Abstract
Introduction
Coronavirus disease 2019 (COVID-19) is a viral respiratory syndrome caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This novel virus was discovered in Wuhan City, Hubei Province, China, in December 2019. As of September 6, 2020, confirmed cases have risen to more than 27,000,000 worldwide and more than 885,000 people have died. Currently, no cure or standard treatment for COVID-19 exists. We conducted a prospective single-arm open-label phase II clinical trial assessing the safety and efficacy of convalescent plasma in hospitalized patients with COVID-19.
Methods
Convalescent plasma with sufficient total anti-SARS-CoV-2 IgG titer (1:320) obtained from recovered donors was administered to adult patients with either severe or critical COVID-19 illness. Primary outcomes were adverse events in association with plasma administration, and hospital mortality. Secondary outcomes included disease progression, recovery, length of stay, and hospital discharge.
Results
Of the 38 patients included in the analysis, 24 (63%) recovered and were discharged, and 14 (37%) died. Patients who received convalescent plasma early in the disease course (severe illness group) as compared to the patients that received convalescent plasma later in the disease progression (critical illness group) had significantly lower hospital mortality 13% vs 55% (p < 0.02) and shorter mean hospital length of stay 15.4 vs 33 days (p < 0.01). One patient experienced a transient transfusion reaction. No other adverse effects of convalescent plasma infusion were observed.
Conclusions
Our results suggest that convalescent plasma with adequate anti-SARS-CoV-2 antibody titer is safe and has the potential for positive impact on clinical outcomes including recovery and survival if given to patients early in the course of COVID-19 disease.
Trial Registration
ClinicalTrials.gov. Identifier, NCT04343261, IND #19805.
Similar content being viewed by others
Coronavirus disease 2019 (COVID-19) is a viral respiratory syndrome caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). |
This novel virus was discovered in Wuhan City, Hubei Province, China, in December 2019, and as of September 6, 2020, confirmed cases have risen to more than 27,000,000 worldwide and more than 885,000 people have died. |
Currently, no cure or standard treatment for COVID-19 exists. |
We conducted a prospective single-arm open label phase II clinical trial assessing the safety and efficacy of convalescent plasma in hospitalized patients with COVID-19. |
Patients who received convalescent plasma with adequate amount of anti-SARS-CoV-2 antibodies early in the disease course (severe illness group) as compared to the patients that received convalescent plasma later in disease progression (critical illness group) had significantly lower hospital mortality 13% vs 55% (p < 0.02) and shorter mean hospital length of stay 15.4 vs 33 days (p < 0.01). |
Of the 38 patients that received convalescent plasma, only one patient experienced a transient transfusion reaction. |
Digital Features
This article is published with digital features to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12933338.
Introduction
Coronavirus disease 2019 (COVID-19) is a viral respiratory syndrome caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This novel virus was discovered in Wuhan City, Hubei Province, China, in December 2019. As of September 6, 2020, confirmed cases have risen to more than 27,000,000 worldwide and more than 885,000 people have died [1]. Currently, no cure or standard treatment for COVID-19 exists.
The majority of people with COVID-19 experience an asymptomatic, mild, or manageable course of disease [2, 3]. The most common symptoms include fever, cough, fatigue, dyspnea, headache, diarrhea, myalgia, and/or loss of taste and smell [4, 5]. However, 19% of those who are infected with the virus become severely or critically ill [2]. Life-threatening illness occurs when the virus triggers a progressive hyper-immune response or “cytokine storm” progressing to acute respiratory distress syndrome (ARDS), cardiac injury, thrombotic complications, septic shock, and/or organ failure [6,7,8,9]. Estimated mortality among patients admitted to the intensive care unit (ICU) with severe or critical illness ranges from 34.8% to 41.6% [10, 11]. Higher mortality rates (48–55%) were observed in patients with ARDS, needing invasive ventilatory support [10, 11]. Risk of death and disease severity increase with older age, obesity, and chronic disease such as hypertension, diabetes, and cardiovascular disease [7,8,9, 12,13,14].
