Abstract
Introduction
Gut permeability is increased in critically ill patients, and associated with the development of the systemic inflammatory response syndrome and multiple organ dysfunction syndrome (MODS). The pathogenetic link(s) and potential therapies are an area of intense research over the last decades.
Methods
We thoroughly reviewed the literature on gut-origin sepsis and MODS in critically ill patients, with emphasis on the implicated pathophysiological mechanisms and therapeutic interventions.
Findings
Intestinal barrier failure leading to systemic bacterial translocation associated with MODS was the predominant pathophysiological theory for several years. However, clinical studies with critically ill patients failed to provide the evidence of systemic spread of gut-derived bacteria and/or their products as a cause of MODS. Newer experimental data highlight the role of the mesenteric lymph as a carrier of gut-derived danger-associated molecular patterns (DAMPs) to the lung and the systemic circulation. These substances are recognized by pattern recognition receptor-bearing cells in diverse tissues and promote proinflammatory pathways and the development MODS. Therefore, the gut becomes a pivotal proinflammatory organ, driving the systemic inflammatory response through DAMPs release in mesenteric lymph, without the need for systemic bacterial translocation.
Conclusions
There is an emerging need for application of sensitive non-invasive and easily measured biomarkers of early intestinal injury (e.g., citrulline, intestinal fatty acid protein, and zonulin) in our everyday clinical practice, guiding the early pharmacological intervention in critically ill patients to restore or prevent intestinal injury and improve their outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The terms bacterial translocation (BT), was first described by Berg and Garlington in 1979, as the phenomenon of passage of viable bacteria from the gastrointestinal tract through the epithelial mucosa into the lamina propria and then to the mesenteric lymph nodes and possibly other normally sterile organs [1]. This initial definition was later widened to include the translocation of non-viable bacteria or their products, namely pathogen-associated molecular patterns (PAMPs), with main representative the intestinal endotoxin. The intestinal tract contains the body’s largest interface between a person and his or her external environment. The complexity of its function is obvious when thinking that, at the same time, the intestine must serve two opposite functions; the selective permeability of needed nutrients from the intestinal lumen into the circulation and into the internal milieu in general and, on the other hand, the prevention of the penetration of harmful entities including microorganisms, luminal antigens, and luminal proinflammatory factors. The latter function is known as barrier function. The present review aims to provide a comprehensive overview of intestinal barrier dysfunction in the critically ill patient, leading to gut-origin sepsis and MODS, with an emphasis on the implicated pathophysiological mechanisms and therapeutic interventions.
Materials and methods
A systematic literature search was conducted using PubMed, PubMed Central, and Google from inception until April 30th, 2018. Several search terms were used to identify relevant literature: “intestinal barrier”, “gut barrier”, “intestinal permeability”, “intestinal barrier dysfunction”, “gut origin sepsis”, “bacterial translocation”, “microbial translocation”, “endotoxemia”, combined with the terms “critically ill”, “ICU”, “trauma”, “sepsis”, “MODS”, “therapy”, and “treatment”. Results were screened for appropriateness by the first author, according to title and abstract. Most relevant papers were further assessed by full content and their references were also reviewed and assessed when were found relevant. Only English language articles were included. Article types included clinical studies, experimental studies, clinical trials, and reviews.
The intestinal barrier
The gut barrier function is comprised by three major lines of defense [2]: (A) The biological barrier, which is made up of normal intestinal flora (gut microbiota). Microbiota displays important metabolic, immunologic, and gut protective functions. Metabolically, microbiota ferments carbohydrates, and indigestible oligosaccharides and synthesizes short-chain fatty acids (SCFA) such as butyrate, propionate, and acetate, which are rich sources of energy for the intestinal epithelium [3]. In addition, gut microbiota synthesizes vitamins B and K, and completes the entero-hepatic cycle of biliary acids. Immunologically, the gut microbiota contribute to gut immunomodulation interacting with both the innate and adaptive immune systems, through production of PAMPs, which are recognized by specific receptors of intestinal immune cells [4]. In addition, intestinal microbiota prevents the growth of potentially pathogenic bacteria through antagonism for nutrients and exerting colonization resistance. (B) The immune barrier, which is composed of gut-associated lymphoid tissue (GALT), effector and regulatory T cells, IgA producing B (plasma) cells, group 3 innate lymphoid cells, and resident macrophages and dendritic cells in the lamina propria. As stated above, the intestinal innate and adaptive immune system is in continuous cross-talk with the intestinal microbiota driving development of tolerance to commensal bacteria, while simultaneously shaping an effective immunological response to potential microbial invaders [5]. Microbiota stimulation leads to B-cell switch to IgA class, regulatory T-cell induction, and T-cell differentiation to Th17 [4]. (C) The mechanical barrier, which is consisted by the closed-lining intestinal epithelial cells and by the capillary endothelial cells in the submucosa. Intestinal epithelial cells come into the closest possible contact in the most apical part of the lateral cell membrane by specific structures named “tight junctions” (TJs), thus forming “kissing points”, which interconnect the cells and restrict the passage of ions, molecules and cells through the paracellular space [2, 6]. Similar intercellular junctions exist in endothelial cells of submucosal vessels, restricting the passage of bacterial products in blood circulation. Beyond the critical role of TJs in the regulation of paracellular permeability, the integrity and continuity of intestinal epithelial lining is dependent on homeostasis between epithelial cell apoptosis and proliferation [2].
