr Opin Pharmacol. 2018;40:59–66.
Al-Dosari MS, Gao X. Nonviral gene delivery: principle, limitations, and recent progress. AAPS J. 2009;11:671–81.
Im GI. Nonviral gene transfer strategies to promote bone regeneration. J Biomed Mater Res A. 2013;101:3009–18.
Giacca M, Zacchigna S. Virus-mediated gene delivery for human gene therapy. J Control Release. 2012;161:377–88.
Appaiahgari MB, Vrati S. Adenoviruses as gene/vaccine delivery vectors: promises and pitfalls. Expert Opin Biol Ther. 2015;15:337–51.
Glorioso JC. Herpes simplex viral vectors: late bloomers with big potential. Hum Gene Ther. 2014;25:83–91.
Miller AD, Jolly DJ, Friedmann T, Verma IM. A transmissible retrovirus expressing human hypoxanthine phosphoribosyltransferase (HPRT): gene transfer into cells obtained from humans deficient in HPRT. Proc Natl Acad Sci U S A. 1983;80:4709–13.
Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–7.
Berns KI, Giraud C. Adenovirus and adeno-associated virus as vectors for gene therapy. Ann N Y Acad Sci. 1995;772:95–104.
Grieger JC, Samulski RJ. Adeno-associated virus vectorology, manufacturing, and clinical applications. Methods Enzymol. 2012;507:229–54.
Kotterman MA, Schaffer DV. Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet. 2014;15:445–51.
Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–5.
Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–42.
Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–108.
Madry H, Cucchiarini M, Terwilliger EF, Trippel SB. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum Gene Ther. 2003;14:393–402.
Nault JC, Datta S, Imbeaud S, Franconi A, Mallet M, Couchy G, et al. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat Genet. 2015;47:1187–93.
Gil-Farina I, Fronza R, Kaeppel C, Lopez-Franco E, Ferreira V, D’Avola D, et al. Recombinant AAV integration is not associated with hepatic genotoxicity in nonhuman primates and patients. Mol Ther. 2016;24:1100–5.
Morisset S, Frisbie DD, Robbins PD, Nixon AJ, McIllwraith CW. IL-1ra/IGF-1 gene therapy modulates repair of microfractured chondral defects. Clin Orthop Relat Res. 2007;462:221–8.
Cucchiarini M, Madry H, Ma C, Thurn T, Zurakowski D, Menger MD, et al. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol Ther. 2005;12:229–38.
Hiraide A, Yokoo N, Xin KQ, Okuda K, Mizukami H, Ozawa K, et al. Repair of articular cartilage defect by intraarticular administration of basic fibroblast growth factor gene, using adeno-associated virus vector. Hum Gene Ther. 2005;16:1413–21.
Cucchiarini M, Orth P, Madry H. Direct rAAV SOX9 administration for durable articular cartilage repair with delayed terminal differentiation and hypertrophy in vivo. J Mol Med (Berl). 2013;91:625–36.
Cucchiarini M, Madry H. Overexpression of human IGF-I via direct rAAV-mediated gene transfer improves the early repair of articular cartilage defects in vivo. Gene Ther. 2014;21:811–9.
Cucchiarini M, Asen AK, Goebel L, Venkatesan JK, Schmitt G, Zurakowski D, et al. Effects of TGF-β overexpression via rAAV gene transfer on the early repair processes in an osteochondral defect model in minipigs. Am J Sports Med. 2018;46:1987–96.
Baltzer AW, Lattermann C, Whalen JD, Wooley P, Weiss K, Grimm M, et al. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000;7:734–9.
Tarkka T, Sipola A, Jämsä T, Soini Y, Ylä-Herttuala S, Tuukkanen J, et al. Adenoviral VEGF-A gene transfer induces angiogenesis and promotes bone formation in healing osseous tissues. J Gene Med. 2003;5:560–6.
Southwood LL, Frisbie DD, Kawcak CE, Ghivizzani SC, Evans CH, McIlwraith CW. Evaluation of Ad-BMP-2 for enhancing fracture healing in an infected defect fracture rabbit model. J Orthop Res. 2004;22:66–72.