In March of 2020, the US Food and Drug Administration (FDA) solicited investigational new drug applications to test the safety and efficacy of convalescent plasma therapy for patients with severe or life-threatening COVID-19 [15]. Convalescent plasma is derived from the blood of recovered patients and is a rich source of antibodies. When administered to patients who are ill with the same disease, the plasma may aid recovery by conferring passive immunity and neutralizing the pathogen [15]. The therapy showed promise during outbreaks of other novel viral respiratory syndromes, including two caused specifically by coronavirus [SARS-CoV in 2003 and Middle East respiratory syndrome (MERS) in 2012] [16, 17]. Data showed that convalescent plasma might be most effective when given earlier in the course of disease, but research was limited to small observational studies and much remains unknown [16, 17].
Preliminary data from clinical trials and observational studies targeting COVID-19 suggest that administration of convalescent plasma may reduce mortality, hospital length of stay, and time on mechanical ventilation with minimal adverse side effects in patients with severe or life-threatening disease [18,19,20,21,22,23]. Consistent with earlier studies, treatment may be most efficacious for severe COVID-19 when administered closer to symptom onset [21,22,23,24,25]. The purpose of this study is to describe the course of illness among 38 patients hospitalized with severe or life-threatening COVID-19 who received convalescent plasma as part of an FDA-approved phase 2 clinical trial. Specifically, the study will assess their hospital course in the context of demographics, disease onset, symptomology, illness severity, and disease progression.
Methods
This study is an FDA-approved prospective single-arm open-label phase II clinical trial (NCT04343261) assessing the safety and efficacy of convalescent plasma (IND #19805) on the clinical course of adult patients hospitalized with severe COVID-19. The study protocol was approved by the Trinity Health of New England Institutional Review Board (#SFH-20-23). The research study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. Informed consent was provided by either the patient or the patient’s legally authorized representative (LAR).
Patients
Subjects were recruited from four regional hospitals in Connecticut and Massachusetts between April 20, 2020 and June 8, 2020. Patients were considered eligible for the study if they were between the ages of 18 and 90, hospitalized, severely or critically ill with confirmed COVID-19 through nasopharyngeal swab real-time PCR (RT-PCR). Illness severity was defined as follows: mild COVID-19 was defined as symptoms with no clinical signs of moderate, severe, or critical disease; moderate illness was defined as respiratory rate ≥ 20 breaths per minute and oxygen saturation > 93%; severe illness was defined as any of the following: respiratory frequency ≥ 30/min, blood oxygen saturation ≤ 93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio < 300, and lung infiltrates > 50% within 24–48 h; evidence of critical illness included respiratory failure, septic shock, or multi-organ dysfunction or failure [15, 26].
Subjects who met eligibility criteria were referred by their treating physicians. Patients were enrolled regardless of previous treatment or therapies for COVID-19, including experimental medications and therapies administered off-label. Informed consent was provided by either the patient or the patient’s LAR. Once a patient or the patient’s LAR provided informed consent and the patient’s ABO blood type was determined, compatible convalescent plasma was administered in two consecutive 200-mL infusions. Each unit was transfused for the duration of 1 h, 1–2 h apart. If the patient received plasma with undetectable total anti-SARS-CoV-2 antibodies, the patient was re-dosed with a unit of plasma with adequate antibody titer (1:320). Recipients were monitored and all adverse reactions or events were recorded whether or not they were related to the plasma infusion. The protocol was approved by the Trinity Health of New England Institutional Review Board (#SFH-20-23).
Convalescent Plasma
Convalescent plasma was obtained from adult donors who were confirmed positive and had recovered from SARS-CoV-2. All donors screened negative for the virus using a nasal swab (RT-PCR) and had total anti-SARS-CoV-2 titer > 6.5 arbitrary units per milliliter (AU/mL; equivalent to 1:320). Plasma was collected by apheresis at an established blood donation center following standard operating procedures and 21 CFR 630.10 requirements. Plasma was frozen within 24 h of collection and labeled for investigational use and ABO typing.