Bacterial translocation in health and disease
BT may be a phenomenon that occurs in healthy individuals and may be a normal physiologic event without deleterious consequences. The baseline rate of translocation in human studies has been reported to be 5–10% [7]. The pathophysiological role of this low level of normal BT has been hypothesized to be the antigenic exposure of gut immune system to be prepared for an effective immune response in case of extensive pathogen invasion, and, on the other hand, develop immune tolerance to several microbial antigens of commensal microflora [7,8,9]. However, an excess level of BT has been demonstrated in several disease states, and has been associated with infectious complications and promotion of a systemic inflammatory response that aggravates the pathophysiological consequences of the underlying disease. Nowadays, we have convincingly shown that gut-derived bacteria and endotoxins translocate in normally sterile extraintestinal sites in patients with ileus, cirrhosis, obstructive jaundice, acute pancreatitis, abdominal and aortic repair surgery, inflammatory bowel disease, bowel transplantation, haemorrhagic shock, burn injury, and those receiving total parenteral nutritional support [10,11,12,13].
The gut-origin sepsis hypothesis of SIRS and MODS in the critically ill patient
In the early 1980s, a considerable amount of preclinical and clinical evidence had demonstrated the presence of the BT phenomenon in diverse clinical states. That time, there was a pathophysiological gap in the sequence of events leading to the development of systemic inflammatory response syndrome (SIRS) and ultimately to MODS in critically ill or injured patients, in the absence of an infectious focus confirmed even at autopsy [14]. In 1985, during a panel discussion of the Surgical Infection Society, Meakins and Marshall proposed the gut might represent the “motor” of MODS. According to this theory, in critically ill patients, the intestinal barrier integrity is disrupted owing to microcirculatory alterations; bacteria and endotoxins translocate to the mesenteric lymph nodes and the portal vein system, gaining finally access to the systemic circulation, after spilling over a dysfunctional liver which cannot clear the portal vein circulation from enteric bacteria or their products [15]. Consequently, a systemic inflammatory response is promoted, which induces deleterious functional and structural alterations in diverse and even distant organs, thus leading to MODS.
The role of bacterial translocation in gut-origin sepsis in the critically ill patient
The attractive theory of gut-derived sepsis and MODS remained to be confirmed in clinical situations. In 1991, a surgical team from Denver attempted to gain insight into the clinical relevance of BT in 20 severely injured patients, most of which (60%) were in shock at presentation [16]. The researchers inserted portal vein catheters for sequential blood sampling in trauma patients and performed consecutive blood cultures and endotoxin measurements. Even though 30% of patients developed MODS, only 2% of portal venous blood cultures turned out positive, while none of the patients had portal or systemic endotoxemia. The findings of this study raised reasonable questions on the accuracy of the “gut hypothesis” of sepsis, but similar studies with severely injured patients were difficult to be repeated. Confirmation of the BT process in trauma patients would require cultures of MLNs or portal vein blood sampling that is very difficult to be performed in this patient population. The most suitable alternative population, to directly study the potential clinical relevance of BT, was surgical patients subjected to major operations for diverse reasons, where sampling of MLNs could be easily performed. From 1998 to 2006, six relevant clinical studies enrolling 2125 surgical patients were conducted [7, 13, 17,18,19,20]; BT was confirmed in 5–21% of patients, but most importantly BT was associated with increased postoperative infectious complications. Specifically, when BT was present, the rate of infectious complications raised to 45% as compared to only 19% in patients without BT. Furthermore, in almost half of patients with BT, the same enteric pathogen that was isolated from the MLNs was also found in the postoperative infectious focus. However, the evidence of the above studies in surgical patients does not directly apply to the critically ill, injured, or septic, patient. After the initial not supportive BT study by Moore et al., several researchers attempted to answer the question of the accuracy of the gut hypothesis of sepsis and MODS. In all these subsequent studies, owing to the difficulty of direct measurements of BT, the researchers seek evidence for gut barrier failure by measuring intestinal permeability. Indeed, in critically ill ICU patients, intestinal permeability was significantly increased, and this finding was interrelated with the development of SIRS, sepsis, and MODS [21, 22]. Moreover, this interrelation seemed to be causative, because improvement of gut barrier function and permeability led to prevention of the above-stated complications [23].