Betz OB, Betz VM, Nazarian A, Pilapil CG, Vrahas MS, Bouxsein ML, et al. Direct percutaneous gene delivery to enhance healing of segmental bone defects. J Bone Joint Surg Am. 2006;88:355–65.
Egermann M, Lill CA, Griesbeck K, Evans CH, Robbins PD, Schneider E, et al. Effect of BMP-2 gene transfer on bone healing in sheep. Gene Ther. 2006;13:1290–9.
Betz OB, Betz VM, Nazarian A, Egermann M, Gerstenfeld LC, Einhorn TA, et al. Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene Ther. 2007;14:1039–44.
Ishihara A, Shields KM, Litsky AS, Mattoon JS, Weisbrode SE, Bartlett JS, et al. Osteogenic gene regulation and relative acceleration of healing by adenoviral-mediated transfer of human BMP-2 or -6 in equine osteotomy and ostectomy models. J Orthop Res. 2008;26:764–71.
Rundle CH, Miyakoshi N, Kasukawa Y, Chen ST, Sheng MH, Wergedal JE, et al. In vivo bone formation in fracture repair induced by direct retroviral-based gene therapy with bone morphogenetic protein-4. Bone. 2003;32:591–601.
Rundle CH, Strong DD, Chen ST, Linkhart TA, Sheng MH, Wergedal JE, et al. Retroviral-based gene therapy with cyclooxygenase-2 promotes the union of bony callus tissues and accelerates fracture healing in the rat. J Gene Med. 2008;10:229–41.
Strohbach CA, Rundle CH, Wergedal JE, Chen ST, Linkhart TA, Lau KH, et al. LMP-1 retroviral gene therapy influences osteoblast differentiation and fracture repair: a preliminary study. Calcif Tissue Int. 2008;83:202–11.
Cucchiarini M, Schetting S, Terwilliger EF, Kohn D, Madry H. rAAV-mediated overexpression of FGF-2 promotes cell proliferation, survival, and alpha-SMA expression in human meniscal lesions. Gene Ther. 2009;16:1363–72.
Cucchiarini M, Schmidt K, Frisch J, Kohn D, Madry H. Overexpression of TGF-β via rAAV-mediated gene transfer promotes the healing of human meniscal lesions ex vivo on explanted menisci. Am J Sports Med. 2015;43:1197–205.
Madry H, Kohn D, Cucchiarini M. Direct FGF-2 gene transfer via recombinant adeno-associated virus vectors stimulates cell proliferation, collagen production, and the repair of experimental lesions in the human ACL. Am J Sports Med. 2013;41:194–202.
Tang JB, Chen CH, Zhou YL, McKeever C, Liu PY. Regulatory effects of introduction of an exogenous FGF2 gene on other growth factor genes in a healing tendon. Wound Repair Regen. 2014;22:111–8.
Tang JB, Wu YF, Cao Y, Chen CH, Zhou YL, Avanessian B, et al. Basic FGF or VEGF gene therapy corrects insufficiency in the intrinsic healing capacity of tendons. Sci Rep. 2016;6:20643.
Gelse K, von der Mark K, Aigner T, Park J, Schneider H. Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis Rheum. 2003;48:430–41.
Kuroda R, Usas A, Kubo S, Corsi K, Peng H, Rose T, et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54:433–42.
Park J, Gelse K, Frank S, von der Mark K, Aigner T, Schneider H. Transgene-activated mesenchymal cells for articular cartilage repair: a comparison of primary bone marrow-, perichondrium/periosteum- and fat-derived cells. J Gene Med. 2006;8:112–25.
Pagnotto MR, Wang Z, Karpie JC, Ferretti M, Xiao X, Chu CR. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007;14:804–13.
Kubo S, Cooper GM, Matsumoto T, Phillippi JA, Corsi KA, Usas A, et al. Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle-derived stem cells. Arthritis Rheum. 2009;60:155–65.
