Abstract
The nanocrystalline ZnS semiconducting thin films of 500 nm thickness have been deposited on glass substrate at different substrate temperatures (T s) by thermal evaporation technique. The structural property of deposited thin films has been measured by X-ray diffraction, scanning electron microscopy, and Energy dispersive analysis of X-ray. The electrical and optical properties of thin films have been determined by D.C. two point probe and ultra-violet visible spectroscopy measurements. The X-ray diffraction patterns show that thin films have cubic structure. The electrical resistivity of thin films has decreased from 0.36 × 106 to 0.15 × 106 Ω cm as substrate temperature increases from 300 to 400 K. It shows that films have semiconducting in nature. The grain size and electrical conductivity of the thin films have increased as the deposition temperature increased while dislocation density, activation energy, and band gap decreased. The minimum band gap 3.43 eV has been found.
Similar content being viewed by others
Introduction
The nanocrystalline zinc sulfide (ZnS) semiconductor has attracted much attention from the viewpoint of fabrication of many optoelectronics devices because of their unique properties such as quantum size effect [1, 2] and abnormal luminescence phenomenon [3–5]. The non-stoichiometry is responsible for conductivity in thin film which is created during deposition of thin films. In recent years, advances in formation of nanometer-sized materials continuously unabated and it show that more exciting opportunities are laying ahead if size of crystal structure can be scaled down to nanometers. The semiconductor zinc sulfide belongs to II–VI group in periodic table which has large energy band gap (3.72–3.77 eV) and cubic structure in the ultra-violet visible (UV)-region. Due to these wide band gap materials, it is suitable for use in the blue light-emitting diodes [6], optoelectronics devices such as electron luminescent display [7], cathode luminescent display [8], and multilayer dielectrics filters [9]. The ZnS semiconductor exists in two phases, i.e., cubic phase (sphalerite) and hexagonal phase (wurtzite). The exciting binding energy (38 MeV) of ZnS is higher than thermal energy (25 MeV) at room temperature which can show excitonic emission. For the fabrication of optoelectronics devices to need high conductivity, high mobility, and low band gap. Many growth techniques have been adopted to prepare ZnS thin films, such as sputtering [10], pulsed-laser deposition [11], metal organic chemical vapor deposition [12–15], electron beam evaporation [16], photochemical deposition [17], chemical bath deposition [18], and thermal evaporation technique. Among these methods, the thermal evaporation technique is the most interesting technique because the advantages of this technique have high stability, high reproducibility, high deposition rate, large area deposition, and non-expansive. This technique provides economical and efficient use of evaporate material enabling constant rate of deposition. The aim of the present study is to investigate the effect of substrate temperature on the structural, electrical, and optical properties of ZnS thin films deposited on glass substrate by thermal evaporation technique. We also studied its variation with substrate temperature.
Experimental
Chemicals
For the preparation of non-stoichiometric semiconducting ZnS thin films, the material used as source is zinc sulfide. All chemicals were purchased from Alfa Aesar, Ltd. USA, which have a high purity of 99.99 %.
Substrate cleaning
The substrate cleaning plays an important role in the deposition of thin films, so commercially available glass substrates with the size of (75 mm × 25 mm × 1 mm) were washed in detergent then in chromic acid and finally washed with double distilled water in ultrasonic cleaner and dried at 400 K in an oven.
Preparation of ZnS thin films
The nanocrystalline ZnS thin films of 500 nm thickness have been deposited on well-cleaned glass substrates at different substrate temperatures (T s = 300–400 K) by the thermal evaporation technique. The films were fabricated under vacuum of 10−4–10−5 Torr. The zinc sulfide is used as the source material. The stoichiometric starting/source materials (ZnS) were taken in molybdenum boat and evaporated in vacuum (~10−5 Torr) in the vacuum system equipped with liquid nitrogen trap. The source materials were kept at the distance of 120 mm from the substrate holder in vacuum chamber. The film deposition rate (2.0–15 nm/s) was adjusted by changing the electrical current. The deposition rate was measured by digital film thickness monitor using a quartz crystal sensor set at 6 MHz (DTM-10). Initially, substrates were not exposed to vapor stream by using shutter, and after obtaining constant rate of evaporation, the shutter was opened. During deposition of thin film, substrate was placed normal to the line of sight from the evaporation surface at different polar angles to obtain uniform deposition. The source material about 30 mg was used for the deposition of each thin film. In each cycle of deposition, fresh source material was kept in molybdenum boat. The film thickness and deposition rate were measured by film thickness monitor (FTM, VICO, DTM-10) with quartz crystal sensor vibrating at a frequency 6 MHz. The sensor of FTM was attached parallel to the substrate. The substrate temperature was measured using digital temperature meter (PTS-9601: GELCO) that was attached with thermocouple sensor. The tip of the thermocouple sensor was in contact with the surface of substrate. The different substrate temperatures were obtained by changing the current of substrate heater.