Reshaping pathophysiology beyond bacterial translocation: the gut-lymph hypothesis
Therefore, accumulating clinical data provided evidence that the critically ill patient in the ICU has a compromised intestinal barrier function and increased gut permeability, which is interrelated with the development of MODS, but translocation of enteric microbes and their products through the portal vein system does not seem to be the connecting mechanism. Then, how could increase of gut permeability and MODS be interrelated in the critically ill patient? An alternative route of translocation of gut-derived pathogens and PAMPs could be the intestinal lymphatics. Mesenteric lymphatics first drain to the cisterna chyli and finally via the thoracic duct empty into the systemic circulation at the left subclavian vein. The pulmonary vasculature is the first vascular bed that is exposed to the mesenteric lymph, while this organ is also the first and most commonly injured organ in the critically ill patient through development of the acute respiratory distress syndrome (ARDS). Therefore, translocation of gut-derived microbes and/or their products through the intestinal lymphatics could theoretically explain the frequently encountered ARDS in critically ill patients, with subsequent development of MODS, and would also provide a convincing explanation for the failure of Moore et al. to detect translocating microbes in the portal circulation. Consequently, the research team of Professor Deitch designed an excellent series of appropriate studies to test the hypothesis that gut-origin sepsis and distant organ injury are mediated by gut-derived factors carried in the intestinal lymphatic system, rather than the portal vein.
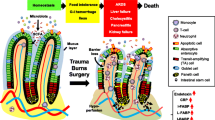
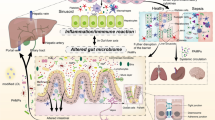
In a trauma/haemorrhagic shock experimental animal model, which mimics the clinical situation of seriously injured critically ill patient in the ICU, they documented that the early lung injury and MODS could be prevented by ligation of the major intestinal lymph duct, which prevents intestinal lymph from reaching the systemic circulation [24, 25]. In vitro studies showed that mesenteric lymph from shocked animals led to neutrophil activation, cardiomyocyte and endothelial cell injury, and red blood cell dysfunction [25]. Furthermore, injection of mesenteric lymph from shocked animals into healthy mice or rats induced a systemic septic state and caused ARDS and MODS [26, 27]. These injurious in vitro and in vivo effects were not observed when portal blood from shocked animals was tested [24]. Mesenteric lymph analysis from experimental animal models of shock showed that lymph did not contain detectable levels of endotoxin or bacterial DNA [28], while the same findings were obtained in a clinical study examining thoracic duct lymph in ICU patients [29]. More detailed investigations into the exact nature of the biologically active factors in lymph suggest that non-microbial and non-cytokine factors act as danger signals (Danger-Associated Molecular Patterns—DAMPs) and exert their adverse systemic effects by stimulating Toll-like receptor-4 and, perhaps, other pattern recognition receptors (PRR) in a fashion similar to bacteria [30, 31]. Considering the above, principally experimental evidence, the “gut-lymph” theory of gut-origin sepsis and MODS was proposed by Deitch in 2006 [27] (the evolution of pathogenetic theories of gut-origin sepsis in the critically ill patient is schematically presented in Fig. 1). According to this theory, critically ill trauma or septic patients develop a structural and functional derangement of the integrity of their gut-barrier function leading to increased intestinal permeability. Intestinal barrier injury is promoted according to the three-hit model; the first insult is splanchnic hypoperfusion or ischemia, the second hit is restoration of intestinal blood flow during resuscitation leading to ischemia–reperfusion injury and the third hit is loss of gut-barrier function which permits luminal bacteria, endotoxin, or both to cross the mucosal barrier [32]. Bacterial translocation can activate first a local gut inflammatory response, even when translocating bacteria and PAMPs are trapped within the gut wall or intestinal lymph nodes and do not reach the systemic circulation. The result of this gut inflammatory response is production of toxic and inflammatory substances which are carried through the mesenteric lymphatics to systemic circulation. These injurious biomolecules (DAMPS) are recognized by PRR-bearing cells of the innate immune system, including macrophages, leukocytes, and dendritic cells as well as vascular cells, fibroblasts, and epithelial cells, to promote proinflammatory and profibrotic pathways [33]. In case that the systemic release of DAMPs is sufficiently great, the result is the promotion of organ injury in diverse organs and the development of MODS, which further aggravate intestinal barrier injury leading to a vicious cycle (Fig. 2). With the evolution of the “gut-lymph” theory of sepsis and MODS, we have passed from the classic view of the “systemic BT”, which means the spread of bacteria and/or their products to the systemic circulation and other normally sterile extraintestinal sites, accounting for a microbe-associated promotion of a systemic inflammatory response, to a new pathophysiological appraisal, which highlights the intestinal barrier failure and the subsequent “local BT”, as the first step in a sequence of events that finally lead the gut becoming a major proinflammatory organ driving the systemic inflammatory response associated with MODS in the critically ill patient.