Matsumoto T, Cooper GM, Gharaibeh B, Meszaros LB, Li G, Usas A, et al. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60:1390–405.
Liu TM, Guo XM, Tan HS, Hui JH, Lim B, Lee EH. Zinc-finger protein 145, acting as an upstream regulator of SOX9, improves the differentiation potential of human mesenchymal stem cells for cartilage regeneration and repair. Arthritis Rheum. 2011;63:2711–20.
Lee JM, Im GI. SOX trio-co-transduced adipose stem cells in fibrin gel to enhance cartilage repair and delay the progression of osteoarthritis in the rat. Biomaterials. 2012;33:2016–24.
Hidaka C, Goodrich LR, Chen CT, Warren RF, Crystal RG, Nixon AJ. Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. J Orthop Res. 2003;21:573–83.
Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J Bone Joint Surg Br. 2007;89:672–85.
Ortved KF, Begum L, Mohammed HO, Nixon AJ. Implantation of rAAV5-IGF-I transduced autologous chondrocytes improves cartilage repair in full-thickness defects in the equine model. Mol Ther. 2015;23:363–73.
Ivkovic A, Pascher A, Hudetz D, Maticic D, Jelic M, Dickinson S, et al. Articular cartilage repair by genetically modified bone marrow aspirate in sheep. Gene Ther. 2010;17:779–89.
Sieker JT, Kunz M, Weißenberger M, Gilbert F, Frey S, Rudert M, et al. Direct bone morphogenetic protein 2 and Indian hedgehog gene transfer for articular cartilage repair using bone marrow coagulates. Osteoarthritis Cartilage. 2015;23:433–42.
Evans CH, Liu FJ, Glatt V, Hoyland JA, Kirker-Head C, Walsh A, et al. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur Cell Mater. 2009;18:96–111.
Betz VM, Keller A, Foehr P, Thirion C, Salomon M, Rammelt S, et al. BMP-2 gene activated muscle tissue fragments for osteochondral defect regeneration in the rabbit knee. J Gene Med. 2017;19:e2972.
Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999;81:905–17.
Kumar S, Wan C, Ramaswamy G, Clemens TL, Ponnazhagan S. Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol Ther. 2010;18:1026–34.
Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11:291–7.
Koefoed M, Ito H, Gromov K, Reynolds DG, Awad HA, Rubery PT, et al. Biological effects of rAAV-caAlk2 coating on structural allograft healing. Mol Ther. 2005;12:212–8.
Betz OB, Betz VM, Abdulazim A, Penzkofer R, Schmitt B, Schröder C, et al. Healing of large segmental bone defects induced by expedited bone morphogenetic protein-2 gene-activated, syngeneic muscle grafts. Hum Gene Ther. 2009;20:1589–96.
Yazici C, Takahata M, Reynolds DG, Xie C, Samulski RJ, Samulski J, et al. Self-complementary AAV2.5-BMP2-coated femoral allografts mediated superior bone healing versus live autografts in mice with equivalent biomechanics to unfractured femur. Mol Ther. 2011;19:1416–25.
Betz VM, Betz OB, Rosin T, Keller A, Thirion C, Salomon M, et al. The effect of BMP-7 gene activated muscle tissue implants on the repair of large segmental bone defects. Injury. 2015;46:2351–8.
Liu F, Ferreira E, Porter RM, Glatt V, Schinhan M, Shen Z, et al. Rapid and reliable healing of critical size bone defects with genetically modified sheep muscle. Eur Cell Mater. 2015;30:118–30.
Betz VM, Betz OB, Rosin T, Keller A, Thirion C, Salomon M, et al. An expedited approach for sustained delivery of bone morphogenetic protein-7 to bone defects using gene activated fragments of subcutaneous fat. J Gene Med. 2016;18:199–207.
Hou Y, Mao Z, Wei X, Lin L, Chen L, Wang H, et al. Effects of transforming growth factor-beta1 and vascular endothelial growth factor 165 gene transfer on Achilles tendon healing. Matrix Biol. 2009;28:324–35.
