Abstract
Three-dimensional printing enables precise modeling of anatomical structures and has been employed in a broad range of applications across medicine. Its earliest use in eye care included orbital models for training and surgical planning, which have subsequently enabled the design of custom-fit prostheses in oculoplastic surgery. It has evolved to include the production of surgical instruments, diagnostic tools, spectacles, and devices for delivery of drug and radiation therapy. During the COVID-19 pandemic, increased demand for personal protective equipment and supply chain shortages inspired many institutions to 3D-print their own eye protection. Cataract surgery, the most common procedure performed worldwide, may someday make use of custom-printed intraocular lenses. Perhaps its most alluring potential resides in the possibility of printing tissues at a cellular level to address unmet needs in the world of corneal and retinal diseases. Early models toward this end have shown promise for engineering tissues which, while not quite ready for transplantation, can serve as a useful model for in vitro disease and therapeutic research. As more institutions incorporate in-house or outsourced 3D printing for research models and clinical care, ethical and regulatory concerns will become a greater consideration. This report highlights the uses of 3D printing in eye care by subspecialty and clinical modality, with an aim to provide a useful entry point for anyone seeking to engage with the technology in their area of interest.
Similar content being viewed by others
3D printing allows for precise, layered construction of anatomical models via imaging modalities such as CT and MRI. |
Printed orbital models enhance surgical planning and allow custom fitting of implants for improved fit, reduced surgical time, and fewer complications. |
Glasses, contact lenses, surgical instruments, diagnostic tools, and drug delivery devices making use of this technology are in development. |
Bioprinting of cell-laden tissues has been demonstrated in other parts of the body, and progress has been made toward printing of corneal and retinal tissues for both research and transplantation. |
Introduction
Additive manufacturing, or “3D printing,” is a process in which three-dimensional computer design files are utilized to produce a physical structure one layer at a time. Standard Triangle Language, or STL, files can be made to represent three-dimensional objects either by manual manipulation or by processing of radiographic images. A range of methods have emerged based on various layering techniques and printing materials. These include fused deposition modeling (FDM), inkjet, stereolithography, and microvalve-based and laser-assisted printing [1]. The technology enables custom-designed products to be created faster, more economically, and more locally than by traditional manufacturing.
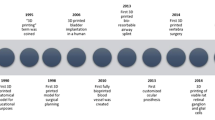
Its earliest applications in medicine were concentrated around educational models and surgical planning. In 1998, the first anatomical model used for planning of craniofacial reconstruction was created from a computed tomography (CT) scan [2]. Medical use of 3D printing technology has since expanded to include a wide array of applications. Recently, considerable research has focused on bioprinting, in which human tissues are synthesized for use in research or implantation. Scaffold materials can be printed, populated with progenitor cells, and infused with necessary growth factors to create synthetic tissues. In 2013, Zopf et al. custom-designed and implanted a 3D-printed airway splint for a child with tracheobronchomalacia [3].
The field of ophthalmology has begun to implement 3D printing in anatomical education, surgical planning, research models, implants, lenses, diagnostics, therapeutic applicators, and most recently, eye protection during the COVID-19 pandemic. The purpose of this review is not to delve into the specific technical parameters of each 3D printing approach. Rather, this report highlights the broad applications of 3D printing in ophthalmology by subspecialty and clinical modality with an aim to provide a useful entry point for anyone seeking to learn more about 3D printing in their area of interest. Several prior authors have reviewed certain applications of 3D printing without covering the full range of eye care topics [4,5,6,7,8,9,10]. This work is the most comprehensive review of 3D printing in ophthalmology to date.
Methods
We reviewed an extensive range of publications related to the application of 3D printing in ophthalmology. We accessed several databases which provided similar data, and we chose to proceed with the database with the most robust search results. Within the PubMed electronic database, we searched using all combinations of the following terms which were likely to return relevant results: eye-related terms including ophthalmology, eye, ocular, ophthalmic, and orbital; 3D printing-related terms including 3D, 3D printing, three-dimensional printing, printing, bioprinting, and additive manufacturing; and targeted anatomical or disease-focused terms including cornea, glaucoma, cataract, lens, retina, oculoplastics, radiation, contact lens, spectacles, glasses, low vision, tool, instrument, surgery, surgical device, prosthesis, implant, transplant, COVID, coronavirus, face shield, and goggle.
Our search criteria expanded to incorporate new terms, as initial results exposed lesser known areas of 3D printing application. We have also incorporated articles which were not uncovered by our search criteria but which were “Cited by” or “Similar” in PubMed and which contributed meaningful content to this review article. Except for a select few cases in which the abstract alone sufficed to offer a novel perspective, we have only included articles in which a full-text English version was available. This method yielded a total of 179 manuscripts for our review. Ethics committee approval was not required for this review article. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Cornea
Corneal Tissue Models
The avascular and immune-privileged nature of the cornea make it an ideal target for transplantation, but there is a dire shortage of donor corneal grafts. A global survey of corneal transplantation found that in 2012, there were only enough grafts for 1 in 70 people needing transplants and that 53% of the world population had no access [11]. Cost and graft rejection increase the demand, while medication history and religious beliefs restrict the supply [12, 13]. Synthetic alternatives such as the Boston Keratoprosthesis are prohibitively expensive for most [12].
In recent years, efforts to design 3D corneas for both in vitro research and in vivo transplantation have expanded within the field of tissue engineering. 3D in vitro models enable analysis of cellular and extracellular matrix interactions, disease processes, tissue healing, and drug testing. These models include organoids, which are small three-dimensional tissue structures composed of stem cells [4]. The term “bioprinting” has been used to describe organotypic approaches for synthesizing corneas in which hydrogel scaffolds composed of organic or synthetic materials are inlaid with cells without the use of a 3D printer [4, 14]. Collagen derived from fish scales has been used to produce corneal scaffolds, though the refractive efficacy remains to be demonstrated, and it is likely that corneal cells will need to be incorporated to maintain structure and optical clarity [12, 15, 16].
Several methods for 3D printing of corneas have used a scaffold into which cells or collagen can be infused. While populating with corneal progenitor cells helps establish a more natural cornea, it may limit the utility of this technology in regions with cost and access barriers [12]. Typically, a 3D image of the cornea is created via ultrasound or optical coherence tomography (OCT), though a Scheimpflug camera can be used as well [12, 17]. The ideal printing medium, referred to as “bioink,” must have the mechanical strength to withstand the shear stress of extrusion through the nozzle, retain adequate transparency, support cell viability, allow diffusion of nutrients and oxygen, be suturable, and be biodegradable [1, 14]. They often must be cross-linked to maintain their structure [18]. Optics is one of the biggest challenges, as the process requires sufficiently high resolution for a smooth surface and involves printing flat layers into a curved structure [1]. Multiple authors have described promising results in meeting the above parameters, but the ultimate challenge remains in building toward a multilayered and fully replicative structure [19].
Stromal equivalents can be printed via the inclusion of keratocytes in type 1 collagen-based bioinks containing various combinations of gelatin, alginate, agarose, and methacrylate [17, 20, 21]. Decellularized porcine stroma and decellularized extracellular matrix can be used as well [22,23,24,25]. Kim et al. found that wide nozzle diameters in extrusion printers led to misaligned collagen fibrils and poor transparency, but narrow nozzles increased shear stress and caused keratocytes to develop fibroblastic characteristics. A medium size allowed for good transparency and stimulation of lattice-patterned collagen production similar to native cornea when implanted in rabbits [23]. Kutlehria used a combination of extrusion and stereolithography printing to produce constructs with good cell marker expression and clarity. Using a support scaffold allowed for high-throughput printing of 6–12 corneas at a time for in vitro drug delivery testing [21].
Epithelial-based models have been produced using similar approaches [26, 27]. Wu et al. demonstrated strong transparency and cell viability and found that adding sodium citrate to their bioink enabled the epithelial cells to degrade the scaffold and proliferate [27]. An endothelial cell model has shown excellent cell structure and function with implantation in rabbits. Endothelial cells were modified to express a pro-angiogenic and pro-mitotic ribonuclease and were printed by extrusion onto an amniotic membrane made to simulate Descemet’s membrane [28]. Sorkio et al. reported the only layered corneal model described to date. Their model used both limbal and adipose-derived stem cells to create epithelium and stroma, respectively. While the epithelial layer bioink contained hyaluronic acid and laminin, the stromal layer bioink contained type I collagen, thrombin, and plasma [18].
Other Ocular Surface Applications
3D printing has been used for corneal procedural training. Lichtenberger et al. used 3D printing to create an eye model which integrates with a face mold and can be mounted at the slit lamp for training in corneal foreign body removal [29]. Famery et al. constructed a model composed of an artificial anterior chamber and 3D-printed iris for training of Descemet membrane endothelial keratoplasty [30].
To date, only one author has described printing of a membrane for conjunctival placement. Dehghani et al. used extrusion printing of elastin, gelatin, and hyaluronic acid to produce a membrane for comparison with amniotic membrane. They reported positive results in terms of tissue integration, tissue reaction, cellular proliferation, and epithelialization [31].