Evolution of pathogenetic theories of gut-origin sepsis in the critically ill patient: Intestinal barrier dysfunction and increased gut permeability is associated with the development of multiple organ dysfunction syndrome (MODS). The initial theory that the pathogenetic link was the translocation of intestinal microbes and/or their products through the portal vein to the liver and the systemic circulation was not confirmed by clinical data. Current pathogenetic aspects, based mainly on experimental evidence, support the gut-lymph theory according to which gut microbes and/or their products gain access to the intestinal submucosa activating the intestinal immunological system of defense. An intestinal proinflammatory response further aggravates intestinal injury and danger-associated molecular patterns (DAMPs) are released in the mesenteric lymphatics, carried to the lung and the systemic circulation. The gut becomes a pivotal proinflammatory organ promoting deleterious effects in even distant organs, through the release of DAMPs, without the need of systemic bacterial translocation
Pathophysiology of gut-origin sepsis and multiple organ dysfunction syndrome (MODS) in the critically ill patient: Splanchnic hypoperfusion, ischemia/reperfusion injury during resuscitation, and food deprivation promote intestinal injury through oxidative stress-mediated mechanisms. Oxidative stress promotes enterocytes’ apoptosis and disruption of intercellular tight junctions, increasing intestinal permeability. Enteric bacteria, endotoxin, or both cross the mucosal barrier and interact with the intestinal immunological system leading to a proinflammatory response, which further aggravates intestinal barrier dysfunction (1st vicious cycle). As a result of intestinal mucosa damage, injurious biomolecules (danger-associated molecular patterns—DAMPS) are released to the mesenteric lymphatics, carried to the lung and subsequently to the systemic circulation. These substances are recognized by pattern recognition receptor (PRR)-bearing cells of the innate immune system, including macrophages, leukocytes, and dendritic cells, as well as vascular cells, fibroblasts, and epithelial cells, and promote proinflammatory pathways (systemic inflammatory response syndrome—SIRS) and multiple organs’ dysfunction (MODS). This systemic inflammatory response further aggravates intestinal barrier injury creating a second selfsustained and potentially lethal vicious cycle
The role of biomarkers of gut-barrier dysfunction in the critically ill patient
Considering the above analyzed pathophysiology, there is an emerging need for reliable and easily applied biomarkers of gut-barrier integrity that may help the intensivist to identify patients with intestinal barrier dysfunction at risk of developing MODS. Several clinical studies have focused on the potential utility of non-invasive biomarkers in the evaluation of gut-barrier dysfunction in the ICU setting [34, 35]. Since the main determinant of intestinal permeability is the gut mechanical barrier, consisted by a monolayer of tightly interconnected epithelial cells, plasma biomarkers of enterocytes’ and TJs’ integrity have been evaluated in the context of critical care. A recent systematic review in this research field highlights the promising role of two enterocyte integrity biomarkers; plasma citrulline, an indicator of the functional enterocyte mass and plasma or urinary intestinal fatty acid-binding protein (I-FABP), a marker of enterocyte damage [36]. Both markers were correlated in diverse studies with intestinal permeability, endotoxemia, systemic inflammation, and even prognosis in the critically ill patient. Another pilot study demonstrated a positive role of plasma zonulin, a modulator of TJs’ integrity and permeability, increasing in plasma in TJs dysfunction, as a biomarker of intestinal barrier failure in the septic ICU patient [37]. Presently, the wide clinical application of these biomarkers is withheld by their limitations. Their measurements are time-consuming and could not be available in real time, the threshold of their prognostic value has not been established and more detailed data are required on their kinetics (half time, metabolism, and clearance) [36].
Therapeutic approaches
Therapeutic approaches aiming at preventing or limiting the BT process in the critically ill patient can be divided into two major categories; (a) treatments aiming to preserve normal intestinal microecology and/or inhibit increased pathogenic bacteria growth and attachment to the intestinal epithelium, which represent the first step in the bacterial translocation process (selective digestive tract decontamination—SDD, probiotics/prebiotics/synbiotics) and (b) therapies aiming at enhancing the integrity of the intestinal epithelial barrier and/or preventing gut injury (early resuscitation, enteral nutrition, immunonutrition, and antioxidants).
Selective digestive tract decontamination
Selective digestive tract decontamination consists of the use of oral non-absorbable antibiotics plus a short course of systemic antibiotics, directed against pathogenic gram negative aerobic enteric bacteria with minimal action against commensal anaerobic bacteria. Suppression of pathogenic intestinal bacterial overgrowth is expected to limit bacterial translocation and gut-derived infections and sepsis. SDD has been consistently shown to reduce infections and ventilator-associated pneumonia (VAP) in ICU patients [38, 39]. A cluster-randomized multicenter trial encompassing 2762 surgical and 3165 non-surgical patients additionally demonstrated a survival advantage of patients subjected to SDD [40]. Regarding the comparison of SDD and SOD strategies, a large open-label, clustered group-randomized crossover study in 13 intensive-care units in the Netherlands, with 5927 patients, showed comparable effects of the two treatments in terms of infection rate and mortality, in parallel with low levels of colonization with antibiotic-resistant pathogens [41].