Basile P, Dadali T, Jacobson J, Hasslund S, Ulrich-Vinther M, Søballe K, et al. Freeze-dried tendon allografts as tissue-engineering scaffolds for Gdf5 gene delivery. Mol Ther. 2008;16:466–73.
Majewski M, Betz O, Ochsner PE, Liu F, Porter RM, Evans CH. Ex vivo adenoviral transfer of bone morphogenetic protein 12 (BMP-12) cDNA improves Achilles tendon healing in a rat model. Gene Ther. 2008;15:1139–46.
Schnabel LV, Lynch ME, van der Meulen MC, Yeager AE, Kornatowski MA, Nixon AJ. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. J Orthop Res. 2009;27:1392–8.
Majewski M, Porter RM, Betz OB, Betz VM, Clahsen H, Flückiger R, et al. Improvement of tendon repair using muscle grafts transduced with TGF-β1 cDNA. Eur Cell Mater. 2012;23:94–101.
Hasslund S, Dadali T, Ulrich-Vinther M, Søballe K, Schwarz EM, Awad HA. Freeze-dried allograft-mediated gene or protein delivery of growth and differentiation factor 5 reduces reconstructed murine flexor tendon adhesions. J Tissue Eng. 2014;5:2041731414528736.
Tan C, Lui PP, Lee YW, Wong YM. Scx-transduced tendon-derived stem cells (tdscs) promoted better tendon repair compared to mock-transduced cells in a rat patellar tendon window injury model. PLoS One. 2014;9:e97453.
Ghivizzani SC, Lechman ER, Kang R, Tio C, Kolls J, Evans CH, et al. Direct adenovirus-mediated gene transfer of interleukin 1 and tumor necrosis factor alpha soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects. Proc Natl Acad Sci U S A. 1998;95:4613–8.
Lechman ER, Jaffurs D, Ghivizzani SC, Gambotto A, Kovesdi I, Mi Z, Evans CH, et al. Direct adenoviral gene transfer of viral IL-10 to rabbit knees with experimental arthritis ameliorates disease in both injected and contralateral control knees. J Immunol. 1999;163:2202–8.
Chan JM, Villarreal G, Jin WW, Stepan T, Burstein H, Wahl SM. Intraarticular gene transfer of TNFR: Fc suppresses experimental arthritis with reduced systemic distribution of the gene product. Mol Ther. 2002;6:727–36.
Zhou X, Shen L, Liu L, Wang C, Qi W, Zhao A, et al. Preclinical safety evaluation of recombinant adeno-associated virus 2 vector encoding human tumor necrosis factor receptor-immunoglobulin Fc fusion gene. Hum Vaccin Immunother. 2016;12:732–9.
Bellavia D, Veronesi F, Carina V, Costa V, Raimondi L, De Luca A, et al. Gene therapy for chondral and osteochondral regeneration: is the future now? Cell Mol Life Sci. 2018;75:649–67.
Cottard V, Valvason C, Falgarone G, Lutomski D, Boissier MC, Bessis N. Immune response against gene therapy vectors: influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J Clin Immunol. 2004;24:162–9.
Mangeat B, Trono D. Lentiviral vectors and antiretroviral intrinsic immunity. Hum Gene Ther. 2005;16:913–20.
Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–90.
Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17:295–304.
Fausther-Bovendo H, Kobinger GP. Pre-existing immunity against Ad vectors: humoral, cellular, and innate response, what’s important? Hum Vaccin Immunother. 2014;10:2875–84.
Schuettrumpf J, Zou J, Zhang Y, Schlachterman A, Liu YL, Edmonson S, et al. The inhibitory effects of anticoagulation on in vivo gene transfer by adeno-associated viral or adenoviral vectors. Mol Ther. 2006;13:88–97.
Robbins PD, Tahara H, Ghivizzani SC. Viral vectors for gene therapy. Trends Biotechnol. 1998;16:35–40.
Campbell EM, Hope TJ. Gene therapy progress and prospects: viral trafficking during infection. Gene Ther. 2005;12:1353–9.