Glaucoma
Using average parameters of the anterior chamber, Wang et al. 3D-printed a 5× magnified eye model and added an inflow and outflow valve to simulate aqueous humor dynamics. They used this model to study the interplay of iris deformation, intraocular pressure, and aqueous outflow; they proposed that it could elucidate the pathogenesis of iris-induced glaucoma mechanisms such as iris bombe, pupillary block, and narrow angles [32]. Current extrusion-based additive manufacturing technology does not have adequate resolution to model the 10 um pores of the trabecular meshwork, but stereolithography printing and use of materials such as sodium alginate and methacrylated gelatin bioinks have shown promise in optimizing resolution [33, 34].
Cataract
Cataract surgery is the most common surgery performed worldwide, and intraocular lens technology has rapidly expanded to offer numerous options for surgeons and patients. At some point, 3D printing may offer a cheaper and more accessible solution for producing tailor-made lenses. Debellemaniere et al. printed a Ridley lens with 75% visible light transmission, but significant surface irregularity [35]. Hinze et al. reported the printing of a trifocal diffractive aspheric intraocular lens (IOL), though we were not able to access a full text of their publication [36].
Li et al. described implantation of 3D-printed poly(acrylamide-co-sodium acrylate) hydrogel-based lenses in rabbits. Their process required 9 days of pretreatment with phosphate buffered saline to stabilize the gel structure. They observed no effect on intraocular pressure, electroretinogram, or optical structure. Both the hydrogel and the standard IOL group experienced elevation in anterior chamber concentrations of the inflammatory mediators TNF-alpha and IL-8, which normalized at 1 month, indicating that no rabbits developed endophthalmitis [37].
Challenges to 3D printing of IOLs include achieving the optimally smooth surface of conventional IOLs and selecting a material with optimal ultraviolet blockage, visual transmissibility, and mechanical properties [5, 37]. In the future, 3D-printed intraocular lenses may be able to be infused with antibiotics, corticosteroids, and nonsteroidal anti-inflammatory drugs to eliminate postoperative drop burden [37].
Retina
Educational Models
Traditionally used models such as Navarro’s schematic eye have inspired the creation of 3D-printed models for research and training. Model eyes have been printed to assess the fundus viewing range of various lenses as well as the quality of wide-angle imaging and OCT instruments [38, 39]. Model eyes have also been printed for retinal laser training [40].
Retinal Tissue Bioprinting
Three-dimensional retinal representations may more accurately model tissue response to therapeutics, as has been shown in the modeling of the blood–retina barrier with scaffolds containing retinal endothelial cells and astrocytes [41]. Retinal ganglion cells can be printed onto an electrospun cell scaffold with brain-derived neurotrophic factor and ciliary neurotrophic factor with retention of electrophysiologic function and radial alignment of growing axons [42]. Scaffolds can be printed to support the proliferation of infused retinal progenitor cells as well [43]. Hydrogels can be used as a printing medium and supportive extracellular matrix in creating a model with both a retinal pigment epithelium (RPE) and photoreceptor layers [44,45,46,47]. Rather than printing cells within hydrogel, dense cell suspensions can also be used as a printing material and secrete their own supportive matrix [44]. This approach has permitted printing of an in vitro retinal research model containing an RPE with demonstrated phagocytosis of photoreceptor outer segments and retinal layers displaying characteristic structural markers [48].
It remains to be seen whether 3D printing can generate a sufficiently functional and safe retinal tissue implant. Rat ganglion and glial cells have been printed using a piezoelectric printer, and a bilayer retinal tissue has been printed using a hydrogel-free approach [44, 49]. The retina, however, is a multilayer, vascularized structure comprising more than 60 cell types [50]. Furthermore, the retinal architecture varies by location, and different diseases demand replacement of different components; while the macula is primarily affected in macular degeneration, broad swaths of the nerve fiber layer may need replacing in glaucoma [6]. Implantation of functional bioprinted retinal tissue would likely need to include the use of angiogenic factors and microchannels to help create diffusion of nutrients and oxygen in the absence of vasculature [51].
Customized Treatment
3D printing has been employed in the treatment of retinal diseases. Using CT-generated virtual models of a patient’s eye, a custom-fit, polyetheretherketone macular buckle can be 3D-printed for implantation in patients with myopic foveoschisis maculopathy [52]. A 3D-printed representation of an OCT scan has been used to show where to start a membrane peel in a patient with an epiretinal membrane [53]. 3D models of OCT-angiography scans can be useful for studying retinal vasculature and choroidal tumors; with further iteration and lower cost, it may become a useful diagnostic tool in diseases such as inflammatory or infiltrative choroidal pathologies [54, 55].
Oculoplastics
Education
3D printing provides a useful method for creating orbital models with similar rigidity as bone for anatomical education and surgical training [56, 57]. Traditional anatomy education with cadavers may be limited by access, cost, and social stigma. 3D-printed orbital models were created by Adams et al. based on 3D designs recreated from cadaveric prosections [58]. However, these designs can also be achieved through the use of magnetic resonance imaging (MRI) and CT studies [59]. Orbital anatomy is complex, difficult to conceptualize externally, and may vary significantly between patients; furthermore, the consequences of violating the intracranial space during surgery include meningitis, cerebrospinal fluid leak, and intracranial hemorrhage. Trainees can hone skills such as lateral wall decompression in a safe and easily reproducible context [56]. Soft tissues are more difficult to model due to less crisp delineation on imaging and lack of conducive printing materials [60]. Soft tissue has been more challenging to print with realistic quality, but can still be of educational value [57, 58]. Adjacent structures such as the pterygopalatine fossa can be modeled as well [61].
Repair of Bone Defects
Patient-specific orbital models can be used for orbital surgical planning of bone defects. Models allow for preoperative familiarization of patient-specific anatomy and enable the surgeon to select the optimal location and material for implants and screws [7, 62]. They have also been shown to improve patient understanding [63].
Accurate sizing of an implant to repair an orbital fracture is technically challenging and typically requires multiple intraoperative implant modifications [64]. Even with experienced surgeons, postoperative complications such as globe malposition can occur [65]. Implant templates can also be printed by virtually mirroring the unaffected orbit on the normal side [66]. Both CT and MRI have been shown to create anatomical models accurately [67, 68]. Templates can be sterilized for intraoperative use without losing their shape [69, 70].
Optimal implant sizing through 3D printing helps prevent globe misalignment, strabismus, blood loss, and other postoperative complications [70,71,72,73]. Preoperative sizing reduces surgical time and limits fatigue of the implant material due to fewer adjustments [74]. It also reduces the risk of misplacement and damage to adjacent structures, such as positioning an implant too posterior and impinging the optic nerve [75]. Furthermore, this approach can be combined with intraoperative navigation systems to guide optimal placement [76, 77].
The orbital literature concerning 3D-printed bony defect templates includes numerous reports of successful use in the orbital floor, medial wall, and lateral wall [67, 78,79,80,81]. Chai et al. published a study of 127 fracture repairs done with the aid of 3D-printed skull models [70]. Oh et al. reported successful outcomes without complication in 104 patients [82]. Tel et al. described 14 orbital floor fractures repaired via a transmaxillary endoscopic approach with CT-guided 3D prints used to fit autologous bone into the defects [71].
Complex, multi-wall fractures have been addressed via this approach as well. Combined inferior and medial wall fractures pose a particular challenge given destruction of the inferomedial orbital strut. Kim et al. used 100 cadaver skulls to create a digital model of a standardized inferomedial orbital strut used in 3D printing of polycaprolactone implants [83]. Interlocking puzzle-piece implants can be used to bridge two or more implants across missing landmarks in cases of adjacent wall fractures; doing so may reduce incisional burden by enabling piece-by-piece placement through the same incision [84,85,86,87].
Several comparative studies have shed light on the impact of this technology in clinical practice. Fan et al. compared 29 cases of fracture repair using 3D-printed technology with 27 cases addressed with conventional methods. They found that patients in the 3D printing group had statistically significantly better implant fit, shorter surgical duration, and better outcomes at their postoperative assessment [88]. Kozakiewicz et al. showed an improvement in binocular single vision and globe motility in a retrospective study of 12 fractures treated with 3D-printed templates and 12 treated with conventional implant manual manipulation [89]. Sigron et al. found that ten patients treated with pre-bent implants based on printed models had lower volume difference between their two orbits and a 42% decrease in surgery time [90]. In a retrospective study of 82 patients, Kim et al. found that the 3D printing group had a smaller bony defect, shorter gap between implant and bony edge, and smaller angle between implant and natural bony contour [91]. Incorporating 3D printing into orbital fracture repair has been found to reduce surgical time by as much as 50% [70, 92].
3D printing has also been used in the bony reconstruction of congenital craniofacial malformations, including orbital defects such as hypertelorism [62, 93]. It has been used to characterize the location of an intraorbital cyst in a patient microphthalmia and design an orbital floor implant [94]. In such cases, 3D-printed models can be particularly useful for explaining the bony deformities and surgical repair to patients and families [62].