Data and Data Sources
Demographic, clinical, and outcomes data were prospectively collected from electronic patient medical records at each of the four hospitals. Descriptive data included sex, age, race, ethnicity, smoking status, functional status, comorbidities, living situation, and means of arrival to the hospital. Initial presentation to the emergency department included self-reported symptoms, vital signs, degree of respiratory distress, and need for oxygen supplementation and resuscitation. Initial chest X-ray findings, and laboratory markers of sepsis, inflammatory response, immune deficiency, and organ dysfunction were recorded. The clinical course during hospital stay was prospectively captured by tracking changes in oxygenation (FiO2), need for invasive ventilation, ICU-level care, and types of essential medications given. Patient clinical status progression and recovery were prospectively monitored by capturing days on invasive ventilation, intubation, extubation, discharge alive, and death during hospitalization.
Outcomes and Data Analysis
Primary clinical outcomes were rate of adverse events associated with convalescent plasma administration, and hospital mortality. Secondary outcomes included disease progression, recovery, length of hospital stay, and hospital discharge. Primary and secondary clinical outcomes were compared between two groups based on severity of illness [15, 26] at the time of plasma infusion: (1) patients with severe illness, who had not progressed to ARDS at the time of enrollment, and (2) patients whose condition had progressed to critical illness at the time of enrollment. Patients were excluded from the analysis if they were transferred to another acute hospital; did not receive convalescent plasma with adequate antibody titer; or care was withdrawn and patient received comfort care only within 5 days of plasma administration.
Statistical Analysis
Descriptive statistics included means, medians, and proportions as appropriate based on variable, sample size, and distribution. Descriptive variables included demographic characteristics, clinical parameters, and time from illness onset and hospitalization to plasma transfusion. As a result of the small sample size, both parametric and nonparametric statistics were used in the analysis as appropriate. Continuous variables were compared using t tests, and categorical variables using chi-square analyses and Fisher’s exact test when cell sizes were small. SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for analyses. Outcomes were considered statistically significant at p < 0.05.
Results
Plasma Recipients
A total of 46 patients (Fig. 1) with RT-PCR-confirmed COVID-19 were enrolled in the study. Eight of 46 (17%) patients were excluded from the analysis for the following reasons: two received convalescent plasma with no detectable antibody titer; one was transferred to another hospital; five were made comfort care only (CMO) and medical care was withdrawn within 5 days of plasma administration. The remaining patients (n = 38) included in this analysis received convalescent plasma with adequate total anti-SARS-CoV-2 antibody titer of 1:320 (32 received 2 units, 5 were re-dosed with 1 unit, 1 received 1 unit).
Patient demographics, clinical presentation, hospital course, and clinical outcomes are shown in Tables 1, 2, 3, 4, and 5. Mean age was 63 years (95% CI 59–70), 53% were male, 34% black, 32% white, and 34% were Hispanic; 61% of the patients were from Connecticut, 37% from Harford County, 21% New Haven County, and 40% were from Hampden County, Massachusetts (Table 1). More than 68% had been diagnosed with hypertension and nearly half (47.4%) with diabetes mellitus; overall, 31.5% had three or more comorbidities (Table 2). As shown in Table 3, mean days from onset of symptoms to hospitalization was 7.3 days (95% CI 6.4–8.2), with the most common symptoms at admission being fever, cough, and dyspnea. With the exception of one patient who arrived in critical condition, subjects presented initially to the hospital with moderate to severe COVID-19 pneumonia without evidence of ARDS or requiring invasive ventilation support at the time of admission (Table 4). The most common laboratory abnormalities on admission included severe rise in inflammatory markers [C-reactive protein (CRP) ≥ 10 mg/dL] (66%), lymphopenia (absolute lymphocyte count < 1000 per microliter) (50%), and hyponatremia (Na < 135 mEq/L) (47%)—see Table 4.