Langer RS, Peppas NA. Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials. 1981;2:201–14.
Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6.
Freed LE, Guilak F, Guo XE, Gray ML, Tranquillo R, Holmes JW, et al. Advanced tools for tissue engineering: scaffolds, bioreactors, and signaling. Tissue Eng. 2006;12:3285–305.
Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, et al. Engineering complex tissues. Tissue Eng. 2006;12:3307–39.
Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, et al. Tissue engineering for articular cartilage repair—the state of the art. Eur Cell Mater. 2013;25:248–67.
Smith BD, Grande DA. The current state of scaffolds for musculoskeletal regenerative applications. Nat Rev Rheumatol. 2015;11:213–22.
Tatara AM, Mikos AG. Tissue engineering in orthopaedics. J Bone Joint Surg Am. 2016;98:1132–9.
Verrier S, Alini M, Alsberg E, Buchman SR, Kelly D, Laschke MW, et al. Tissue engineering and regenerative approaches to improving the healing of large bone defects. Eur Cell Mater. 2016;32:87–110.
Armiento AR, Stoddart MJ, Alini M, Eglin D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018;65:1–20.
Kim BS, Cho CS. Injectable hydrogels for regenerative medicine. Tissue Eng Regen Med. 2018;15:511–2.
Oh HJ, Kim SH, Cho JH, Park SH, Min BH. Mechanically reinforced extracellular matrix scaffold for the application of cartilage tissue engineering. Tissue Eng Regen Med. 2018;15:287–99.
Patel M, Park S, Lee HJ, Jeong B. Polypeptide thermogels as three-dimensional scaffolds for cells. Tissue Eng Regen Med. 2018;15:521–30.
Mason JM, Breitbart AS, Barcia M, Porti D, Pergolizzi RG, Grande DA. Cartilage and bone regeneration using gene-enhanced tissue engineering. Clin Orthop Relat Res. 2000;379:S171–8.
Grande DA, Mason J, Light E, Dines D. Stem cells as platforms for delivery of genes to enhance cartilage repair. J Bone Joint Surg Am. 2003;85-A Suppl 2:111–6.
Yokoo N, Saito T, Uesugi M, Kobayashi N, Xin KQ, Okuda K, et al. Repair of articular cartilage defect by autologous transplantation of basic fibroblast growth factor gene-transduced chondrocytes with adeno-associated virus vector. Arthritis Rheum. 2005;52:164–70.
Cao L, Yang F, Liu G, Yu D, Li H, Fan Q, et al. The promotion of cartilage defect repair using adenovirus mediated Sox9 gene transfer of rabbit bone marrow mesenchymal stem cells. Biomaterials. 2011;32:3910–20.
Klinger P, Surmann-Schmitt C, Brem M, Swoboda B, Distler JH, Carl HD, et al. Chondromodulin 1 stabilizes the chondrocyte phenotype and inhibits endochondral ossification of porcine cartilage repair tissue. Arthritis Rheum. 2011;63:2721–31.
Qi BW, Yu AX, Zhu SB, Zhou M, Wu G. Chiotosan/poly(vinyl alcohol) hydrogel combined with Ad-hTGF-β1 transfected mesenchymal stem cells to repair rabbit articular cartilage defects. Exp Biol Med (Maywood). 2013;238:23–30.
Rose T, Peng H, Shen HC, Usas A, Kuroda R, Lill H, et al. The role of cell type in bone healing mediated by ex vivo gene therapy. Langenbecks Arch Surg. 2003;388:347–55.
Shen HC, Peng H, Usas A, Gearhart B, Fu FH, Huard J. Structural and functional healing of critical-size segmental bone defects by transduced muscle-derived cells expressing BMP4. J Gene Med. 2004;6:984–91.
Peterson B, Zhang J, Iglesias R, Kabo M, Hedrick M, Benhaim P, et al. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120–9.
Hsu WK, Sugiyama O, Park SH, Conduah A, Feeley BT, Liu NQ, et al. Lentiviral-mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone. 2007;40:931–8.