Characterization of thin films
Structural characterization
The X-ray diffraction (XRD) patterns of deposited thin films have been recorded by Rigaku diffractometer using graphite-filtered CuKα1 radiation (λ = 1.54 Å) at 40 kV, 100 mA with a scanning rate of 3°/min (2θ = 20°–60°). The composition of deposited films was analyzed using scanning electron microscope, with attached energy dispersive analysis of X-rays (EDAX). The surface morphology of thin films was studied by scanning electron microscopy measurement. The crystallite size and dislocation density of deposited thin films were calculated by using Debye–Scherrer’s formula [19].
where λ is wavelength of radiation used, θ is diffraction angle of the concern diffraction peak, β is the full width at half maximum (FWHM) of the diffraction peak corresponding to a particular crystal plane.
Electrical characterization
The electrical resistivity and conductivity of semiconducting ZnS thin filmswere measured by using the D.C. two point probe method. The electrical resistivity and conductivity of thin films were determined by the following equation [20].
where ρ is resistivity, K is Boltzmann constant, and T is absolute temperature.
Optical characterization
The optical absorption spectra were recorded by a shimadzu double beam double monochromator spectrophotometer (UV-2550) in the wavelength range of 300–1500 nm at room temperature with unpolarized radiation on thin films which were deposited on the glass substrate at different substrate temperatures. The absorption coefficient (α) was calculated for deposited thin film in the region of strong absorption using the relation [21].
where α is absorption coefficient at particular wavelength, T the transmittance at same wavelength, and d is film thickness.
The direct band gap of thin film has calculated by using Tauc relation [20].
where hv is photon energy, E g is band gap, A is constant, and n = ½ for direct band gap material.
Results and discussion
Structural analysis of thin films
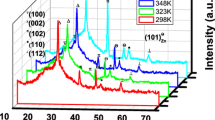
The X-ray diffraction patterns of the deposited semiconducting ZnS thin films are shown in Figs. 1, 2, 3, 4 and 5. The XRD measurement reveals that the nanocrystalline of ZnS thin films is in cubic structure. The cubic phase of the crystals has identified from the agreement of peak position with standard JCPDS data card no. 65–1691. The nano-crystals of ZnS have different planes (111), (220), and (311) with preferential orientation along (111) plane. Figures 1, 2, 3, 4, and 5, show that intensity of XRD peaks was increased with the increase of substrate temperature 300–400 K. This indicates that a full width half maximum (FWHM) of diffraction peaks was decreased. For this, particle size of deposited film was increased with the increase of substrate temperature. Figure 1 shows that low grain size and high dislocation density while Fig. 5 has highest grain size and lowest dislocation density. Thus, crystallinity is improved with the increase of substrate temperature. The experimental and standard d values of ZnS materials are given in Table 1. The calculated grain size and dislocation density of thin films are given in Table 2.
From the EDAX, films were non-stoichiometric with the composition Zn = 52.46 % and S = 47.54 %. There were small differences between composition of Zn and S in films and source material nearly equal to 1 %.
The variations of grain size or particle size (nm) and dislocation density of semiconducting thin films with different substrate temperatures are shown in Fig. 6. It was cleared from Fig. 6; the grain size of the films have been increased from 36.12 to 43.82 nm as the substrate temperature increased from 300 to 400 K while dislocation density decreased from 0.76 × 1015 to 0.52 × 1015 line/m2. The maximum grain size 43.82 nm and minimum dislocation density 0.52 × 1015 line/m2 have been obtained and its value nearly saturated above at 400 K. This indicates that the growth in grain size was constant above the temperature 400 K. The highest grain size of thin film indicates the highest conductivity and lowest resistivity of film. Thus, crystallinity of thin films has improved with the increase of substrate temperature. This may be due to collapse of nano-particles with each other and decrease crystal defects in thin films at higher substrate temperature.