Probiotics, prebiotics, and synbiotics
Maintenance of intestinal microflora with the use of probiotics, prebiotics, and synbiotics is another treatment option. Probiotics are living non-pathogenic microorganisms, which have demonstrated well-documented beneficial health effects administered in optimum amounts via promoting a healthy gut microbiome, prebiotics are specific plant fibres that promote the growth of useful bacteria and synbiotics are a combination of the two [42]. Probiotics have been widely tested, with positive results, as a measure to reduce the rate of postoperative infections in patients undergoing elective major abdominal procedures [43, 44]. However, important skepticism on their wide use rose in the scientific community, after the results of a randomized control trial on the value of their prophylactic use in patients with severe acute pancreatitis; probiotics not only failed to reduce infectious complications but also increased mortality from 6 to 16% [45]. In critically ill ICU patients, probiotics were shown to reduce the incidence of VAP by 40%, although no survival benefit was demonstrated [46]. A randomized clinical trial with 72 multiple trauma patients showed that synbiotics decrease the risk for sepsis by bloodstream infections and the occurrence of VAP by A. baumannii [47]. Taking into consideration these contradictory results, it seems that probiotics administration requires a careful selection of the candidate patient in order to apply the Hippocratic Oath “first do not harm”. Their prophylactic use in stable patients before any damage of their intestinal barrier integrity occur, e.g., in the preoperative period to reduce postoperative infections, is reasonable. In contrast, their use in critically ill patients with the established gut injury and increased gut permeability may predispose these patients to probiotic strains translocation, thus promoting a systemic inflammatory response deteriorating the patient’s clinical state.
Early hemodynamic resuscitation
Gut hypoperfusion has been suggested as a critical initiative event leading to intestinal injury and gut-barrier dysfunction in the critically ill patient. Decrease of adequate blood supply to the intestine induces several injurious effects associated with disruption of the mucosal barrier and this injury is further aggravated during reperfusion through oxidative stress-mediated mechanisms [48, 49]. Increased enterocytes’ apoptosis, decreased proliferative response, and loss of tight junctions’ integrity are some of the most important cellular alterations associated with intestinal barrier dysfunction in this setting [50]. Therefore, the early resuscitation to maintain the intravascular volume and cardiac supply is a pivotal therapeutic manipulation. Conventional aggressive fluid therapy has been challenged with the early evidence supporting balanced, restricted fluid, and early vasopressor use, which is anticipated to reduce hypervolemic state-induced intestinal mucosal edema, but more trials are needed before any conclusion can be made on this issue [51]. To prevent the oxidative stress-related reperfusion injury, several studies have been conducted with antioxidant volume resuscitative therapies with positive results [52, 53].
Enteral feeding
The gut has specific nutritional needs to preserve its normal structure and function. Experimental and clinical studies have shown that deprivation of the digestive tract from food nutrients and their associated gastric and pancreaticobiliary secretions induces mucosal atrophy and compromises the integrity of the gut barrier, thus promoting bacterial translocation [54]. Enteral feeding as compared to total parenteral nutrition was associated with reduced rates of infectious complications, SIRS, MODS, and mortality in critically ill patients with severe acute pancreatitis [55,56,57]. A recent metanalysis of 18 RCTs with a total number of 3347 critically ill adult patients showed that enteral nutrition is superior to total parenteral nutrition in terms of infectious complications and ICU length of stay, but no difference in mortality was observed [58].
Immunonutrition
The term “immunonutrition” refers to the enteral or parenteral administration of pharmacologically active nutrients (pharmaconutrients) that may modulate the metabolic and inflammatory response to surgery or critical illness and enhance immune function. Enteral immunonutrition refers to the enteral administration of the normal constituents of the basic nutrition enriched with these specific immunomodulating substrates, to directly supply enterocytes and prevent gut-barrier injury. The most well-studied immunonutrients are glutamine, arginine, ω-3 fatty acids, γ-linoleic acid, and nucleotides [59]. These nutritional components have been shown to exert pleiotropic actions on the intestinal mucosa, including proliferative, antiapoptotic, antioxidant, and antiinflammatory effects, thus enhancing the mechanical (enterocytes and tight junctions) and immunological integrity of the gut barrier and preventing BT [60,61,62]. These positive effects have been mainly derived from animal studies and clinical studies of elective surgery for gastrointestinal cancer, whereas, in the critically ill patient, the evidence has been controversial [63, 64]. Though the early single-centre studies with ICU patients demonstrated some clinical benefits, recent multicentre trials have shown no benefit or even negative effects in terms of mortality or other clinical endpoints [65,66,67,68]. A large randomized trial enrolling 1223 critically ill adults in 40 intensive-care units (ICUs) in Canada, the United States, and Europe showed that the early administration of glutamine in critically ill patients with multiorgan failure was associated with increased mortality [69].
Antioxidants
Since oxidative stress has been shown to be a crucial factor contributing to intestinal injury in the critically ill patient, the therapeutic trial of antioxidants for reversing gut-barrier dysfunction seems reasonable. However, clinical studies with antioxidants supplementation in this patient population have shown inconsistent results. In 2012, a meta-analysis analyzed all randomized clinical trials about the effects of micronutrients and antioxidants, as pharmaceutical agents, on clinical outcome in critically ill patients showed that high dose of parenteral selenium reduces mortality especially in patients with high risk of death [70]. Two later large randomized-controlled trials, using combinations of antioxidants, failed to show any benefit of antioxidants in the clinical outcome of ICU patients [69, 71]. On the other hand, some recent metanalyses on high-dose selenium use in severely ill septic patients have shown a 28-day survival benefit [72, 73]. It is obvious that, on the basis of the above-stated results, no evidence-based recommendation can be made regarding the use of antioxidants in critically ill patients. These contradictory findings might be explained on the different doses or combinations of antioxidants used, diverse stages of patients’ illness, or immunological function (e.g., hyperinflammatory or immunoparalysis stages of sepsis). We think that we should probably reshape our beliefs on oxidative stress as a uniformly considered harmful mediator of cellular and tissue damage. Reactive oxygen species are also crucial molecules regulating essential cellular signaling pathways. The negative results of antioxidants in critical illness might be explained by disruption of the normal signaling processes that regulate an effective host defense to severe infection [74].