In addition to using printed models as a template to size implants, custom patient-specific implants can be produced directly using 3D printing technology. Implants have been produced by casting polymethyl methacrylate in printed injection molds [95]. Implants can also be printed themselves. Several authors have reported successful repairs using printed polyetheretherketone [77, 96, 97]. Titanium implants can be printed as well [76, 77, 98,99,100]. Kitabata et al. printed a rigid titanium floor implant with reduced risk of buckling, but note that the process took 1 day and was more expensive than a traditional flexible titanium implant [101]. Interlocking puzzle-piece implants have also been printed [77, 85, 87]. Printing of implants results in a more rigid titanium implant; it also produces softer edges than those of traditional implants which are cut from titanium mesh, thereby reducing the risk of soft tissue entrapment [85]. In a series of 34 orbital fractures, custom-fit 3D-printed implants showed a significant advantage over pre-bent implants in terms of volume difference between the operative and unaffected orbit [102].
Limitations to 3D printing for the repair of bony implants include cost and time, with directly printed implants taking as long as 5 days to produce [85]. Manipulation of preformed implants may still be required in complex fractures with missing landmarks and unpredictable final orbital shape or to avoid neurovascular structures [64, 103]. While most printing materials can withstand sterilization, melting point is a concern for some [104]. There are regulatory concerns as well. Custom-made implants are not subject to the same rigorous process of regulatory approval as mass-produced medical devices, which may create liabilities for institutions which print and use them [105, 106].
Repair of Anophthalmic Sockets
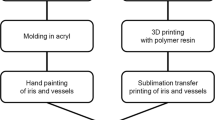
Production of an ocular prosthesis typically entails the impression of hydrocolloid in the patient’s orbit, then fitting a scleral shell into this space [107]. A prosthesis can be printed based on a CT scan of a molded wax model [108]. However, the molding process is uncomfortable for the patient and can shift the soft tissues and decrease accuracy [107]. Several authors have demonstrated methods of virtually modeling of the patient’s orbit for prosthesis printing. 3D-printed scleral shells can be printed from either CT or corneoscleral topography scans and undergo standard post-fitting modifications such as iris art and acrylic finish [109,110,111]. Two authors have described a method for avoiding the manual artwork by printing a shell and layering on an iris with a sublimation transfer method [112, 113]. The resulting product met safety standards including cytotoxicity and tissue reactivity and had strength testing comparable to that of conventional prostheses [112]. Huang et al. printed a custom-designed surgical guide to optimize the placement of titanium osseointegrated implants for retention of an orbital prosthesis [114].
3D printing can streamline the production of ocular implants (the space-occupying item behind the visible prosthesis). A series of ten patients underwent implantation of a 3D-printed sphere following evisceration. None of the patients developed systemic or local toxicity, infection, inflammation, extrusion, or exposure [115]. Implant migration can cause poor prosthesis fit and cosmesis, often necessitating a secondary dermis fat graft. Dave et al. used a CT-guided virtual skull model to 3D-print a secondary implant to recenter an inferotemporally displaced primary implant [116].
Following enucleation, a conformer must be placed to prevent tissue contraction, symblepharon, and forniceal shortening while the tissues heal. Poor conformer fit prevents proper healing and may cause conformers to fall out or be removed by patients. Custom conformers have been printed for this purpose [117]. Serial 3D printing has also been used to produce successive socket conformers in orbital expansion therapy for patients with microphthalmia [118].
Weisson et al. custom-printed orbital exenteration prostheses based on facial topography scans for three patients who had stopped wearing their initial prosthesis due to issues with fit, color, or degradation. The exenteration prosthesis can integrate with a separately produced ocular prosthesis [119]. Similarly, a 3D-printed orbital mold can be used for casting of a polydimethylsiloxane exenteration prosthesis [120].
Eyelids
Sun et al. used external measurements in a patient with ptosis to guide custom 3D printing of inexpensive, adjustable, spectacle-mounted eyelid crutches [121]. Acetyl-hexapeptide-3 is an effective anti-wrinkle peptide which requires microneedle patch delivery due to poor skin permeability; a bespoke patch can be printed using a face imaging model [122]. A novel heat and massage eyelid device in development for meibomian gland dysfunction reportedly incorporates 3D printing for personalized fitting [123].
Radiation
Radiation-induced cataracts are an important consideration for patients receiving treatment to orbital and periorbital tissues and for providers delivering the radiation. While lead glasses are effective at reducing exposure, scatter from adjacent tissues remains a concern [124]. In recent years, the threshold radiation dose for cataract formation has been revised significantly downward. In response, multiple eye lens dosimetry models have been created to quantify off-target radiation. This modeling requires attention to the energy dose, the angular dependence of the rays, and their passage through the cornea and aqueous humor to the sensitive portion of the lens [125]. Several 3D-printed dosimetry models have been described [124,125,126]. Similarly, 3D printing has been used to create custom-fit lead face shields by either direct printing or molding to a printed template; this can eliminate the need to intubate and live-mold a shield over the patient’s nose and mouth [127, 128].
3D-printed parts have also begun to be incorporated into the delivery of radiation therapy near the eye. Plaque brachytherapy applicators for ocular radiation have been printed [129, 130]. 3D printing of an external applicator for delivery of radiation to an orbital fibrous solitary tumor has been demonstrated [131]. Boluses, used in radiation therapy to smooth uneven surfaces to achieve optimal radiation of superficial tumors, have been printed for the orbit [132]. Basal and squamous cell tumors near the medial and lateral canthi have been successfully treated using this method [133]. Furdova et al. used CT and MRI to generate a 3D-printed model eye with uveal melanoma for planning of radiation therapy in 150 patients [134].
Contact Lenses
In 2017, Johnson and Johnson Vision Care (Jacksonville, FL) entered into collaborations with the University of Waterloo to develop 3D-printed contact lenses [135]. Rigid gas-permeable contact lenses are used in patients with keratoconus, corneal transplants, or high refractive error. An irregular corneal surface can necessitate multiple trial fits. Simulation software can help improve lens fitting, but cannot account for the weight of the lens and the effect of the eyelid on lens fit. With 3D printing, topographic data can be converted to a 3D, digital file and a model can be printed for accurate fitting. This approach can reduce the number of fits required and decrease the risk of infection or epithelial damage. Dixon et al. have proposed a novel method for measuring oxygen transmissibility of contact lenses which uses 3D printing to produce components of a diffusion cell containing the oxygen-sensitive compound cysteamine [136]. Future research will likely focus on refining printing material choice as well as incorporating tear film and eyelid into the printed model [137, 138].
Spectacles
Additive manufacturing of spectacle lenses has the potential to streamline production and reduce costs. It can eliminate the need to store lens blanks, reduce the waste associated with starting with blanks, and obviate the need for grinding and edging machines. Post-printing processing typically requires nothing more than assembling printed frame parts with hinges and adding any desired lens coatings [8]. Production times have been shown to decrease by as much as 80% from traditional manufacturing [139]. The technology theoretically holds unique benefits to both ends of the socioeconomic market: it allows highly customizable fittings for high-end purchases while also enabling local and efficient production in remote, low-resource settings [8]. It has been shown to produce lenses of comparable surface roughness and wavefront aberrations as conventionally produced lenses [140].
Thus far, the literature on 3D-printed spectacles applies mostly to niche situations, such as patients with facial deformities. Spectacles were printed for a 5-year-old child with Goldenhar syndrome who could not wear conventional glasses due to a nasal deformity and wide interpupillary distance and who was not a candidate for contact lenses or refractive surgery due to limbal dermoids. The printed pair had better fit and cosmesis for the patient, resulting in her wearing them more and decreasing her risk of amblyopia [141]. Similarly, 3D printing offered a solution for an infant who was left aphakic after congenital cataract extraction and could not use conventional glasses due to craniofacial malformations or contacts due to microcornea and lid malformations [142].
In both of the aforementioned cases, printing the glasses required the costly and time-consuming step of first printing a model of the child’s face. While this is not practical to apply on a broad scale, printing of a face model is not always necessary. Many companies have begun printing glasses and sunglasses frames for off-the-shelf purchases. One company has focused on customized printing for complex prescriptions, such as in patients who require higher lens powers than are traditionally available [143]. In this case, glasses can be virtually fitted in digital format to a scan of the patient’s face prior to printing [8].
Limitations currently include the need to equip and train in low-resource settings, certification of spectacles and lenses as commercial grade, and the ability to print frames using multiple materials. In the future, businesses may focus on producing lens holders or molds to aid in frame production and to allow for simple design modifications in response to evolving styles. Future applications might also enable users to print repair parts or tools or to print industry-grade safety goggles [8].
Low Vision
Prismatic devices have been printed to achieve field expansion and help prevent collisions in patients with homonymous hemianopia, monocular status, or peripheral field loss [144, 145]. Researchers at Michigan Technological University (Houghton, Michigan) are developing a 3D-printed, ultrasound-guided navigation bracelet for the blind [146].
Diagnostics
Smart Contact Lenses
In recent years, contact lenses have been designed for noninvasive monitoring in diseases such as diabetes. The electronic sensors incorporated into the lenses can be interconnected using 3D-printed components [147]. 3D-printed molds can also be employed to create microchannels in the hydrogel found in smart contact lenses [148]. Alam et al. also described 3D printing of an entire smart contact lens with embedded diagnostic sensors [149].