Virk MS, Conduah A, Park SH, Liu N, Sugiyama O, Cuomo A, et al. Influence of short-term adenoviral vector and prolonged lentiviral vector mediated bone morphogenetic protein-2 expression on the quality of bone repair in a rat femoral defect model. Bone. 2008;42:921–31.
Wojtowicz AM, Templeman KL, Hutmacher DW, Guldberg RE, García AJ. Runx2 overexpression in bone marrow stromal cells accelerates bone formation in critical-sized femoral defects. Tissue Eng Part A. 2010;16:2795–808.
Li J, Zhao Q, Wang E, Zhang C, Wang G, Yuan Q. Transplantation of Cbfa1-overexpressing adipose stem cells together with vascularized periosteal flaps repair segmental bone defects. J Surg Res. 2012;176:e13–20.
Sonnet C, Simpson CL, Olabisi RM, Sullivan K, Lazard Z, Gugala Z, et al. Rapid healing of femoral defects in rats with low dose sustained BMP2 expression from PEGDA hydrogel microspheres. J Orthop Res. 2013;31:1597–604.
Duan C, Liu J, Yuan Z, Meng G, Yang X, Jia S, et al. Adenovirus-mediated transfer of VEGF into marrow stromal cells combined with PLGA/TCP scaffold increases vascularization and promotes bone repair in vivo. Arch Med Sci. 2014;10:174–81.
Ho CY, Sanghani A, Hua J, Coathup M, Kalia P, Blunn G. Mesenchymal stem cells with increased stromal cell-derived factor 1 expression enhanced fracture healing. Tissue Eng Part A. 2015;21:594–602.
Yin C, Chen J, Chen Z, Zeng Z, Qiu J. hBMP-2 and hTGF-β1 expressed in implanted BMSCs synergistically promote the repairing of segmental bone defects. J Orthop Sci. 2015;20:717–27.
Bougioukli S, Jain A, Sugiyama O, Tinsley BA, Tang AH, Tan MH, et al. Combination therapy with BMP-2 and a systemic RANKL inhibitor enhances bone healing in a mouse critical-sized femoral defect. Bone. 2016;84:93–103.
Yan X, Zhou Z, Guo L, Zeng Z, Guo Z, Shao Q, et al. BMP7-overexpressing bone marrow-derived mesenchymal stem cells (BMSCs) are more effective than wild-type BMSCs in healing fractures. Exp Ther Med. 2018;16:1381–8.
Otabe K, Nakahara H, Hasegawa A, Matsukawa T, Ayabe F, Onizuka N, et al. Transcription factor Mohawk controls tenogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. J Orthop Res. 2015;33:1–8.
Xu K, Sun Y, Kh Al-Ani M, Wang C, Sha Y, Sung KP, et al. Synergistic promoting effects of bone morphogenetic protein 12/connective tissue growth factor on functional differentiation of tendon derived stem cells and patellar tendon window defect regeneration. J Biomech. 2018;66:95–102.
Fang J, Zhu YY, Smiley E, Bonadio J, Rouleau JP, Goldstein SA, et al. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc Natl Acad Sci U S A. 1996;93:5753–8.
Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–9.
Bonadio J. Tissue engineering via local gene delivery: update and future prospects for enhancing the technology. Adv Drug Deliv Rev. 2000;44:185–94.
Im GI, Kim HJ, Lee JH. Chondrogenesis of adipose stem cells in a porous PLGA scaffold impregnated with plasmid DNA containing SOX trio (SOX-5,-6 and -9) genes. Biomaterials. 2011;32:4385–92.
Pannier AK, Shea LD. Controlled release systems for DNA delivery. Mol Ther. 2004;10:19–26.
Gower RM, Shea LD. Biomaterial scaffolds for controlled, localized gene delivery of regenerative factors. Adv Wound Care (New Rochelle). 2013;2:100–6.
Jang JH, Schaffer DV, Shea LD. Engineering biomaterial systems to enhance viral vector gene delivery. Mol Ther. 2011;19:1407–15.
