Abstract
Background:
The urinary tract can be affected by both congenital abnormalities as well as acquired disorders, such as cancer, trauma, infection, inflammation, and iatrogenic injuries, all of which may lead to organ damage requiring eventual reconstruction. As a gold standard, gastrointestinal segment is used for urinary bladder reconstruction. However, one major problem is that while bladder tissue prevents reabsorption of specific solutes, gastrointestinal tissue actually absorbs them. Therefore, tissue engineering approach had been attempted to provide an alternative tissue graft for urinary bladder reconstruction.
Methods:
Human adipose-derived stem cells isolated from fat tissues were differentiated into smooth muscle cells and then seeded onto a triple-layered PLGA sheet to form a bladder construct. Adult athymic rats underwent subtotal urinary bladder resection and were divided into three treatment groups (n = 3): Group 1 (“sham”) underwent anastomosis of the remaining basal region, Group 2 underwent reconstruction with the cell-free scaffold, and Group 3 underwent reconstruction with the tissue-engineered bladder construct. Animals were monitored on a daily basis and euthanisation was performed whenever a decline in animal health was detected.
Results:
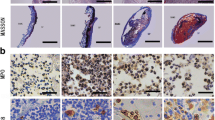
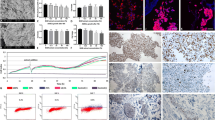
All animals in Groups 1, 2 and 3 survived for at least 7 days and were followed up to a maximum of 12 weeks post-operation. It was found that by Day 14, substantial ingrowth of smooth muscle and urothelial cells had occurred in Group 2 and 3. In the long-term follow up of group 3 (tissue-engineered bladder construct group), it was found that the urinary bladder wall was completely regenerated and bladder function was fully restored. Urodynamic and radiological evaluations of the reconstructed bladder showed a return to normal bladder volume and function.Histological analysis revealed the presence of three muscular layers and a urothelium similar to that of a normal bladder. Immunohistochemical staining using human-specific myocyte markers (myosin heavy chain and smoothelin) confirmed the incorporation of the seeded cells in the newly regenerated muscular layers.
Conclusion:
Implantation of PLGA construct seeded with smooth muscle cells derived from human adipose stem cells can lead to regeneration of the muscular layers and urothelial ingrowth, leading to formation of a completely functional urinary bladder.




Similar content being viewed by others
References
Yoo JJ, Meng J, Oberpenning F, Atala A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology. 1998;51:221–5.
Lewis SA. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol. 2000;278:F867–74.
Soergel TM, Cain MP, Misseri R, Gardner TA, Koch MO, Rink RC. Transitional cell carcinoma of the bladder following augmentation cystoplasty for the neuropathic bladder. J Urol. 2004;172:1649–51.
Lam Van Ba O, Aharony S, Loutochin O, Corcos J. Bladder tissue engineering: a literature review. Adv Drug Deliv Rev. 2015;82–83:31–7.
Atala A. Tissue engineering of human bladder. Br Med Bull. 2011;97:81–104.
Drewa T, Sir J, Czajkowski R, Wozniak A. Scaffold seeded with cells is essential in urothelium regeneration and tissue remodeling in vivo after bladder augmentation using in vitro engineered graft. Transplant Proc. 2006;38:133–5.
Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–6.
Lin HK, Cowan R, Moore P, Zhang Y, Yang Q, Peterson JA Jr, et al. Characterization of neuropathic bladder smooth muscle cells in culture. J Urol. 2004;171:1348–52.
Akbal C, Lee SD, Packer SC, Davis MM, Rink RC, Kaefer M. Bladder augmentation with acellular dermal biomatrix in a diseased animal model. J Urol. 2006;176:1706–11.
Iizuka K, Muraishi O, Maejima T, Kitami Y, Xu YM, Watanabe K, et al. Total replacement of urethral mucosa with oral mucosa in dogs. J Urol. 1996;156:498–501.
Drewa T, Joachimiak R, Kaznica A, Sarafian V, Pokrywczynska M. Hair stem cells for bladder regeneration in rats: preliminary results. Transplant Proc. 2009;41:4345–51.
Wu R, Liu G, Bharadwaj S, Zhang Y. Isolation and myogenic differentiation of mesenchymal stem cells for urologic tissue engineering. Methods Mol Biol. 2013;1001:65–80.