Clinical Equipment
Hopkins et al. used a $500 printer with included software to print a glare acuity tester, disposable pinhole cover paddle, various ophthalmic equipment repair items, lens thickness caliper, NoIR filter lens blank flipper, Optivisor face plate, EasyPocket ID lanyard holder, dome magnifier handle, and phoropter near card holder. These items took anywhere from 20 to 100 minutes to design, and 12 minutes to 5 hours to print; per-unit material costs were all under $1 [150].
Imaging
Ocular imaging systems have benefitted from the incorporation of 3D-printed components. Smartphone retinal imaging adapters have been designed to couple the camera system to a standard indirect ophthalmoscopy lens [151, 152]. One teenager designed an artificial intelligence-based screening app paired with a 3D-printed lens for diabetic retinopathy screening [153]. A similar combination of software and printed adapter has been employed in the Portable Eye Examination Kit [154]. An analogous anterior segment imaging phone adapter can be printed and assembled in 35 minutes for less than NZD 20.00 [155]. Researchers in Seoul have developed a 3D-printed headmount for anterior segment imaging and disease tracking in mice [156]. Lee et all designed and printed a head-mounted gaze tracker, with applications in assistive devices, virtual reality devices, disabled person communication, human–computer interaction, and research in fields ranging from neuroscience to marketing [157].
Drug Delivery
Contact Lenses
Drug-eluting bandage contact lenses have the potential to lengthen exposure time and improve compliance. Silicone hydrogels are typically used due to optimal mechanics, oxygen permeability, and transparency. Gelatin hydrogels are a potentially drug-eluting material which can be processed through 3D printers. One group found that incorporating polyethylene glycol diacrylate into drug-eluting lenses prolonged dexamethasone release. Some drugs can compromise lens clarity, so a hollow center may be recommended [158]. Phan et al. used 3D printing to produce a model eye which incorporates tear film simulation and a blink model for testing of drug delivery via contact lenses [159, 160].
Other Devices
3D printing can be used to produce delivery devices or the molds used to cast them [161]. Kojima et al. described their development of a periocular, transscleral, 3D-printed delivery device containing human RPE cells and providing sustained release of brain-derived neurotrophic factor to the retina. In vitro data showed continuous secretion for at least 16 days [162]. Lee et al. designed an intravitreal, transscleral-sutured delivery device consisting of micro- and nanochannels and a reservoir system whose components were produced using 3D-printed molds [163]. Won et al. demonstrated promising preclinical data in rabbits with intravitreal injection of a printed rod containing bevacizumab and dexamethasone [164].
Surgical Devices
Canabrava et al. described the 3D printing of the first intraocular device, Cana’s ring, a 300° pupil expansion ring [165]. Navajas et al. demonstrated that a customizable trocar-cannula system could be printed for vitreoretinal surgery with a minimum size of 21–22 gauge; although one trocar broke upon insertion in a pig eye, the idea showed promise for future iteration [166]. Choi et al. created a 3D-printed micro-vane modification for phacoemulsifier sleeves which induces swirl in the irrigation inflow and dissipates the shearing forces responsible for corneal endothelial cell damage. They found that the flow velocity drops significantly 2 mm from the phacoemulsifier tip and then remains at about 70% of that with the traditional sleeve [167]. Ruzza et al. 3D-printed a smart storage glide to store and deliver the posterior lenticule in Descemet stripping automated endothelial keratoplasty procedures [168].
Personal Protective Equipment During the COVID-19 Pandemic
Faced with global supply shortages of personal protective equipment (PPE) and supply chain disruption during the COVID-19 pandemic, people across the world have turned to 3D printing. Eye protection has been recommended due to the well-documented ability to spread aerosols through ocular exposure. Face shield and goggle components can be printed cheaply and quickly and reused with simple sterilization protocols [9, 169,170,171,172,173,174,175,176]. For example, the surgery and anesthesiology departments at the University of Nebraska were able to make 112 face shields in 3 days by printing headbands and chinpieces and assembling a final product with a verified sterilization protocol [169]. A team at Brigham and Women’s Hospital in Boston had made 3000 face shields as of December 2020 [176]. Surgical helmets have been converted into powered air-purifying respirators (PAPr) that meet high-efficiency particulate air (HEPA) standards, and headlights have been modified into face shields with the aid of additive manufacturing [177, 178].
3D printers previously used to produce anatomic models have been repurposed internally, but printers and raw materials have also been sourced from hobbyists and companies through the efforts of coalitions such as the National Security Innovation Network [171, 174, 175]. Large corporations such as Nike, Ford, and Apple have printed large quantities using their existing equipment [175]. Manufacturers such as Prusa, YouMagine, and 3DVerkstan have made their software open-source [171, 173]. The low cost and ease of production mean that institutions may continue to print PPE even as global supplies recover [172].
Conclusions
3D printing has demonstrated a broad range of applications within ophthalmology over the past decade. The technology allows precise modeling of bony anatomy for surgical planning and custom-fit prostheses in the field of oculoplastic surgery. It has been used in various ways to design surgical instruments, diagnostic tools, drug delivery devices, and components of personal protective equipment. Perhaps its most alluring applications reside in the possibility of printing tissues at a cellular level to address unmet needs in the world of corneal and retinal diseases. Early models toward this end have shown promise for engineering tissues which, while not quite ready for transplantation, can serve as a useful model for in vitro disease and therapeutic research. While this technology may allow us to reduce animal testing, the transformative idea of printing tissues and eventually organs brings up ethical and regulatory concerns which will warrant consideration [19, 179].
3D printing technology is rapidly advancing and access is now widespread, though the equipment varies considerably in design and quality. Even in scenarios when equipment is not available or is not of sufficient quality, designers can upload their three-dimensional files to a third party for outsourced printing. [180]. As the technology and access continue to improve, 3D printing will assume a greater role in the field of ophthalmology.
References
Khalili M, Asadi M, Kahroba H, Soleyman MR, Andre H, Alizadeh E. Corneal endothelium tissue engineering: an evolution of signaling molecules, cells, and scaffolds toward 3D bioprinting and cell sheets. J Cell Physiol. 2020;236:3275–303.
Eppley BL, Sadove AM. Computer-generated patient models for reconstruction of cranial and facial deformities. J Craniofac Surg. 1998;9(6):548–56.
Zopf DA, Hollister SJ, Nelson ME, Ohye RG, Green GE. Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med. 2013;368(21):2043–5.
Shiju TM, Carlos de Oliveira R, Wilson SE. 3D in vitro corneal models: a review of current technologies. Exp Eye Res. 2020;200:108213.
Al-Kinani AA, Zidan G, Elsaid N, Seyfoddin A, Alani AWG, Alany RG. Ophthalmic gels: past, present and future. Adv Drug Deliv Rev. 2018;126:113–26.
Lorber B, Hsiao WK, Martin KR. Three-dimensional printing of the retina. Curr Opin Ophthalmol. 2016;27(3):262–7.
Ruiters S, Mombaerts I. Applications of three-dimensional printing in orbital diseases and disorders. Curr Opin Ophthalmol. 2019;30(5):372–9.
Lee L, Burnett AM, Panos JG, Paudel P, Keys D, Ansari HM, et al. 3-D printed spectacles: potential, challenges and the future. Clin Exp Optom. 2020;103(5):590–6.
Rendeki S, Nagy B, Bene M, Pentek A, Toth L, Szanto Z, et al. An overview on personal protective equipment (PPE) fabricated with additive manufacturing technologies in the era of COVID-19 pandemic. Polymers (Basel). 2020;12(11):2703.
Sommer AC, Blumenthal EZ. Implementations of 3D printing in ophthalmology. Graefes Arch Clin Exp Ophthalmol. 2019;257(9):1815–22.
Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134(2):167–73.
Ludwig PE, Huff TJ, Zuniga JM. The potential role of bioengineering and three-dimensional printing in curing global corneal blindness. J Tissue Eng. 2018;9:2041731418769863.
Fuest M, Yam GH, Mehta JS, Duarte Campos DF. Prospects and challenges of translational corneal bioprinting. Bioengineering (Basel). 2020;7(3):71.
Zhang B, Xue Q, Li J, Ma L, Yao Y, Ye H, et al. 3D bioprinting for artificial cornea: challenges and perspectives. Med Eng Phys. 2019;71:68–78.
van Essen TH, van Zijl L, Possemiers T, Mulder AA, Zwart SJ, Chou CH, et al. Biocompatibility of a fish scale-derived artificial cornea: cytotoxicity, cellular adhesion and phenotype, and in vivo immunogenicity. Biomaterials. 2016;81:36–45.
Yuan F, Wang L, Lin CC, Chou CH, Li L. A cornea substitute derived from fish scale: 6-month followup on rabbit model. J Ophthalmol. 2014;2014:914542.
Isaacson A, Swioklo S, Connon CJ. 3D bioprinting of a corneal stroma equivalent. Exp Eye Res. 2018;173:188–93.
Sorkio A, Koch L, Koivusalo L, Deiwick A, Miettinen S, Chichkov B, et al. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials. 2018;171:57–71.
Ruiz-Alonso S, Villate-Beitia I, Gallego I, Lafuente-Merchan M, Puras G, Saenz-Del-Burgo L, et al. Current insights into 3D bioprinting: an advanced approach for eye tissue regeneration. Pharmaceutics. 2021;13(3):308.