Conclusions
The gut is the motor of sepsis and MODS in severely ill patients, but the pathophysiological explanation for this pivotal role of the gut has changed over time. Splanchnic hypoperfusion and enteric microcirculatory disturbances are a critical first step leading to intestinal injury and disruption of its barrier function. Gut microbes gain access to the intestinal submucosa activating the intestinal immunological system of defense. An intestinal proinflammatory response further aggravates intestinal injury and DAMPs are released in the mesenteric lymphatics, passing subsequently to the systemic circulation (Fig. 2). This is the second critical step promoting a systemic inflammatory response associated with MODS, irrespectively of translocation of intestinal microbes or their products beyond the gut or the mesenteric lymph nodes. Consequently, in the critically ill patient, the gut exerts a pivotal role as a proinflammatory organ that drives the systemic inflammatory response associated with MODS. In this respect, we have reached a point where we should highlight in our medical care the vital role of the gut in the clinical outcome of ICU patients. Towards this direction, there is an emerging need for sensitive non-invasive and easily measured biomarkers of the early intestinal injury that will guide the early hemodynamic or pharmacological intervention to restore or prevent intestinal barrier dysfunction. Research efforts in this field should be intensified and promising biomarkers (e.g., citrulline, intestinal fatty acid protein, and zonulin) should be further tested to overwhelm their current limitations and become readily available for clinical application. Intensivist should also pay attention in preventing their patients’ intestines from injury during critical illness, by applying well-demonstrated treatment strategies like selective gut decontamination, early hemodynamic resuscitation to prevent visceral-microcirculatory disturbances, enteral nutrition to improve microcirculation, prevent mucosal atrophy, and provide important nutrients for enterocytes. In conclusion, the Hippocratic quote “all disease begins in the gut” seems to be particularly true, over 2000 years later, for the critically ill patient.
References
Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23:403–11.
Assimakopoulos SF, Scopa CD, Vagianos CE. Pathophysiology of increased intestinal permeability in obstructive jaundice. World J Gastroenterol. 2007;13:6458–64.
Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–803.
Becattini S, Taur Y, Pamer EG. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med. 2016;22:458–78.
Nutsch KM, Hsieh CS. T cell tolerance and immunity to commensal bacteria. Curr Opin Immunol. 2012;24:385–91.
Assimakopoulos SF, Papageorgiou I, Charonis A. Enterocytes’ tight junctions: from molecules to diseases. World J Gastrointest Pathophysiol. 2011;2:123–37.
Sedman PC, Macfie J, Sagar P, Mitchell CJ, May J, Mancey-Jones B, et al. The prevalence of gut translocation in humans. Gastroenterology. 1994;107:643–9.
Vaishnavi C. Translocation of gut flora and its role in sepsis. Indian J Med Microbiol. 2013;31:334–42.
Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209.
Wiest R, Rath HC. Gastrointestinal disorders of the critically ill. Bacterial translocation in the gut. Best Pract Res Clin Gastroenterol. 2003;17:397–425.
Ambrose NS, Johnson M, Burdon DW, Keighley MR. Incidence of pathogenic bacteria from mesenteric lymph nodes and ileal serosa during Crohn’s disease surgery. Br J Surg. 1984;71:623–5.
Deitch EA. Simple intestinal obstruction causes bacterial translocation in man. Arch Surg. 1989;124:699–701.
Woodcock NP, Sudheer V, El-Barghouti N, Perry EP, MacFie J. Bacterial translocation in patients undergoing abdominal aortic aneurysm repair. Br J Surg. 2000;87:439–42.
Goris RJ, te Boekhorst TP, Nuytinck JK, Gimbrere JS. Multiple-organ failure. Generalized autodestructive inflammation? Arch Surg. 1985;120:1109–15.
Deitch EA. Bacterial translocation of the gut flora. J Trauma. 1990;30:184-9.
Moore FA, Moore EE, Poggetti R, McAnena OJ, Peterson VM, Abernathy CM, et al. Gut bacterial translocation via the portal vein: a clinical perspective with major torso trauma. J Trauma. 1991;31:629–36. discussion 36 – 8.
O’Boyle CJ, MacFie J, Mitchell CJ, Johnstone D, Sagar PM, Sedman PC. Microbiology of bacterial translocation in humans. Gut. 1998;42:29–35.
MacFie J, O’Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223–8.
Chin KF, Kallam R, O’Boyle C, MacFie J. Bacterial translocation may influence the long-term survival in colorectal cancer patients. Dis Colon Rectum. 2007;50:323–30.