The maximum grain size 43.82 nm and minimum dislocation density 0.52 × 1015 line/m2 have been observed for the ZnS thin film deposited at temperature 400 K whereas Haque et al. [22] have reported the grain size of 34.08 nm and dislocation density 0.86 × 1015 line/m2 for ZnS thin films by R F Magnetron sputtering. Borah et al. [23] have reported the grain size 7.0 nm for ZnS crystal by a chemical route method. Chauhan et al. [24] and Lu et al. [25] have reported the grain size 4.0 and 3.0 nm, respectively, for ZnS nanoparticles by chemical precipitation method.
The surface morphology of ZnS thin films is shown in Figs. 7, 8, 9, 10, and 11. It was cleared from Figs. 7, 8, 9, 10, and 11 that the fabricated thin films were homogenous, without crack and pin holes. In scanning electron micrograph as shown in Figs. 7, 8, 9, 10 and 11, crystal or grain size has been increased with the increases of substrate temperature from 300 to 400 K. The grain size was lowest while dislocation density and strain were highest in Fig. 7. The grain size in Fig. 11 was highest while dislocation density and strain were lowest at 400 K than other surface micrographs. This is a good agreement with X-ray diffraction of thin films.
Electrical analysis of thin films
The calculated value of electrical resistivity, electrical conductivity, and activation energy of thin films is given in Table 2. The electrical resistivity of thin films decrease with the increase of substrate temperature. This shows that the film was semiconductor in nature. The decrease in electrical resistivity is due to the improvement in crystallinity of film as the substrate temperature was increased from 300 to 400 K. The activation energy of semiconducting ZnS thin films has been determined from slope of log of resistivity (log ρ) with reciprocal of temperature (1/T) × 103 K. The variation of electrical resistivity and conductivity of thin films with different substrate temperatures is shown in Fig. 12.
It is clear from Fig. 12 that electrical resistivity of film decreased from 0.36 × 106 to 0.15 × 106 Ω cm as substrate temperature increased from 300 K to 400 K. The electrical conductivity increased from 2.77 × 10−6 to 6.66 × 10−6 1/Ω cm with the increase of substrate temperature from 300 K to 400 K. This indicates that films are semiconducting in nature. The decrease in electrical resistivity is due to the improvement in crystallinity of thin films, mobility of charge carrier, and concentration of charge carrier with substrate temperature. The minimum electrical resistivity 0.15 × 106 Ω cm and high electrical conductivity 6.66 × 10−6 1/Ω cm have been obtained at T s = 400 K. The value of electrical resistivity and conductivity of thin films were nearly saturated above 400 K. This may be due to increment in grain size has constant with increase of substrate temperature.
The variation of activation energy of thin films with different substrate temperatures is shown in Fig. 13. It is cleared from Fig. 13 that the activation energy has been decreased from 1.24 to 0.86 eV as the substrate temperature increased from 300 to 400 K and nearly saturated above at 400 K. This may be due to distance between valence band and conductance has decreased with increase of substrate temperature from 300 to 400 K and above 400 K, it has constant. The minimum activation energy obtained at T s = 400 K was 0.86 eV.
The lowest resistivity 0.15 × 106 Ω cm and high electrical conductivity 6.66 × 10−6 1/Ω cm have been found for the ZnS thin films deposited at temperature of 400 K, whereas Ozutok et al. [26] have reported the resistivity 0.49 × 106 Ω cm and conductivity 2.05 × 10−6 1/Ω cm for ZnS thin film deposited by Spray pyrolysis technique. Ubale et al. [20] have reported the resistivity 0.18 × 106 Ω cm and activation energy 1.29 eV for ZnS thin films deposited by Chemical bath technique. Shinde et al [27] have reported the activation energy 0.89 eV for ZnS film deposited by chemical bath deposition.
Optical analysis of thin films
The optical absorption spectra of ZnS thin films at different substrate temperatures are shown in Fig. 14. It is clear from Fig. 14 that the optical absorption decreases with the increase of wavelength and minimum absorption obtained at 1500 nm. The optical absorption also decreased with the increase of substrate temperature because grain boundary scattering was reduced with the increase of substrate temperature.