Jack GS, Zhang R, Lee M, Xu Y, Wu BM, Rodríguez LV. Urinary bladder smooth muscle engineered from adipose stem cells and a three dimensional synthetic composite. Biomaterials. 2009;30:3259–70.
Damaser MS, Lehman SL. The effect of urinary bladder shape on its mechanics during filling. J Biomech. 1995;28:725–32.
Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). 2011;3:1377–97.
Salem SA, Hwei NM, Saim AB, Ho CC, Sagap I, Singh R, et al. Polylactic-co-glycolic acid mesh coated with fibrin or collagen and biological adhesive substance as a prefabricated, degradable, biocompatible, and functional scaffold for regeneration of the urinary bladder wall. J Biomed Mater Res A. 2013;101:2237–47.
Salem SA, Hwie AN, Saim A, Chee Kong CH, Sagap I, Singh R, et al. Human adipose tissue derived stem cells as a source of smooth muscle cells in the regeneration of muscular layer of urinary bladder wall. Malays J Med Sci. 2013;20:80–7.
Souza AB, Suaid HJ, Suaid CA, Tucci S Jr, Cologna AJ, Martins AC. Comparison of two experimental models of urodynamic evaluation in female rats. Acta Cir Bras. 2008;23(1):59–65.
Salem SA, Hwei NM, Ho CC, Sagap I, Idrus RB, Md Zainuddin Z. Combining radiographic, urodynamic and ultrasonographic technique for the evaluation of the urinary bladder structure and function in an animal model. J Surg Acad. 2011;1:25–32.
Roth CC, Bell CH, Woodson B, Schultz AD, Palmer BW, Frimberger D, et al. Temporal differentiation and maturation of regenerated rat urothelium. BJU Int. 2000;103:836–41.
Ng MH, Chowdhury SR, Morshed M, Tan KK, Tan GH, Phang MY, et al. Effective cell seeding and three-dimensional cell culture for bone tissue engineering. J Biomater Tissue Eng. 2014;4:573–8.
Shoae-Hassani A, Sharif S, Seifalian AM, Mortazavi-Tabatabaei SA, Rezaie S, Verdi J. Endometrial stem cell differentiation into smooth muscle cell: a novel approach for bladder tissue engineering in women. BJU Int. 2013;112:854–63.
Zhao J, Zeiai S, Ekblad A, Nordenskjöld A, Hilborn J, Götherström C, et al. Transdifferentiation of autologous bone marrow cells on a collagen-poly(ε-caprolactone) scaffold for tissue engineering in complete lack of native urothelium. J R Soc Interface. 2014;11:20140233.
Wu S, Cheng Z, Liu G, Zhao X, Zhong L, Zhu Y, et al. Urothelial differentiation of human umbilical cord-derived mesenchymal stromal cells in vitro. Anal Cell Pathol (Amst). 2013;36:63–9.
Shi JG, Fu WJ, Wang XX, Xu YD, Li G, Hong BF. Transdifferentiation of human adipose-derived stem cells intourothelial cells: potential for urinary tract tissue engineering. Cell Tissue Res. 2012;347:737–46.
Acknowledgements
The study was funded by Universiti Kebangsaan Malaysia Medical Centre Grants: GUP-2017-092, FF-173-2010 and Young Researcher Grant: GGPM-2011-079.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no financial conflicts of interest regarding the publication of this paper.
Ethical statement
Human samples used in this study were obtained and processed with informed consent from patients undergoing abdominoplasty according to procedures approved by the Institutional Research and Ethics Committee (Approval code: FF-173-2010). Experimentation on rats in this study followed guidelines and procedures approved by the Institutional Animal Ethics Committee (Approval code: PP/SURG/2010/ZULKIFLI/17-MARCH/292-MARCH-2010 DECEMBER-2011).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salem, S.A., Rashidbenam, Z., Jasman, M.H. et al. Incorporation of Smooth Muscle Cells Derived from Human Adipose Stem Cells on Poly(Lactic-co-Glycolic Acid) Scaffold for the Reconstruction of Subtotally Resected Urinary Bladder in Athymic Rats. Tissue Eng Regen Med 17, 553–563 (2020). https://doi.org/10.1007/s13770-020-00271-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-020-00271-7