MacFie J, Reddy BS, Gatt M, Jain PK, Sowdi R, Mitchell CJ. Bacterial translocation studied in 927 patients over 13 years. Br J Surg. 2006;93:87–93.
Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, Meddings JB. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998;158:444–51.
Herbert MK, Holzer P. Standardized concept for the treatment of gastrointestinal dysmotility in critically ill patients–current status and future options. Clin Nutr. 2008;27:25–41.
De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med. 2005;33:1125–35.
Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228:518–27.
Upperman JS, Deitch EA, Guo W, Lu Q, Xu D. Post-hemorrhagic shock mesenteric lymph is cytotoxic to endothelial cells and activates neutrophils. Shock. 1998;10:407–14.
Senthil M, Brown M, Xu DZ, Lu Q, Feketeova E, Deitch EA. Gut-lymph hypothesis of systemic inflammatory response syndrome/multiple-organ dysfunction syndrome: validating studies in a porcine model. J Trauma. 2006;60:958–65. discussion 65 – 7.
Deitch EA, Xu D, Kaise VL. Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci. 2006;11:520–8.
Adams CA Jr, Xu DZ, Lu Q, Deitch EA. Factors larger than 100 kd in post-hemorrhagic shock mesenteric lymph are toxic for endothelial cells. Surgery. 2001;129:351–63.
Lemaire LC, van Lanschot JB, Stoutenbeek CP, van Deventer SJ, Dankert J, Oosting H, et al. Thoracic duct in patients with multiple organ failure: no major route of bacterial translocation. Ann Surg. 1999;229:128–36.
Reino DC, Pisarenko V, Palange D, Doucet D, Bonitz RP, Lu Q, et al. Trauma hemorrhagic shock-induced lung injury involves a gut-lymph-induced TLR4 pathway in mice. PLoS One. 2011;6:e14829.
Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5.
Deitch EA. Bacterial translocation or lymphatic drainage of toxic products from the gut: what is important in human beings? Surgery. 2002;131:241–4.
Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–36.
Lieberman JM, Marks WH, Cohn S, Jaicks R, Woode L, Sacchettini J, et al. Organ failure, infection, and the systemic inflammatory response syndrome are associated with elevated levels of urinary intestinal fatty acid binding protein: study of 100 consecutive patients in a surgical intensive care unit. J Trauma. 1998;45:900–6.
Piton G, Manzon C, Monnet E, Cypriani B, Barbot O, Navellou JC, et al. Plasma citrulline kinetics and prognostic value in critically ill patients. Intensive Care Med. 2010;36:702–6.
Piton G, Capellier G. Biomarkers of gut barrier failure in the ICU. Curr Opin Crit Care. 2016;22:152–60.
Klaus DA, Motal MC, Burger-Klepp U, Marschalek C, Schmidt EM, Lebherz-Eichinger D, et al. Increased plasma zonulin in patients with sepsis. Biochem Med (Zagreb). 2013;23:107–11.
Stoutenbeek CP, van Saene HK, Little RA, Whitehead A. The effect of selective decontamination of the digestive tract on mortality in multiple trauma patients: a multicenter randomized controlled trial. Intensive Care Med. 2007;33:261–70.
Veelo DP, Bulut T, Dongelmans DA, Korevaar JC, Spronk PE, Schultz MJ. The incidence and microbial spectrum of ventilator-associated pneumonia after tracheotomy in a selective decontamination of the digestive tract-setting. J Infect. 2008;56:20–6.
Melsen WG, de Smet AM, Kluytmans JA, Bonten MJ. Selective decontamination of the oral and digestive tract in surgical versus non-surgical patients in intensive care in a cluster-randomized trial. Br J Surg. 2012;99:232–7.
de Smet AM, Kluytmans JA, Blok HE, Mascini EM, Benus RF, Bernards AT, et al. Selective digestive tract decontamination and selective oropharyngeal decontamination and antibiotic resistance in patients in intensive-care units: an open-label, clustered group-randomised, crossover study. Lancet Infect Dis. 2011;11:372–80.
Manzanares W, Lemieux M, Langlois PL, Wischmeyer PE. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care. 2016;19:262.
Kotzampassi K, Stavrou G, Damoraki G, Georgitsi M, Basdanis G, Tsaousi G, et al. A four-probiotics regimen reduces postoperative complications after colorectal surgery: a randomized, double-blind, placebo-controlled study. World J Surg. 2015;39:2776–83.
Pitsouni E, Alexiou V, Saridakis V, Peppas G, Falagas ME. Does the use of probiotics/synbiotics prevent postoperative infections in patients undergoing abdominal surgery? A meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2009;65:561–70.
Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–9.
Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Crit Care Med. 2010;38:954–62.
Giamarellos-Bourboulis EJ, Bengmark S, Kanellakopoulou K, Kotzampassi K. Pro- and synbiotics to control inflammation and infection in patients with multiple injuries. J Trauma. 2009;67:815–21.
Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–83.