This indicates that grain size of crystal increased with increase of substrate temperature. This may be increase in transmittance of thin films with substrate temperature. The variation of (αhν)2 with photon energy (eV) is shown in Fig. 15. The extrapolation of straight-line portion of plot gives the value of direct band gap. Using these spectra, we determined the band gap of ZnS thin films. The band gap obtained was 3.49–3.43 eV at temperature range 350–400 K. The minimum band gap obtained was 3.43 eV at 400 K. This shows that band gap of thin film decreased with the increase of substrate temperature.
The minimum direct band gap (3.43 eV) has been observed for the ZnS thin film deposited at substrate temperature 400 K. Shinde et al [27] have reported the band gap 3.51 eV for ZnS thin films deposited by chemical bath deposition. Kumar et al. [28] have reported the band gap 3.50 eV for ZnS thin film by vacuum evaporation technique.
The structural, electrical, and optical properties of deposited film studies support this decrease in this resistivity due to improvement in crystallinity of the film which would increase the charge carrier, mobility, and decrease in defect levels with increase in the temperature.
Conclusion
The non-stoichiometric semiconducting ZnS thin films have been deposited on glass substrate by thermal evaporation technique at different substrate temperatures. The maximum grain size 43.82 nm has been obtained. The SEM shows the agglomeration of nano-crystals. The XRD analysis shows that films have cubic structure. The minimum resistivity 0.15 × 106 Ω cm and maximum conductivity 6.66 × 10−6 1/Ω cm of film have been obtained. The measurement of optical absorption spectra indicates that band gap decreased with the increase of substrate temperature. The minimum band gap 3.43 eV has been observed.
References
Li, L.-P., Qiu, X.-Q., Li, G.-S.: Correlation between size-induced lattice variations and yellow emission shift in ZnO nanostructures. Appl. Phys. Lett. 87(12), 124101–124103 (2005)
Schmidt, H.M., Weller, H.: Synthesis of ZnS nano particles by solid liquid chemicals method. Chem. Phys. Lett. 129(6), 615–618 (1986)
Xin, X.-S., Zhou, B.-B., Du, H., Xu, X.-Q., Su, W.-H.: Preparation of ZnS nano particles and study its optical property. Chem. Res. Appl. 17(3), 303–306 (2005)
Cheng, X.W., Li, X., Zhai, F.-F., Yu, X., Long, X., Lin, P.-T.: Fabrication and Photoluminescence study on ZnO nanostructured particles. J. Aeronaut. Mater. 29(1), 92–95 (2009)
Nasuno, Y., Kohama, N., Nishimura, K., Hayakawa, T., Taniguchi, H., Shimizu, M.: Size-dependent melting behavior of Zn nanowire arrays. Appl. Phys. Lett. 88(7), 1909–1911 (2006)
Katyama, H., Oda, S., Kulimato, H.: Study of ZnS nano particals. Appl. Lett. 27, 657–661 (1975)
Mach, R., Muller, G.O.: Physical concept of high filed, thin film of ZnS. Phys. Status Solidi A 69, 11–17 (1982)
Ledger, A.M.: Inhomogeneous interface laser mirror coatings. Appl. Opt. 18, 2979–2983 (1979)
Jones, P.L., Moore, D.M., Smith, S.C.: A new method for melting and recrystallization of lanthanum ZnS nanoparticle. J Phys E 9, 312–316 (1976)
Nitta, A., Tanakab, K., Maekawab, Y., Kusabirakib, M., Aozasa, M.: Effects of gas impurities in the sputtering environment on the stoichiometry and crystallinity of ZnS: Mn electroluminescent-device active layers. Thin Solid Films 384, 261–268 (2001)
Yano, S., Schroeder, R., Ullrich, B., Sakai, H.: Absorption and photocurrent properties of thin ZnS films formed by pulsed-laser deposition on quartz. Thin Solid Films 423, 273–276 (2003)