Duarte Campos DF, Rohde M, Ross M, Anvari P, Blaeser A, Vogt M, et al. Corneal bioprinting utilizing collagen-based bioinks and primary human keratocytes. J Biomed Mater Res A. 2019;107(9):1945–53.
Kutlehria S, Dinh TC, Bagde A, Patel N, Gebeyehu A, Singh M. High-throughput 3D bioprinting of corneal stromal equivalents. J Biomed Mater Res B Appl Biomater. 2020;108(7):2981–94.
Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935.
Kim H, Jang J, Park J, Lee KP, Lee S, Lee DM, et al. Shear-induced alignment of collagen fibrils using 3D cell printing for corneal stroma tissue engineering. Biofabrication. 2019;11(3):035017.
Kim H, Park MN, Kim J, Jang J, Kim HK, Cho DW. Characterization of cornea-specific bioink: high transparency, improved in vivo safety. J Tissue Eng. 2019;10:2041731418823382.
Kim BS, Kim H, Gao G, Jang J, Cho DW. Decellularized extracellular matrix: a step towards the next generation source for bioink manufacturing. Biofabrication. 2017;9(3):034104.
Zhang B, Xue Q, Hu HY, Yu MF, Gao L, Luo YC, et al. Integrated 3D bioprinting-based geometry-control strategy for fabricating corneal substitutes. J Zhejiang Univ Sci B. 2019;20(12):945–59.
Wu Z, Su X, Xu Y, Kong B, Sun W, Mi S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci Rep. 2016;6:24474.
Kim KW, Lee SJ, Park SH, Kim JC. Ex vivo functionality of 3D bioprinted corneal endothelium engineered with ribonuclease 5-overexpressing human corneal endothelial cells. Adv Healthc Mater. 2018;7(18):e1800398.
Lichtenberger JP, Tatum PS, Gada S, Wyn M, Ho VB, Liacouras P. Using 3D printing (additive manufacturing) to produce low-cost simulation models for medical training. Mil Med. 2018;183(suppl_1):73–7.
Famery N, Abdelmassih Y, El-Khoury S, Guindolet D, Cochereau I, Gabison EE. Artificial chamber and 3D printed iris: a new wet lab model for teaching Descemet’s membrane endothelial keratoplasty. Acta Ophthalmol. 2019;97(2):e179–83.
Dehghani S, Rasoulianboroujeni M, Ghasemi H, Keshel SH, Nozarian Z, Hashemian MN, et al. 3D-Printed membrane as an alternative to amniotic membrane for ocular surface/conjunctival defect reconstruction: an in vitro & in vivo study. Biomaterials. 2018;174:95–112.
Wang W, Qian X, Song H, Zhang M, Liu Z. Fluid and structure coupling analysis of the interaction between aqueous humor and iris. Biomed Eng Online. 2016;15(Suppl 2):133.
Lu R, Soden PA, Lee E. Tissue-engineered models for glaucoma research. Micromachines (Basel). 2020;11(6):612.
In vitro 3D bioprinting trabecular meshwork models using organic hydrogels [Internet]. 2017. https://mountainscholar.org/handle/11124/171213. Accessed 30 May 2021.
Debellemanière G, Flores M, Montard M, Delbosc B, Saleh M. Three-dimensional printing of optical lenses and ophthalmic surgery: challenges and perspectives. J Refract Surg. 2016;32(3):201–4.
Hinze U, El-Tamer A, Reiß S, Stolz H, Guthoff R, Stachs O, et al. Implant design by means of multiphoton polymerization. Klin Monbl Augenheilkd. 2015;232(12):1381–5.
Li JW, Li YJ, Hu XS, Gong Y, Xu BB, Xu HW, et al. Biosafety of a 3D-printed intraocular lens made of a poly(acrylamide-co-sodium acrylate) hydrogel. Int J Ophthalmol. 2020;13(10):1521–30.
Xie P, Hu Z, Zhang X, Li X, Gao Z, Yuan D, et al. Application of 3-dimensional printing technology to construct an eye model for fundus viewing study. PLoS ONE. 2014;9(11):e109373.
Corcoran A, Muyo G, van Hemert J, Gorman A, Harvey AR. Application of a wide-field phantom eye for optical coherence tomography and reflectance imaging. J Mod Opt. 2015;62(21):1828–38.
Pugalendhi A, Ranganathan R, Venkatapathy N, Narendran K, Shah PK. Design and development of model eye for retina laser by using additive manufacturing. Proc Inst Mech Eng H. 2021;235(1):89–98.
Beharry KD, Cai CL, Valencia GB, Lazzaro D, Valencia AM, Salomone F, et al. Human retinal endothelial cells and astrocytes cultured on 3-D scaffolds for ocular drug discovery and development. Prostaglandins Other Lipid Mediat. 2018;134:93–107.
Kador KE, Grogan SP, Dorthé EW, Venugopalan P, Malek MF, Goldberg JL, et al. Control of retinal ganglion cell positioning and neurite growth: combining 3D printing with radial electrospun scaffolds. Tissue Eng Part A. 2016;22(3–4):286–94.
Worthington KS, Wiley LA, Kaalberg EE, Collins MM, Mullins RF, Stone EM, et al. Two-photon polymerization for production of human iPSC-derived retinal cell grafts. Acta Biomater. 2017;55:385–95.
Masaeli E, Marquette C. Direct-write bioprinting approach to construct multilayer cellular tissues. Front Bioeng Biotechnol. 2019;7:478.
Shi P, Edgar TYS, Yeong WY, Laude A. Hybrid three-dimensional (3D) bioprinting of retina equivalent for ocular research. Int J Bioprint. 2017;3(2):008.
Shi P, Tan YSE, Yeong WY, Li HY, Laude A. A bilayer photoreceptor-retinal tissue model with gradient cell density design: a study of microvalve-based bioprinting. J Tissue Eng Regen Med. 2018;12(5):1297–306.
Wang P, Li X, Zhu W, Zhong Z, Moran A, Wang W, et al. 3D bioprinting of hydrogels for retina cell culturing. Bioprinting. 2018;11:e00029.
Masaeli E, Forster V, Picaud S, Karamali F, Nasr-Esfahani MH, Marquette C. Tissue engineering of retina through high resolution 3-dimensional inkjet bioprinting. Biofabrication. 2020;12(2):025006.
Lorber B, Hsiao WK, Hutchings IM, Martin KR. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication. 2014;6(1):015001.
Masland RH. Cell populations of the retina: the Proctor lecture. Invest Ophthalmol Vis Sci. 2011;52(7):4581–91.
Murphy SV, De Coppi P, Atala A. Opportunities and challenges of translational 3D bioprinting. Nat Biomed Eng. 2020;4(4):370–80.
Pappas G, Vidakis N, Petousis M, Maniadi A. Individualized ophthalmic exoplants by means of reverse engineering and 3D printing technologies for treating high myopia complications with macular buckles. Biomimetics (Basel). 2020;5(4):54.
Choi SW, Kwon HJ, Song WK. Three-dimensional printing using open source software and JPEG images from optical coherence tomography of an epiretinal membrane patient. Acta Ophthalmol. 2018;96(3):e399–402.
Maloca PM, Spaide RF, Rothenbuehler S, Scholl HPN, Heeren T, Ramos de Carvalho JE, et al. Enhanced resolution and speckle-free three-dimensional printing of macular optical coherence tomography angiography. Acta Ophthalmol. 2019;97(2):e317–9.
Maloca PM, Tufail A, Hasler PW, Rothenbuehler S, Egan C, Ramos de Carvalho JE, et al. 3D printing of the choroidal vessels and tumours based on optical coherence tomography. Acta Ophthalmol. 2019;97(2):e313–6.
Scawn RL, Foster A, Lee BW, Kikkawa DO, Korn BS. Customised 3D printing: an innovative training tool for the next generation of orbital surgeons. Orbit. 2015;34(4):216–9.
Lichtenstein JT, Zeller AN, Lemound J, Lichtenstein TE, Rana M, Gellrich NC, et al. 3D-printed simulation device for orbital surgery. J Surg Educ. 2017;74(1):2–8.
Adams JW, Paxton L, Dawes K, Burlak K, Quayle M, McMenamin PG. 3D printed reproductions of orbital dissections: a novel mode of visualising anatomy for trainees in ophthalmology or optometry. Br J Ophthalmol. 2015;99(9):1162–7.
McMenamin PG, Quayle MR, McHenry CR, Adams JW. The production of anatomical teaching resources using three-dimensional (3D) printing technology. Anat Sci Educ. 2014;7(6):479–86.
Mottl-Link S, Hübler M, Kühne T, Rietdorf U, Krueger JJ, Schnackenburg B, et al. Physical models aiding in complex congenital heart surgery. Ann Thorac Surg. 2008;86(1):273–7.
Bannon R, Parihar S, Skarparis Y, Varsou O, Cezayirli E. 3D printing the pterygopalatine fossa: a negative space model of a complex structure. Surg Radiol Anat. 2018;40(2):185–91.
Engel M, Hoffmann J, Castrillon-Oberndorfer G, Freudlsperger C. The value of three-dimensional printing modelling for surgical correction of orbital hypertelorism. Oral Maxillofac Surg. 2015;19(1):91–5.