Cucchiarini M. Human gene therapy: novel approaches to improve the current gene delivery systems. Discov Med. 2016;21:495–506.
Rey-Rico A, Cucchiarini M. Controlled release strategies for rAAV-mediated gene delivery. Acta Biomater. 2016;29:1–10.
Raftery RM, Walsh DP, Castaño IM, Heise A, Duffy GP, Cryan SA, et al. Delivering nucleic-acid based nanomedicines on biomaterial scaffolds for orthopedic tissue repair: challenges, progress and future perspectives. Adv Mater. 2016;28:5447–69.
Rey-Rico A, Cucchiarini M. Recent tissue engineering-based advances for effective rAAV-mediated gene transfer in the musculoskeletal system. Bioengineered. 2016;7:175–88.
Rey-Rico A, Cucchiarini M, Madry H. Hydrogels for precision meniscus tissue engineering: a comprehensive review. Connect Tissue Res. 2017;58:317–28.
Venkatesan JK, Falentin-Daudré C, Leroux A, Migonney V, Cucchiarini M. Controlled release of gene therapy constructs from solid scaffolds for therapeutic applications in orthopedics. Discov Med. 2018;25:195–203.
Díaz-Rodríguez P, Rey-Rico A, Madry H, Landin M, Cucchiarini M. Effective genetic modification and differentiation of hMSCs upon controlled release of rAAV vectors using alginate/poloxamer composite systems. Int J Pharm. 2015;496:614–26.
Rey-Rico A, Venkatesan JK, Frisch J, Rial-Hermida I, Schmitt G, Concheiro A, et al. PEO–PPO–PEO micelles as effective rAAV-mediated gene delivery systems to target human mesenchymal stem cells without altering their differentiation potency. Acta Biomater. 2015;27:42–52.
Rey-Rico A, Venkatesan JK, Frisch J, Schmitt G, Monge-Marcet A, Lopez-Chicon P, et al. Effective and durable genetic modification of human mesenchymal stem cells via controlled release of rAAV vectors from self-assembling peptide hydrogels with a maintained differentiation potency. Acta Biomater. 2015;18:118–27.
Rey-Rico A, Frisch J, Venkatesan JK, Schmitt G, Rial-Hermida I, Taboada P, et al. PEO–PPO–PEO carriers for rAAV-mediated transduction of human articular chondrocytes in vitro and in a human osteochondral defect model. ACS Appl Mater Interfaces. 2016;8:20600–13.
Rey-Rico A, Babicz H, Madry H, Concheiro A, Alvarez-Lorenzo C, Cucchiarini M. Supramolecular polypseudorotaxane gels for controlled delivery of rAAV vectors in human mesenchymal stem cells for regenerative medicine. Int J Pharm. 2017;531:492–503.
Rey-Rico A, Venkatesan JK, Schmitt G, Concheiro A, Madry H, Alvarez-Lorenzo C, et al. rAAV-mediated overexpression of TGF-β via vector delivery in polymeric micelles stimulates the biological and reparative activities of human articular chondrocytes in vitro and in a human osteochondral defect model. Int J Nanomedicine. 2017;12:6985–96.
Rey-Rico A, Venkatesan JK, Schmitt G, Speicher-Mentges S, Madry H, Cucchiarini M. Effective remodelling of human osteoarthritic cartilage by sox9 gene transfer and overexpression upon delivery of rAAV vectors in polymeric micelles. Mol Pharm. 2018;15:2816–26.
Brunger JM, Huynh NP, Guenther CM, Perez-Pinera P, Moutos FT, Sanchez-Adams J, et al. Scaffold-mediated lentiviral transduction for functional tissue engineering of cartilage. Proc Natl Acad Sci U S A. 2014;111:E798–806.
Glass KA, Link JM, Brunger JM, Moutos FT, Gersbach CA, Guilak F. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials. 2014;35:5921–31.