Severe and Critical Illness Groups
At the time of plasma infusion, 16 patients (42%) met criteria for severe illness. These patients were enrolled in the study and received convalescent plasma earlier in their hospital and disease course on average 4.6 days (95% CI 2.9–6.3) following hospital admission, and 12.6 days (95% CI 10–15.2) following symptoms’ onset while on high-flow oxygen supplementation prior to any evidence of ARDS. The remaining 22 patients (58%) met the criteria for critical illness at the time of convalescent plasma therapy. They enrolled in the study and received convalescent plasma later in their hospital and disease course on average 16.4 days (95% CI 13–19.8) following hospital admission, and 23.1 days (95% CI 19.5–26.7) following symptoms’ onset after developing ARDS and had been on ventilation support for an average of 10.6 days (95% CI 7.3–13.9).
The two cohorts were comparable in demographics; comorbidities and home medications; pre-illness functional status; onset of symptoms to seeking hospital care; initial clinical presentation and findings; initial disease severity; and the care they received during their hospitalization including essential medications (see Tables 1, 2, 3, 4 and 5). Clinically, hyponatremia on initial hospital presentation was more prevalent in the severe illness group (p = 0.047). Vasopressors (p < 0.01), hydroxychloroquine (p < 0.01), and antibiotics (p < 0.01) were more frequently used during hospitalization in the critical illness group. Renal replacement therapy was utilized at higher rate in the critical illness group but did not reach statistical significance (p = 0.05).
Primary Outcomes
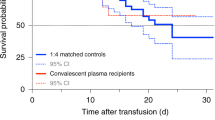
One patient in the severe illness group experienced a transient transfusion reaction (fever and hematuria) within 2 h of plasma infusion. No other adverse effects of convalescent plasma infusion were observed. Of the 38 patients included in the analysis, 24 (63%) recovered and were discharged from the hospital, and 14 (37%) died. Patients who died included two in the severe illness group and 12 in the critical illness group. The difference in mortality (13% severe vs 55% critical) was statistically significant (p = 0.02). Overall, patients who survived (n = 24) regardless of disease severity at time of infusion received convalescent plasma earlier in their course of disease (mean 15.3 days, SD 6.9) and hospital stay (8.4 days, SD 6.8) compared to those who died (n = 14) with mean durations of (24.5 days, SD 9.6), (16.6 days, SD 9.5) respectively.
Secondary Outcomes
Among patients with severe illness at the time of convalescent plasma therapy, 25% (4/16) progressed to ARDS after receiving convalescent plasma (Table 5). Three of the four required mechanical ventilation and two of the four died. One of those patients received convalescent plasma 18 days following onset of symptoms and died of refractory shock in ICU while on ventilator support. The other patient received convalescent plasma 16 days following symptom onset, developed respiratory failure secondary to ARDS, and was placed on comfort measures at the request of the family. The remainder (14/16, 88%) did not progress to ARDS, recovered with resolution of COVID-19 pneumonia, and were discharged from the hospital.
In the patients with critical illness at the time plasma therapy, 10/22 (45%) recovered with resolution of ARDS and restoration of organ function and left the hospital. Of the 12/22 (55%) who died, six died of refractory shock while on ventilator support with evidence of pneumoperitonium in four of them; three patients died of refractory respiratory failure with terminal extubation; two died of complications of upper airway edema; and one patient died of an acute cardiac complication.
Mean hospital length of stay was 25.6 days (95% CI 20.8–30.4) (Table 5). Length of stay was significantly shorter in the severe illness group (15.4 days, 95% CI 9.3–21.6) compared to patients in the critical illness group (33.0 days, 95% CI 27.3–38.7) (p < 0.01). Statistical analyses showed that patients treated earlier in the course of COVID-19 disease (severe group) had significantly lower hospital mortality (p = 0.02) and shorter hospital length of stay (p < 0.01) after convalescent plasma therapy compared to patients that were treated later in their disease course in presence of ARDS (critical group) (Table 5). Other prognostic factors that were significantly associated with good clinical outcomes included shorter durations between symptoms onset and convalescent plasma administration (p < 0.01), and hospital admission and administration of convalescent plasma (p < 0.01).