D’Urso PS, Barker TM, Earwaker WJ, Bruce LJ, Atkinson RL, Lanigan MW, et al. Stereolithographic biomodelling in cranio-maxillofacial surgery: a prospective trial. J Craniomaxillofac Surg. 1999;27(1):30–7.
Kozakiewicz M, Elgalal M, Loba P, Komuński P, Arkuszewski P, Broniarczyk-Loba A, et al. Clinical application of 3D pre-bent titanium implants for orbital floor fractures. J Craniomaxillofac Surg. 2009;37(4):229–34.
Brucoli M, Arcuri F, Cavenaghi R, Benech A. Analysis of complications after surgical repair of orbital fractures. J Craniofac Surg. 2011;22(4):1387–90.
Zhang X, Chen W, Luo TY, Ma J, Dong Z, Cao G, et al. Application of three-dimensional printing technology in the orbital blowout fracture reconstruction. J Craniofac Surg. 2019;30(6):1825–8.
Cooper T, Schmutz B, Hsu E, Lynham A. Magnetic resonance imaging for three-dimensional printing of the bony orbit: is clinical use imminent? Int J Oral Maxillofac Surg. 2020;49(4):483–90.
Callahan AB, Campbell AA, Petris C, Kazim M. Low-cost 3D printing orbital implant templates in secondary orbital reconstructions. Ophthalmic Plast Reconstr Surg. 2017;33(5):376–80.
Park SW, Choi JW, Koh KS, Oh TS. Mirror-imaged rapid prototype skull model and pre-molded synthetic scaffold to achieve optimal orbital cavity reconstruction. J Oral Maxillofac Surg. 2015;73(8):1540–53.
Chai G, Zhang D, Hua W, Yin J, Jin Y, Chen M. Theoretical model of pediatric orbital trapdoor fractures and provisional personalized 3D printing-assisted surgical solution. Bioact Mater. 2021;6(2):559–67.
Tel A, Sembronio S, Costa F, Stenico AS, Bagatto D, D’Agostini S, et al. Endoscopically assisted computer-guided repair of internal orbital floor fractures: an updated protocol for minimally invasive management. J Craniomaxillofac Surg. 2019;47(12):1943–51.
Martelli N, Serrano C, van den Brink H, Pineau J, Prognon P, Borget I, et al. Advantages and disadvantages of 3-dimensional printing in surgery: a systematic review. Surgery. 2016;159(6):1485–500.
Rogers-Vizena CR, Sporn SF, Daniels KM, Padwa BL, Weinstock P. Cost-benefit analysis of three-dimensional craniofacial models for midfacial distraction: a pilot study. Cleft Palate Craniofac J. 2017;54(5):612–7.
Pang SSY, Fang C, Chan JYW. Application of three-dimensional printing technology in orbital floor fracture reconstruction. Trauma Case Rep. 2018;17:23–8.
Xue R, Lai Q, Sun S, Lai L, Tang X, Ci J, et al. Application of three-dimensional printing technology for improved orbital-maxillary-zygomatic reconstruction. J Craniofac Surg. 2019;30(2):e127–31.
Rana M, Gellrich MM, Gellrich NC. Customised reconstruction of the orbital wall and engineering of selective laser melting (SLM) core implants. Br J Oral Maxillofac Surg. 2015;53(2):208–9.
Baumann A, Sinko K, Dorner G. Late reconstruction of the orbit with patient-specific implants using computer-aided planning and navigation. J Oral Maxillofac Surg. 2015;73(12 Suppl):S101–6.
Vehmeijer M, van Eijnatten M, Liberton N, Wolff J. A novel method of orbital floor reconstruction using virtual planning, 3-dimensional printing, and autologous bone. J Oral Maxillofac Surg. 2016;74(8):1608–12.
Nekooei S, Sardabi M, Razavi ME, Nekooei A, Kiarudi MY. Implantation of customized, preshaped implant for orbital fractures with the aid of three-dimensional printing. Middle East Afr J Ophthalmol. 2018;25(1):56–8.
Mahendru S, Jain R, Garg S, Singh H, Jain A, Sarin D, et al. “Hybrid reconstruction” for zygomaticomaxillary complex defect using CAD/CAM: a case report. Plast Reconstr Surg Glob Open. 2020;8(9):e3140.
Kang S, Kwon J, Ahn CJ, Esmaeli B, Kim GB, Kim N, et al. Generation of customized orbital implant templates using 3-dimensional printing for orbital wall reconstruction. Eye (Lond). 2018;32(12):1864–70.
Oh TS, Jeong WS, Chang TJ, Koh KS, Choi JW. Customized orbital wall reconstruction using three-dimensionally printed rapid prototype model in patients with orbital wall fracture. J Craniofac Surg. 2016;27(8):2020–4.
Kim JH, Lee IG, Lee JS, Oh DY, Jun YJ, Rhie JW, et al. Restoration of the inferomedial orbital strut using a standardized three-dimensional printing implant. J Anat. 2020;236(5):923–30.
Kim YC, Min KH, Choi JW, Koh KS, Oh TS, Jeong WS. Patient-specific puzzle implant preformed with 3D-printed rapid prototype model for combined orbital floor and medial wall fracture. J Plast Reconstr Aesthet Surg. 2018;71(4):496–503.
Mommaerts MY, Büttner M, Vercruysse H, Wauters L, Beerens M. Orbital wall reconstruction with two-piece puzzle 3D printed implants: technical note. Craniomaxillofac Trauma Reconstr. 2016;9(1):55–61.
Gonzalez Alvarez A, Ananth S, Dovgalski L, Evans PL. Custom three-dimensional printed orbital plate composed of two joined parts with variable thickness for a large orbital floor reconstruction after post-traumatic zygomatic fixation. Br J Oral Maxillofac Surg. 2020;58(10):e341–2.
Day KM, Phillips PM, Sargent LA. Correction of a posttraumatic orbital deformity using three-dimensional modeling, virtual surgical planning with computer-assisted design, and three-dimensional printing of custom implants. Craniomaxillofac Trauma Reconstr. 2018;11(1):78–82.
Fan B, Chen H, Sun YJ, Wang BF, Che L, Liu SY, et al. Clinical effects of 3-D printing-assisted personalized reconstructive surgery for blowout orbital fractures. Graefes Arch Clin Exp Ophthalmol. 2017;255(10):2051–7.
Kozakiewicz M, Elgalal M, Piotr L, Broniarczyk-Loba A, Stefanczyk L. Treatment with individual orbital wall implants in humans - 1-Year ophthalmologic evaluation. J Craniomaxillofac Surg. 2011;39(1):30–6.
Sigron GR, Rüedi N, Chammartin F, Meyer S, Msallem B, Kunz C, et al. Three-dimensional analysis of isolated orbital floor fractures pre- and post-reconstruction with standard titanium meshes and “hybrid” patient-specific implants. J Clin Med. 2020;9(5):1579.
Kim YC, Jeong WS, Park TK, Choi JW, Koh KS, Oh TS. The accuracy of patient specific implant prebented with 3D-printed rapid prototype model for orbital wall reconstruction. J Craniomaxillofac Surg. 2017;45(6):928–36.
Weadock WJ, Heisel CJ, Kahana A, Kim J. Use of 3D printed models to create molds for shaping implants for surgical repair of orbital fractures. Acad Radiol. 2020;27(4):536–42.
Laure B, Louisy A, Joly A, Travers N, Listrat A, Pare A. Virtual 3D planning of osteotomies for craniosynostoses and complex craniofacial malformations. Neurochirurgie. 2019;65(5):269–78.
Mourits DL, Wolff J, Forouzanfar T, Ridwan-Pramana A, Moll AC, de Graaf P, et al. 3D orbital reconstruction in a patient with microphthalmos and a large orbital cyst—a case report. Ophthalmic Genet. 2016;37(2):233–7.
Martinez-Seijas P, Díaz-Galvis LA, Hernando J, Leizaola-Cardesa IO, Aguilar-Salvatierra A, Gómez-Moreno G. Polymethyl methacrylate custom-made prosthesis: a novel three-dimension printing-aided fabrication technique for cranial and/or orbital reconstruction. J Craniofac Surg. 2018;29(5):e438–40.
Herford AS, Miller M, Lauritano F, Cervino G, Signorino F, Maiorana C. The use of virtual surgical planning and navigation in the treatment of orbital trauma. Chin J Traumatol. 2017;20(1):9–13.
Rudman K, Hoekzema C, Rhee J. Computer-assisted innovations in craniofacial surgery. Facial Plast Surg. 2011;27(4):358–65.
Smeets M, Snel R, Sun Y, Dormaar T, Politis C. Late reconstruction of extensive orbital floor fracture with a patient-specific implant in a bombing victim. J Korean Assoc Oral Maxillofac Surg. 2020;46(5):353–7.
Le Clerc N, Baudouin R, Carlevan M, Khoueir N, Verillaud B, Herman P. 3D titanium implant for orbital reconstruction after maxillectomy. J Plast Reconstr Aesthet Surg. 2020;73(4):732–9.
Stoor P, Suomalainen A, Lindqvist C, Mesimäki K, Danielsson D, Westermark A, et al. Rapid prototyped patient specific implants for reconstruction of orbital wall defects. J Craniomaxillofac Surg. 2014;42(8):1644–9.