Moutos FT, Glass KA, Compton SA, Ross AK, Gersbach CA, Guilak F, et al. Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing. Proc Natl Acad Sci U S A. 2016;113:E4513–22.
Rowland CR, Glass KA, Ettyreddy AR, Gloss CC, Matthews JRL, Huynh NPT, et al. Regulation of decellularized tissue remodeling via scaffold-mediated lentiviral delivery in anatomically-shaped osteochondral constructs. Biomaterials. 2018;177:161–75.
Uemura T, Kojima H. Bone formation in vivo induced by Cbfa1-carrying adenoviral vectors released from a biodegradable porous β-tricalcium phosphate (β-TCP) material. Sci Technol Adv Mater. 2011;12:034405.
Nasu T, Ito H, Tsutsumi R, Kitaori T, Takemoto M, Schwarz EM, et al. Biological activation of bone-related biomaterials by recombinant adeno-associated virus vector. J Orthop Res. 2009;27:1162–8.
Dupont KM, Boerckel JD, Stevens HY, Diab T, Kolambkar YM, Takahata M, et al. Synthetic scaffold coating with adeno-associated virus encoding BMP2 to promote endogenous bone repair. Cell Tissue Res. 2012;347:575–88.
Xue J, Lin H, Bean A, Tang Y, Tan J, Tuan RS, et al. One-step fabrication of bone morphogenetic protein-2 gene-activated porous poly-l-lactide scaffold for bone induction. Mol Ther Methods Clin Dev. 2017;7:50–9.
Evans CH, Ghivizzani SC, Robbins PD. Arthritis gene therapy is becoming a reality. Nat Rev Rheumatol. 2018;14:381–2.
Cherian JJ, Parvizi J, Bramlet D, Lee KH, Romness DW, Mont MA. Preliminary results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-β1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthritis Cartilage. 2015;23:2109–18.
Ha CW, Cho JJ, Elmallah RK, Cherian JJ, Kim TW, Lee MC, et al. A multicenter, single-blind, phase IIa clinical trial to evaluate the efficacy and safety of a cell-mediated gene therapy in degenerative knee arthritis patients. Hum Gene Ther Clin Dev. 2015;26:125–30.
Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene therapy clinical trials worldwide to 2017: an update. J Gene Med. 2018;20:e3015.
Bozo IY, Deev RV, Drobyshev AY, Isaev AA, Eremin II. World’s first clinical case of gene-activated bone substitute application. Case Rep Dent. 2016;2016:8648949.
Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–85.
Cunniffe GM, Gonzalez-Fernandez T, Daly A, Sathy BN, Jeon O, Alsberg E, et al. Three-dimensional bioprinting of polycaprolactone reinforced gene activated bioinks for bone tissue engineering. Tissue Eng Part A. 2017;23:891–900.
Daly AC, Freeman FE, Gonzalez-Fernandez T, Critchley SE, Nulty J, Kelly DJ. 3D bioprinting for cartilage and osteochondral tissue engineering. Adv Healthc Mater. 2017;6:1700298.
Doudna JA, Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096.
Adkar SS, Brunger JM, Willard VP, Wu CL, Gersbach CA, Guilak F. Genome engineering for personalized arthritis therapeutics. Trends Mol Med. 2017;23:917–31.
Almarza D, Cucchiarini M, Loughlin J. Genome editing for human osteoarthritis—a perspective. Osteoarthritis Cartilage. 2017;25:1195–8.
Kim M, Yun HW, Park DY, Choi BH, Min BH. Three-dimensional spheroid culture increases exosome secretion from mesenchymal stem cells. Tissue Eng Regen Med. 2018;15:427–36.
Acknowledgement
This work was supported by a Grant from the Deutsche Forschungsgemeinschaft (DFG VE 1099/1-1 to JKV and MC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Venkatesan, J.K., Rey-Rico, A. & Cucchiarini, M. Current Trends in Viral Gene Therapy for Human Orthopaedic Regenerative Medicine. Tissue Eng Regen Med 16, 345–355 (2019). https://doi.org/10.1007/s13770-019-00179-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-019-00179-x