Feng, Q.J., Shen, D.Z., Zhang, J.Y., Liang, H.W., Zhao, D.X., Lua, Y.M., Fan, X.W.: Highly aligned ZnS nanorods grown by plasma-assisted metalorganic chemical vapor deposition. J. Crystal Growth 285, 561–565 (2005)
Zhang, Z., Shen, D.Z., Zhang, J.Y., Shan, C.X., Lu, Y.M., Liu, Y.C., Li, B.H., Zhao, D.X., Yao, B., Fan, X.W.: The growth of single cubic phase ZnS thin films on silica glass by plasma-assisted metalorganic chemical vapor deposition. Thin Solid Films 513, 114–117 (2006)
Gunasekaran, M., Gopalakrishnan, R., Ramasamy, P.: Deposition of ZnS thin films by photochemical deposition technique. Mater. Lett. 58, 67–70 (2003)
Zhang, X.T., Liu, Y.C., Zhang, L.G., Zhang, J.Y., Lu, Y.M., Shen, D.Z., Xu, W., Zhong, G.Z., Fan, X.W., Kong, Z.G.: Photoluminescence and optically pumped ultraviolet lasing from nanocrystalline ZnO thin films prepared by thermal oxidation of high-quality ZnS thin films. Chin. Phys. Lett. 19, 127–130 (2002)
Wang, S., Fu, X., Xia, G., Wang, J., Shao, J., Fan, Z.: Structure and optical properties of ZnS thin films grown by glancing angle deposition. Appl. Surf. Sci. 252, 8734–8737 (2006)
Fathy, N., Kobayashi, R., Ichimura, M.: Preparation of ZnS thin films by the pulsed electrochemical deposition. Mater. Sci. Eng., B 107, 271–276 (2004)
Roy, P., Ota, J.R., Srivastava, S.K.: Crystalline ZnS thin films by chemical bath deposition method and its characterization. Thin Solid Films 515, 1912–1917 (2006)
Ramasamy, V., Praba, K., Murugadoss, G.: Study of optical and thermal properties in nical doped ZnS nanoparticles using Surfactants. Superlattices Microstruct. 51, 699–714 (2012)
Ubale, A.U., Kulkarni, D.K.: Preparation and study of thickness dependent electrical characteristics of zinc sulfide thin films. Bull. Mater. Sci. 28(1), 43–47 (2005)
Cottrell, A.: An introduction to metallurgy, 2nd edn, pp. 173–178. Edward Arnold Ltd, London (1975)
Haque, F., Rahman, K.S., Islam, M.A., Rasid, M.J., Alam, M.M., Alothman, Z.A., Soplan, K., Amin, N.: Growth optimization of ZnS thin films by R F Magnetran sputtering as prospective buffer layer in thin film solar cell. Chalcogenide Lett. 11, 189–197 (2014)
Borah, J.P., Barman, J., Sarma, K.C.: Structural and optical properties of ZnS nanoparticles. Chalcogenide Lett. 5, 201–208 (2008)
Chauhan, R., Kumar, A., Chaudhary, R.P.: Characterization of chemically synthesized Mn doped ZnS nanoparticals. Chalcogenide Lett. 9, 151–156 (2012)
Lu, S.W., Lee, B.I., Wang, Z.L., Tong, W., Wagner, B.K., Park, W., Summers, C.J.: Synthesis and photoluminescence enhancement of Mn doped ZnS nanocrystals. J. Luminensence 92, 73–78 (2001)
Ozutok, F., Erturk, K., Bilgin, V.: Growth, electrical and optical properties of ZnS: Mn thin films. Acta Phys. Pol., A 121, 221–223 (2012)
Shinde, M.S., Ahirrao, P.B., Patil, I.J., Patil, R.S.: Studies on nanocrystalline ZnS thin films prepared by modified chemical bath deposition method. Indian J. Pure Appl. Phys. 49, 765–768 (2011)
Kumar, P., Kumar, A., Dixit, P.N., Sharma, T.P.: Optical, structural and electrical properties of zinc sulphide vacuum evaporated thin films. Indian J. Pure Appl. Phys. 44, 690–693 (2006)
Acknowledgments
I am thankful to our institute for providing XRD and SEM facilities. I am also thankful to our friend for help in preparation of thin films.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Vishwakarma, R. Effect of substrate temperature on ZnS films prepared by thermal evaporation technique. J Theor Appl Phys 9, 185–192 (2015). https://doi.org/10.1007/s40094-015-0177-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40094-015-0177-5