Kitabata R, Uno K, Sakamoto Y. Reconstruction of combined orbital floor and medial wall fractures using custom-made titanium alloy implant. J Craniofac Surg. 2021;4:e388–9.
Rana M, Chui CH, Wagner M, Zimmerer R, Gellrich NC. Increasing the accuracy of orbital reconstruction with selective laser-melted patient-specific implants combined with intraoperative navigation. J Oral Maxillofac Surg. 2015;73(6):1113–8.
Copperman TS, Idowu OO, Jalaj S, Winn BJ, Pham C, Setabutr P, et al. Patient-specific implants in oculofacial plastic surgery. Ophthalmic Plast Reconstr Surg. 2020;37:241–7.
Hoang D, Perrault D, Stevanovic M, Ghiassi A. Surgical applications of three-dimensional printing: a review of the current literature & how to get started. Ann Transl Med. 2016;4(23):456.
Otero JJ, Vijverman A, Mommaerts MY. Use of fused deposit modeling for additive manufacturing in hospital facilities: European certification directives. J Craniomaxillofac Surg. 2017;45(9):1542–6.
Morrison RJ, Kashlan KN, Flanangan CL, Wright JK, Green GE, Hollister SJ, et al. Regulatory considerations in the design and manufacturing of implantable 3D-printed medical devices. Clin Transl Sci. 2015;8(5):594–600.
Hoods-Moonsammy VJ, Owen P, Howes DG. A comparison of the accuracy of polyether, polyvinyl siloxane, and plaster impressions for long-span implant-supported prostheses. Int J Prosthodont. 2014;27(5):433–8.
Alam MS, Sugavaneswaran M, Arumaikkannu G, Mukherjee B. An innovative method of ocular prosthesis fabrication by bio-CAD and rapid 3-D printing technology: a pilot study. Orbit. 2017;36(4):223–7.
Ruiters S, Sun Y, de Jong S, Politis C, Mombaerts I. Computer-aided design and three-dimensional printing in the manufacturing of an ocular prosthesis. Br J Ophthalmol. 2016;100(7):879–81.
Ruiters S, Shujaat S, de Faria VK, Shaheen E, Jacobs R, Mombaerts I. Three-dimensional design of a geometric model for an ocular prosthesis in ex vivo anophthalmic socket models. Acta Ophthalmol. 2021;99(2):221–6.
Sanchez-Tena MA, Alvarez-Peregrina C, Santos-Arias F, Villa-Collar C. Application of 3D printing technology in scleral cover shell prosthesis. J Med Syst. 2019;43(6):149.
Kim BR, Kim SH, Ko J, Baek SW, Park YK, Kim YJ, et al. A pilot clinical study of ocular prosthesis fabricated by three-dimensional printing and sublimation technique. Korean J Ophthalmol. 2021;35(1):37–43.
Ko J, Kim SH, Baek SW, Chae MK, Yoon JS. Semi-automated fabrication of customized ocular prosthesis with three-dimensional printing and sublimation transfer printing technology. Sci Rep. 2019;9(1):2968.
Huang YH, Seelaus R, Zhao L, Patel PK, Cohen M. Virtual surgical planning and 3D printing in prosthetic orbital reconstruction with percutaneous implants: a technical case report. Int Med Case Rep J. 2016;9:341–5.
Kormann RB, Mörschbächer R, Moreira H, Akaishi P. A three-dimensional printed photopolymer resin implant for orbital rehabilitation for evisceration. Arq Bras Oftalmol. 2019;82(6):471–5.
Dave TV, Gaur G, Chowdary N, Joshi D. Customized 3D printing: a novel approach to migrated orbital implant. Saudi J Ophthalmol. 2018;32(4):330–3.
Mourits DL, Remmers JS, Tan SH, Moll AC, Hartong DT. An individualized 3-dimensional designed and printed conformer after dermis fat grafting for complex sockets. Ophthalmic Plast Reconstr Surg. 2018;34(4):390–2.
Kuijten MMP, Remmers JS, Mourits DL, de Graaf P, Hartong DT. Three-dimensionally printed conformers for treatment of congenital anophthalmos. Ophthalmic Plast Reconstr Surg. 2017;33(5):394–5.
Weisson EH, Fittipaldi M, Concepcion CA, Pelaez D, Grace L, Tse DT. Automated noncontact facial topography mapping, 3-dimensional printing, and silicone casting of orbital prosthesis. Am J Ophthalmol. 2020;220:27–36.
Nyberg EL, Farris AL, Hung BP, Dias M, Garcia JR, Dorafshar AH, et al. 3D-printing technologies for craniofacial rehabilitation, reconstruction, and regeneration. Ann Biomed Eng. 2017;45(1):45–57.
Sun MG, Rojdamrongratana D, Rosenblatt MI, Aakalu VK, Yu CQ. 3D printing for low cost, rapid prototyping of eyelid crutches. Orbit. 2019;38(4):342–6.
Lim SH, Tiew WJ, Zhang J, Ho PC, Kachouie NN, Kang L. Geometrical optimisation of a personalised microneedle eye patch for transdermal delivery of anti-wrinkle small peptide. Biofabrication. 2020;12(3):035003.
Han S, Zhou J, He C, Liang Q, Yang Y. Design and implementation of user-oriented auxiliary treatment instrument for meibomian gland dysfunction. Zhongguo Yi Liao Qi Xie Za Zhi. 2021;45(1):11–6.
Homolka P, Figl M, Wartak A, Glanzer M, Dünkelmeyer M, Hojreh A, et al. Design of a head phantom produced on a 3D rapid prototyping printer and comparison with a RANDO and 3M lucite head phantom in eye dosimetry applications. Phys Med Biol. 2017;62(8):3158–74.
Santos MF, Cassola V, Kramer R, Costa JV, Andrade MEA, Asfora VK, et al. Development of a realistic 3D printed eye lens dosemeter using CAD integrated with Monte Carlo simulation. Biomed Phys Eng Express. 2019;6(1):015009.
Santos MF, Vasconcelos Filho WC, Melo G, Asfora VK, Khoury HJ, Barros VSM. Evaluation of a 3D printed OSL eye lens dosimeter for photon dosimetry. J Radiol Prot. 2020;40:1247–57.
Sharma A, Sasaki D, Rickey DW, Leylek A, Harris C, Johnson K, et al. Low-cost optical scanner and 3-dimensional printing technology to create lead shielding for radiation therapy of facial skin cancer: first clinical case series. Adv Radiat Oncol. 2018;3(3):288–96.
Zemnick C, Woodhouse SA, Gewanter RM, Raphael M, Piro JD. Rapid prototyping technique for creating a radiation shield. J Prosthet Dent. 2007;97(4):236–41.
Sim L. Novel application of 3D printing in brachytherapy using MED610 3D printed insert for I-125 ROPES eye plaque. Australas Phys Eng Sci Med. 2016;39(4):863–70.
Subashi E, Jacobs C, Hood R, Kirsch DG, Craciunescu O. A design process for a 3D printed patient-specific applicator for HDR brachytherapy of the orbit. 3D Print Med. 2020;6(1):15.
Poulsen M, Lindsay C, Sullivan T, D’Urso P. Stereolithographic modelling as an aid to orbital brachytherapy. Int J Radiat Oncol Biol Phys. 1999;44(3):731–5.
Dipasquale G, Poirier A, Sprunger Y, Uiterwijk JWE, Miralbell R. Improving 3D-printing of megavoltage X-rays radiotherapy bolus with surface-scanner. Radiat Oncol. 2018;13(1):203.
Łukowiak M, Jezierska K, Boehlke M, Więcko M, Łukowiak A, Podraza W, et al. Utilization of a 3D printer to fabricate boluses used for electron therapy of skin lesions of the eye canthi. J Appl Clin Med Phys. 2017;18(1):76–81.
Furdová A, Sramka M, Thurzo A. Early experiences of planning stereotactic radiosurgery using 3D printed models of eyes with uveal melanomas. Clin Ophthalmol. 2017;11:267–71.
Saunders S. Johnson & Johnson announces new collaborations to develop biomedical innovation and advance 3D printing technology in healthcare. 2017. https://www.3dprint.com/178341/johnson-johnson-collaboration/. Accessed May 2021.
Dixon P, Christopher K, Jovic N, Chauhan A. Spectroscopy of oxygen-sensitive material for measuring contact lens oxygen transmissibility. Curr Eye Res. 2019;44(5):514–21.
Zhao F, Zhao G, Weijie F, Chen L. Application of 3D printing technology in RGPCL simulation fitting. Med Hypotheses. 2018;113:74–6.
Zhao F, Wang J, Wang L, Chen L. An approach for simulating the fitting of rigid gas-permeable contact lenses using 3D printing technology. Cont Lens Anterior Eye. 2019;42(2):165–9.
Mendoza HR. Safilo and the Stratasys J750 3D Printer: Making Beautiful Eyewear Together. 2016. https://3dprint.com/154296/safilostratasys-eyewear/. Accessed May 2021.
Gawedzinski J, Pawlowski ME, Tkaczyk TS. Quantitative evaluation of performance of 3D printed lenses. Opt Eng. 2017;56(8):1.