Discussion
Among this group of hospitalized patients with severe or critical COVID-19 who received convalescent plasma with adequate total anti-SARS-CoV-2 antibody titer (1:320), only one patient experienced a transient transfusion reaction. This low rate of adverse event secondary to convalescent plasma therapy is consistent with recent published literature [22, 23]. The overall hospital mortality among our study patients was 37%. However, patients who received convalescent plasma early in the disease course (severe illness group) as compared to the patients that received convalescent plasma later in disease progression (critical illness group) had significantly lower hospital mortality 13% vs 55% (p < 0.02) and shorter mean hospital length of stay 15.4 vs 33 days (p < 0.01). In addition, only four patients (25%) in the severe illness group developed ARDS, with three of them needing invasive ventilation support following convalescent plasma therapy. Two of the three recovered and were discharged.
It is important to understand the timeline and dynamics of COVID-19 hospitalizations in Connecticut and Western Massachusetts at the time when we launched our research study. Our study patients presented initially to the hospital with an average of 7.3 days (95% CI 6.4–8.2) from symptoms’ onset to hospitalization, and 97% (37/38) of the patients had moderate to severe disease without evidence of ARDS or urgent need for invasive ventilation support upon admission. By the time we enrolled our first patient in late April 2020, hospitals participating in the study were at their peak COVID-19 census, with a large number of seriously ill patients who had been hospitalized for an average of 2 weeks, and were not improving with supportive care or medications (see Table 5). Some of those patients deteriorated and needed ICU care and ventilator support for an average of 7 days prior to enrollment. Many had severe lung damage and multi-organ failure. In the early phase of our study, physicians enrolled mostly patients in this critical illness category. In the majority of cases, patients died as a result of secondary irreversible complications of COVID-19. As our study progressed, physicians started enrolling patients earlier in their disease course and hospital stay before respiratory status deterioration. Recently published interim analysis from the Mayo Clinic Expanded Access Program for convalescent plasma in the USA reported similar experience [23].
Upon admission to the hospital, the two cohorts were similar in their demographic characteristics, pre-illness functional status, comorbidities, initial clinical presentation to the hospital, and initial disease severity. However, at the time of convalescent plasma administration, the groups diverged on their disease severity and duration from disease onset to plasma therapy. The patients in our study who received convalescent plasma earlier in their disease course (severe illness group) had significantly more favorable primary and secondary clinical outcomes as compared to the critical group. We speculate that convalescent plasma given earlier in the disease course, and with adequate anti-SARS-CoV-2 antibodies arrested the progression to irreversible complications like ARDS or organ failure. In addition, we found that patients who survived in both groups had shorter times between onset of symptoms and convalescent plasma administration compared to those that died. Our patients received convalescent plasma with at least 1:320 titer of total anti-SARS-CoV-2 antibody, with majority of them receiving two consecutive units of 200 mL. This was done to maximize the potential therapeutic effect of the neutralizing anti-SARS-CoV-2 antibody. To our knowledge, there are no published reports with similar treatment strategy. The combination of using two units of convalescent plasma and high anti-SARS-CoV-2 antibody titer could have accounted for their relatively large therapeutic effect relative to other studies.
The literature suggests that convalescent plasma may be more beneficial when administered sooner to disease onset [16, 17]. Data recently published on COVID-19 suggested favorable clinical outcomes when convalescent plasma is given earlier in the course of disease [21,22,23,24,25, 27,28,29], and with higher content of anti-SARS-CoV-2 antibody [27, 29]. Our finding is consistent with the literature that treating patients with COVID-19 disease with convalescent plasma earlier in the disease course, and within the first 2 weeks following symptom onset may promote recovery [30]. Perhaps earlier treatment with convalescent plasma with adequate anti-SARS-CoV-2 antibodies allows antibodies to neutralize the virus before irreversible complications [19]. Vasopressor, antibiotics, and renal replacement therapy were utilized at higher rate in the critical group—we speculate that these therapies were proxies for serious and refractory complications among critically ill patients that could not be reversed by administration of convalescent plasma. We also speculate that the difference in hydroxychloroquine utilization between the two groups is likely a reflection of the change of evidence in association with hydroxychloroquine efficacy and safety and subsequent change in practice during our study.