Medeiros Ada C, Araujo-Filho I, Torres ML, Sa Cde V, Jacome DT, Rego AC. Ischemic preconditioning in different times and its effect on bacterial translocation induced by intestinal ischemia and reperfusion in rats. Rev Col Bras Cir. 2013;40:55–9.
Fernandes de Mattos Dourado S, Barbeiro DF, Koike MK, Barbeiro HV, Pinheiro da Silva F, Cesar Machado MC. Diazoxide reduces local and remote organ damage in a rat model of intestinal ischemia reperfusion. J Surg Res. 2018;225:118–24.
Williams JM, Keijzers G, Macdonald SP, Shetty A, Fraser JF. Review article: sepsis in the emergency department—part 3: treatment. Emerg Med Australas. 2018;30:144–51.
Zhang C, Sheng ZY, Hu S, Gao JC, Li JY, Liu Y. The role of oxygen-free radical in the apoptosis of enterocytes in scalded rats after delayed resuscitation. J Trauma. 2004;56:611–7.
Yang H, Sheng Z, Guo Z, Shi Z, Lu J, Chai J, et al. Oxygen free radical injury and its relation to bacterial and endotoxin translocation after delayed fluid resuscitation: clinical and experimental study. Chin Med J (Engl). 1997;110:118–24.
MacFie J. Enteral versus parenteral nutrition: the significance of bacterial translocation and gut-barrier function. Nutrition. 2000;16:606–11.
Kalfarentzos F, Kehagias J, Mead N, Kokkinis K, Gogos CA. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. Br J Surg. 1997;84:1665–9.
Al-Omran M, Albalawi ZH, Tashkandi MF, Al-Ansary LA. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010;1:CD002837.
Olah A, Romics L Jr. Enteral nutrition in acute pancreatitis: a review of the current evidence. World J Gastroenterol. 2014;20:16123–31.
Elke G, van Zanten AR, Lemieux M, McCall M, Jeejeebhoy KN, Kott M, et al. Enteral versus parenteral nutrition in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. Crit Care. 2016;20:117.
Montejo JC, Zarazaga A, Lopez-Martinez J, Urrutia G, Roque M, Blesa AL, et al. Immunonutrition in the intensive care unit. A systematic review and consensus statement. Clin Nutr. 2003;22:221–33.
Kim MH, Kim H. The roles of glutamine in the intestine and its implication in intestinal diseases. Int J Mol Sci. 2017;18:1051.
Liu Y, Wang X, Hu CA. Therapeutic potential of amino acids in inflammatory bowel disease. Nutrients. 2017;9:920.
Generoso Sde V, Rodrigues NM, Trindade LM, Paiva NC, Cardoso VN, Carneiro CM, et al. Dietary supplementation with omega-3 fatty acid attenuates 5-fluorouracil induced mucositis in mice. Lipids Health Dis. 2015;14:54.
Braga M. Perioperative immunonutrition and gut function. Curr Opin Clin Nutr Metab Care. 2012;15:485–8.
Braga M, Wischmeyer PE, Drover J, Heyland DK. Clinical evidence for pharmaconutrition in major elective surgery. J Parenter Enter Nutr. 2013;37:66S–72S.
Martindale RG, McClave SA, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition: Executive Summary. Crit Care Med. 2009;37:1757–61.
Marik PE, Zaloga GP. Immunonutrition in critically ill patients: a systematic review and analysis of the literature. Intensive Care Med. 2008;34:1980–90.
Annetta MG, Pittiruti M, Vecchiarelli P, Silvestri D, Caricato A, Antonelli M. Immunonutrients in critically ill patients: an analysis of the most recent literature. Minerva Anestesiol. 2016;82:320–31.
Heyland DK, Samis A. Does immunonutrition in patients with sepsis do more harm than good? Intensive Care Med. 2003;29:669–71.
Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, et al. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368:1489–97.
Manzanares W, Dhaliwal R, Jiang X, Murch L, Heyland DK. Antioxidant micronutrients in the critically ill: a systematic review and meta-analysis. Crit Care. 2012;16:R66.
van Zanten AR, Sztark F, Kaisers UX, Zielmann S, Felbinger TW, Sablotzki AR, et al. High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high-protein enteral nutrition and nosocomial infections in the ICU: a randomized clinical trial. JAMA. 2014;312:514–24.
Huang TS, Shyu YC, Chen HY, Lin LM, Lo CY, Yuan SS, et al. Effect of parenteral selenium supplementation in critically ill patients: a systematic review and meta-analysis. PLoS One. 2013;8:e54431.
Landucci F, Mancinelli P, De Gaudio AR, Virgili G. Selenium supplementation in critically ill patients: a systematic review and meta-analysis. J Crit Care. 2014;29:150–6.
Jain M, Chandel NS. Rethinking antioxidants in the intensive care unit. Am J Respir Crit Care Med. 2013;188:1283–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Assimakopoulos, S.F., Triantos, C., Thomopoulos, K. et al. Gut-origin sepsis in the critically ill patient: pathophysiology and treatment. Infection 46, 751–760 (2018). https://doi.org/10.1007/s15010-018-1178-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-018-1178-5