Ayyildiz O. Customised spectacles using 3-D printing technology. Clin Exp Optom. 2018;101(6):747–51.
Altinkurt E, Ceylan NA, Altunoglu U, Turgut GT. Manufacture of custom-made spectacles using three-dimensional printing technology. Clin Exp Optom. 2020;103(6):902–4.
Luxexcel. Ophthalmic Products. https://www.luxexcel.com/ophthalmic-product/. Accessed May 2021.
Jung JH, Castle R, Kurukuti NM, Manda S, Peli E. Field expansion with multiplexing prism glasses improves pedestrian detection for acquired monocular vision. Transl Vis Sci Technol. 2020;9(8):35.
Peli E, Vargas-Martin F, Kurukuti NM, Jung JH. Multi-periscopic prism device for field expansion. Biomed Opt Express. 2020;11(9):4872–89.
Petsiuk AL, Pearce JM. Low-cost open source ultrasound-sensing based navigational support for the visually impaired. Sensors (Basel). 2019;19(17):3783.
Kim H, Kim J, Kang J, Song YW. Three-dimensionally printed interconnects for smart contact lenses. ACS Appl Mater Interfaces. 2018;10(33):28086–92.
Chen Y, Zhang S, Cui Q, Ni J, Wang X, Cheng X, et al. Microengineered poly(HEMA) hydrogels for wearable contact lens biosensing. Lab Chip. 2020;20(22):4205–14.
Alam F, Elsherif M, AlQattan B, Salih A, Lee SM, Yetisen AK, et al. 3D printed contact lenses. ACS Biomater Sci Eng. 2021;7(2):794–803.
Hopkins GR, Irvin BC. Optometric applications for three-dimensional printing: a technical report from low vision rehabilitation practice. Optom Vis Sci. 2019;96(3):213–20.
Hong SC. 3D printable retinal imaging adapter for smartphones could go global. Graefes Arch Clin Exp Ophthalmol. 2015;253(10):1831–3.
Myung D, Jais A, He L, Blumenkranz M, Chang R. 3D printed smartphone indirect lens adapter for rapid, high quality retinal imaging. J Mobile Technol Med. 2014;3(1):9–15.
Saunders S. Teenager uses AI, a 3D printed Lens, and a smartphone to develop portable system to diagnose a common eye disease 2017. 2017. https://3dprint.com/183144/portableeye-diagnostic-system/. Accessed May 2021.
O'Neal B. Peek Vision. 2015. https://3dprint.com/69937/peek-smart-app-eye-exam/. Accessed May 2021.
Chiong HS, Fang JL, Wilson G. Tele-manufactured affordable smartphone anterior segment microscope. Clin Exp Optom. 2016;99(6):580–2.
Paulson B, Lee S, Jue M, Lee K, Kim GB, Moon Y, et al. Stereotaxic endoscopy for the ocular imaging of awake, freely moving animal models. J Biophotonics. 2020;13(5):e201960188.
Lee KF, Chen YL, Yu CW, Chin KY, Wu CH. Gaze tracking and point estimation using low-cost head-mounted devices. Sensors (Basel). 2020;20(7):1917.
Zidan G, Greene CA, Etxabide A, Rupenthal ID, Seyfoddin A. Gelatine-based drug-eluting bandage contact lenses: effect of PEGDA concentration and manufacturing technique. Int J Pharm. 2021;599:120452.
Phan CM, Walther H, Gao H, Rossy J, Subbaraman LN, Jones L. Development of an in vitro ocular platform to test contact lenses. J Vis Exp. 2016;110:e53907.
Phan CM, Shukla M, Walther H, Heynen M, Suh D, Jones L. Development of an in vitro blink model for ophthalmic drug delivery. Pharmaceutics. 2021;13(3):300.
Norman J, Madurawe RD, Moore CM, Khan MA, Khairuzzaman A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv Drug Deliv Rev. 2017;108:39–50.
Kojima H, Raut B, Chen LJ, Nagai N, Abe T, Kaji H. A 3D printed self-sustainable cell-encapsulation drug delivery device for periocular transplant-based treatment of retinal degenerative diseases. Micromachines (Basel). 2020;11(4):436.
Lee JH, Pidaparti RM, Atkinson GM, Moorthy RS. Design of an implantable device for ocular drug delivery. J Drug Deliv. 2012;2012:527516.
Won JY, Kim J, Gao G, Jang J, Park YH, Cho DW. 3D printing of drug-loaded multi-shell rods for local delivery of bevacizumab and dexamethasone: a synergetic therapy for retinal vascular diseases. Acta Biomater. 2020;116:174–85.
Canabrava S, Diniz-Filho A, Schor P, Fagundes DF, Lopes A, Batista WD. Production of an intraocular device using 3D printing: an innovative technology for ophthalmology. Arq Bras Oftalmol. 2015;78(6):393–4.
Navajas EV, Ten Hove M. Three-dimensional printing of a transconjunctival vitrectomy trocar-cannula system. Ophthalmologica. 2017;237(2):119–22.
Choi JW, Yamashita M, Sakakibara J, Kaji Y, Oshika T, Wicker RB. Combined micro and macro additive manufacturing of a swirling flow coaxial phacoemulsifier sleeve with internal micro-vanes. Biomed Microdevices. 2010;12(5):875–86.
Ruzza A, Parekh M, Ferrari S, Salvalaio G, Nahum Y, Bovone C, et al. Preloaded donor corneal lenticules in a new validated 3D printed smart storage glide for Descemet stripping automated endothelial keratoplasty. Br J Ophthalmol. 2015;99(10):1388–95.
Armijo PR, Markin NW, Nguyen S, Ho DH, Horseman TS, Lisco SJ, et al. 3D printing of face shields to meet the immediate need for PPE in an anesthesiology department during the COVID-19 pandemic. Am J Infect Control. 2021;49(3):302–8.
Wierzbicki J, Nowacki M, Chrzanowska M, Matkowski R, Ziętek M, Nowacka K, et al. Additive manufacturing technologies enabling rapid and interventional production of protective face shields and masks during the COVID-19 pandemic. Adv Clin Exp Med. 2020;29(9):1021–8.
Manero A, Smith P, Koontz A, Dombrowski M, Sparkman J, Courbin D, et al. Leveraging 3D printing capacity in times of crisis: recommendations for COVID-19 distributed manufacturing for medical equipment rapid response. Int J Environ Res Public Health. 2020;17(13):4634.
Chaturvedi S, Gupta A, Krishnan SV, Bhat AK. Design, usage and review of a cost effective and innovative face shield in a tertiary care teaching hospital during COVID-19 pandemic. J Orthop. 2020;21:331–6.
Zhang PC, Ahmed Y, Hussein IM, Afenu E, Feasson M, Daud A. Optimization of community-led 3D printing for the production of protective face shields. 3D Print Med. 2020;6(1):35.
Gomes BA, Queiroz FLC, Pereira PLO, Barbosa TV, Tramontana MB, Afonso FAC, et al. In-house three-dimensional printing workflow for face shield during COVID-19 pandemic. J Craniofac Surg. 2020;31(6):e652–3.
Throckmorton AL, Bass EJ, Ferrick B, Ramakrishnan A, Eichmann S, Catucci N, et al. A Cross University-led COVID-19 rapid-response effort: design, build, and distribute drexel AJFlex face shields. Ann Biomed Eng. 2021;49(3):950–8.
Mostaghimi A, Antonini MJ, Plana D, Anderson PD, Beller B, Boyer EW, et al. Regulatory and safety considerations in deploying a locally fabricated, reusable face shield in a hospital responding to the COVID-19 pandemic. Med (N Y). 2020;1(1):139-51.e4.
Erickson MM, Richardson ES, Hernandez NM, Bobbert DW, Gall K, Fearis P. Helmet modification to PPE with 3D printing during the COVID-19 pandemic at Duke University Medical Center: a novel technique. J Arthroplasty. 2020;35(7S):S23–7.
Viera-Artiles J, Valdiande JJ. 3D-printable headlight face shield adapter. Personal protective equipment in the COVID-19 era. Am J Otolaryngol. 2020;41(5):102576.
Gilbert F, O’Connell CD, Mladenovska T, Dodds S. Print me an organ? Ethical and regulatory issues emerging from 3D bioprinting in medicine. Sci Eng Ethics. 2018;24(1):73–91.
Valding B, Zrounba H, Martinerie S, May L, Broome M. Should you buy a three-dimensional printer? A study of an orbital fracture. J Craniofac Surg. 2018;29(7):1925–7.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship contributions
Conceptualization: Cristos Ifantides. Methodology: Ryan Larochelle, Scott Mann and Cristos Ifantides. Formal analysis and investigation: Ryan Larochelle. Draft preparation: Ryan Larochelle. Review and editing: Ryan Larochelle, Scott Mann, and Cristos Ifantides.
Disclosures
Cristos Ifantides is an inventor with university-assigned intellectual property pertaining to 3D printing of spectacles. RL and SM have nothing to declare.
Compliance with ethics guidelines
Ethics committee approval was not required for this review article. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Larochelle, R.D., Mann, S.E. & Ifantides, C. 3D Printing in Eye Care. Ophthalmol Ther 10, 733–752 (2021). https://doi.org/10.1007/s40123-021-00379-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-021-00379-6