The published literature on mortality of hospitalized patients with COVID-19 and ARDS needing invasive ventilatory support is evolving, as earlier published reports significantly underestimated hospital mortality when significant numbers of patients included in the analyses were still in the hospital [10, 14, 31]. Recent reports from Germany, Italy, and the UK estimated the mortality rate in this population to be at least 48–55%, which is consistent with our experience [31,32,33]. It is important to note that in our study, we only enrolled patients with severe or critical disease per the emergency IND issued by the US FDA. Many patients in the critical illness group had been hospitalized for an extended period and sustained serious lung damage prior to receiving convalescent plasma, and many remained hospitalized for weeks prior to death or discharge. Our enrolled patients may have been sicker and further along in the course of disease compared to other non-enrolled patients with COVID-19 in the ICU. Similar to the Mayo CP project, our study was pragmatic, and patients were enrolled on the basis of the judgment of their treating physicians.
As a result, our study may have been subject to “compassionate selection bias” toward sicker, and some were dying patients for whom nothing else could be done. This is an important limitation of our study and other studies that were approved under the emergency IND for convalescent plasma solicited by the FDA. Other important limitations in this study include its open-label design, no placebo control group, and modest sample size. However, despite the study design and power limitations, one interpretation of this result is that the therapeutic effect of convalescent plasma, when given early in disease course and in adequate amount, is so strong that signals of efficacy break through all the confounding noise to reduce mortality. Our research is important as it meaningfully contributes to the question whether convalescent plasma is safe, and it sheds light on important factors that are associated with favorable outcomes including recovery and survival.
Conclusions
For patients with severe or critical COVID-19 disease, convalescent plasma from recovered patients with COVID-19 is safe and has the potential for positive impact on clinical outcomes including recovery and survival if given early in the course of the disease, and in adequate amount. Our study makes a strong case for the importance of pursing a randomized placebo controlled trial focused on enrolling patients early in the course of their disease to further explore experimentally the efficacy and effectiveness of convalescent plasma with adequate anti-SARS-CoV-2 antibodies in COVID-19.
References
Johns Hopkins University & Medicine. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html. Accessed 6 Sept 2020.
Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Accessed 3 Aug 2020.
He J, Guo Y, Mao R, Zhang J. Proportion of asymptomatic coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Med Virol. 2020. https://doi.org/10.1002/jmv.26326.
Park M, Cook AR, Lim JT, Sun Y, Dickens BL. A systematic review of COVID-19 epidemiology based on current evidence. J Clin Med. 2020;9(4):967.
Gautier JF, Ravussin Y. A new symptom of COVID-19: loss of taste and smell. Obesity (Silver Spring). 2020;28(5):848.
Lu L, Zhang H, Zhan M, et al. Preventing mortality in COVID-19 patients: which cytokine to target in a raging storm? Front Cell Dev Biol. 2020;8:677.
Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–9.
Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020;133(11):1261–7.
Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020;133(9):1032–8.
Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID-19: a systematic review and meta-analysis of observational studies. Anaesthesia. 2020;75(10):1340–9.
Quah P, Li A, Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit Care. 2020;24(1):285.
Chen J, Qi T, Liu L, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80(5):e1–e6.
Bonow RO, Hernandez AF, Turakhia M. Hydroxychloroquine, coronavirus disease 2019, and QT prolongation. JAMA Cardiol. 2020.. https://doi.org/10.1001/jamacardio.2020.1782.
Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020. https://doi.org/10.1001/jamainternmed.2020.3596.
US Department of Health and Human Services Food and Drug Administration (FDA). Investigational COVID-19 convalescent plasma: emergency INDs. March 2020. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/investigational-covid-19-convalescent-plasma. Accessed 10 Apr 2020.
Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90.
Sun M, Xu Y, He H, et al. Potential effective treatment for COVID-19: systematic review and meta-analysis of the severe infectious disease with convalescent plasma therapy. Int J Infect Dis. 2020;98:334–46.
Zhang L, Pang R, Xue X, et al. Anti-SARS-CoV-2 virus antibody levels in convalescent plasma of six donors who have recovered from COVID-19. Aging (Albany NY). 2020;12(8):6536–42.
Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490–6.
Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–9.
Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–70.
Joyner MJ, Wright RS, Fairweather D, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791–7.
Joyner MJ, Bruno KA, Klassen SA, et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95(9):1888–97.
Hartman WR, Hess AS, Connor JP. Hospitalized COVID-19 patients treated with convalescent plasma in a mid-size city in the midwest. Res Sq. 2020. https://doi.org/10.21203/rs.3.rs-39447/v1.
Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680–90.
US Department of Health and Human Services Food and Drug Administration (FDA). COVID-19: developing drugs and biological products for treatment or prevention guidance for industry, May 2020. https://www.fda.gov/media/137926/download. Accessed 26 July 2020.
Salazar E, Christensen PA, Graviss EA, et al. Treatment of COVID-19 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol. 2020:S0002-9440(20)30370-9.
Perotti C, Baldanti F, Bruno R, et al. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica. 2020:261784.
Joyner MJ, Senefeld JW , Klassen SA, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. medRxiv. https://doi.org/10.1101/2020.08.12.20169359.
Rojas M, Rodríguez Y, Monsalve DM, et al. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev. 2020:102554.
Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020:3539.
Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;S2213–2600(20):30316–7.
Intensive Care National Audit Research Center (ICNARC). ICNARC report on COVID-19 in critical care, July 2020. https://www.icnarc.org/DataServices/Attachments/Download/. Accessed 6 Sep 2020.
Acknowledgements
We thank the donors, patients and their families for contributing to this work. We also thank the physicians from the Trinity Health of New England Hospitals who enrolled patients in this study at Johnson Memorial Hospital, Stafford, Connecticut (Ian Tucker); Mercy Medical Center, Springfield Massachusetts (Robert Roose, Laurie Loiacono, and Vikram Sondi); Saint Francis Hospital and Medical Center, Hartford Connecticut (Phillip Roland, Jessica Abrantes-Figueiredo, Daniel Gerardi, Prashant Grover, and Gagandeep Singh), and St. Mary’s Hospital in Waterbury, Connecticut (Paul Porter and Bethel Shiferaw); the New York Blood Center and Rhode Island Blood Center for assisting with the plasma collection process; Ernst J. Schaeffer and Margaret R Diffenderfer, Boston Heart Diagnostics in Massachusetts, for serology testing; Collen Lima, Jessica McKenzie, Lisa Cook, Jennifer Puff, Mary Onoroski, and Patricia Nabors for their work with donors, specimen collection and plasma delivery; Kelly Batch, Donna Sobinski, Cynthia Considine, Brian Masthay, Christina Maxwell and Robert Wilke from the Trinity Health of New England EPIC team for building the study in the electronic health record and assisting with study implementation.
Funding
This study was supported by funds from Trinity Health of New England, a not-for-profit healthcare organization. The Rapid Service Fees were funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Danyal Ibrahim, Latha Dulipsingh, Lisa Zapatka, Reginald Eadie, Rebecca Crowell, Kendra Williams, Dorothy B. Wakefield, Lisa Cook, Jennifer Puff and Syed A. Hussain declare that they have no conflicts of interests and no competing interests.
Compliance with Ethics Guidelines
The study protocol was approved by the Trinity Health of New England Institutional Review Board (#SFH-20-23). The research study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. Informed consent was provided by either the patient or the patient’s legally authorized representative (LAR).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12933338.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ibrahim, D., Dulipsingh, L., Zapatka, L. et al. Factors Associated with Good Patient Outcomes Following Convalescent Plasma in COVID-19: A Prospective Phase II Clinical Trial. Infect Dis Ther 9, 913–926 (2020). https://doi.org/10.1007/s40121-020-00341-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-020-00341-2