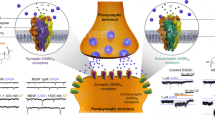
Abstract
Postpartum depression is one of the most common complications of childbirth. Untreated postpartum depression can have substantial adverse effects on the well-being of the mother and child, negatively impacting child cognitive, behavioral, and emotional development with lasting consequences. There are a number of therapeutic interventions for postpartum depression including pharmacotherapy, psychotherapy, neuromodulation, and hormonal therapy among others, most of which have been adapted from the treatment of major depressive disorder outside of the peripartum period. Current evidence of antidepressant treatment for postpartum depression is limited by the small number of randomized clinical trials, underpowered samples, and the lack of long-term follow-up. The peripartum period is characterized by rapid and significant physiological change in plasma levels of endocrine hormones, peptides, and neuroactive steroids. Evidence supporting the role of neuroactive steroids and γ-aminobutyric acid (GABA) in the pathophysiology of postpartum depression led to the investigation of synthetic neuroactive steroids and their analogs as potential treatment for postpartum depression. Brexanolone, a soluble proprietary intravenous preparation of synthetic allopregnanolone, has been developed. A recent series of open-label and placebo-controlled randomized clinical trials of brexanolone in postpartum depression demonstrated a rapid reduction in depressive symptoms, and has led to the submission for regulatory approval to the US Food and Drug Administration (decision due in March 2019). SAGE-217, an allopregnanolone analog, with oral bioavailability, was recently tested in a randomized, double-blind, placebo-controlled phase III study in severe postpartum depression, with reportedly positive results. Finally, a 3β-methylated synthetic analog of allopregnanolone, ganaxolone, is being tested in both intravenous and oral forms, in randomized, double-blind, placebo-controlled phase II studies in severe postpartum depression.
Similar content being viewed by others
References
Hahn-Holbrook J, Cornwell-Hinrichs T, Anaya I. Economic and health predictors of national postpartum depression prevalence: a systematic review, meta-analysis, and meta-regression of 291 studies from 56 countries. Front Psychiatry. 2017;8:248. https://doi.org/10.3389/fpsyt.2017.00248.
Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev. 2011;14(1):1–27. https://doi.org/10.1007/s10567-010-0080-1.
O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180:502–8.
Madigan S, Oatley H, Racine N, Fearon RMP, Schumacher L, Akbari E, et al. A meta-analysis of maternal prenatal depression and anxiety on child socio-emotional development. J Am Acad Child Adolesc Psychiatry. 2018;57(9):645–57.e8. https://doi.org/10.1016/j.jaac.2018.06.012.
Cox EQ, Sowa NA, Meltzer-Brody SE, Gaynes BN. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77(9):1189–200. https://doi.org/10.4088/JCP.15r10174.
Dietz PM, Williams SB, Callaghan WM, Bachman DJ, Whitlock EP, Hornbrook MC. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164(10):1515–20. https://doi.org/10.1176/appi.ajp.2007.06111893.
Takehara K, Tachibana Y, Yoshida K, Mori R, Kakee N, Kubo T. Prevalence trends of pre- and postnatal depression in Japanese women: a population-based longitudinal study. J Affect Disord. 2018;225:389–94. https://doi.org/10.1016/j.jad.2017.08.008.
Anokye R, Acheampong E, Budu-Ainooson A, Obeng EI, Akwasi AG. Prevalence of postpartum depression and interventions utilized for its management. Ann Gen Psychiatry. 2018;17:18. https://doi.org/10.1186/s12991-018-0188-0.
Bell AF, Carter CS, Davis JM, Golding J, Adejumo O, Pyra M, et al. Childbirth and symptoms of postpartum depression and anxiety: a prospective birth cohort study. Arch Womens Ment Health. 2016;19(2):219–27. https://doi.org/10.1007/s00737-015-0555-7.
Franca UL, McManus ML. Frequency, trends, and antecedents of severe maternal depression after three million U.S. births. PLoS One. 2018;13(2):e0192854. https://doi.org/10.1371/journal.pone.0192854.
Shakeel N, Sletner L, Falk RS, Slinning K, Martinsen EW, Jenum AK, et al. Prevalence of postpartum depressive symptoms in a multiethnic population and the role of ethnicity and integration. J Affect Disord. 2018;241:49–58. https://doi.org/10.1016/j.jad.2018.07.056.
Rasmussen MH, Strom M, Wohlfahrt J, Videbech P, Melbye M. Risk, treatment duration, and recurrence risk of postpartum affective disorder in women with no prior psychiatric history: a population-based cohort study. PLoS Med. 2017;14(9):e1002392. https://doi.org/10.1371/journal.pmed.1002392.
Ko JY, Rockhill KM, Tong VT, Morrow B, Farr SL. Trends in postpartum depressive symptoms: 27 states, 2004, 2008 and 2012. MMWR Morb Mort Wkly Rep. 2017;66:153–8. https://doi.org/10.15585/mmwr.mm6606a1.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Hanusa BH, Scholle SH, Haskett RF, Spadaro K, Wisner KL. Screening for depression in the postpartum period: a comparison of three instruments. J Womens Health (Larchmt). 2008;17(4):585–96. https://doi.org/10.1089/jwh.2006.0248.
Beck CT, Gable RK. Postpartum depression screening scale: development and psychometric testing. Nurs Res. 2000;49(5):272–82.
Brodey BB, Goodman SH, Baldasaro RE, Brooks-DeWeese A, Wilson ME, Brodey IS, et al. Development of the perinatal depression inventory (PDI)-14 using item response theory: a comparison of the BDI-II, EPDS, PDI, and PHQ-9. Arch Womens Ment Health. 2016;19(2):307–16. https://doi.org/10.1007/s00737-015-0553-9.
Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6.
Flynn HA, Sexton M, Ratliff S, Porter K, Zivin K. Comparative performance of the Edinburgh Postnatal Depression Scale and the Patient Health Questionnaire-9 in pregnant and postpartum women seeking psychiatric services. Psychiatry Res. 2011;187(1–2):130–4. https://doi.org/10.1016/j.psychres.2010.10.022.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
Stewart DE, Robertson E, Dennis C-L, Grace SL, Wallington T. Risk factors for postpartum depression. In: Postpartum depression: literature review of risk factors and interventions. Geneva: Department of Mental Health and Substance Abuse: World Health Organization; 2003: p. 1–63. Ed. University Health Network Women’s Health Program. https://www.who.int/mental_health/prevention/suicide/lit_review_postpartum_depression.pdf.
Centers for Disease Control and Prevention (CDC) Morbidity and Mortality Weekly Report. Prevalence of self-reported postpartum depressive symptoms: 17 States, 2004–2005. Department of Health and Human Services, Atlanta (GA): 2008. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5714a1.htm.
Sharma V, Khan M, Corpse C, Sharma P. Missed bipolarity and psychiatric comorbidity in women with postpartum depression. Bipolar Disord. 2008;10(6):742–7. https://doi.org/10.1111/j.1399-5618.2008.00606.x.
Vesga-Lopez O, Blanco C, Keyes K, Olfson M, Grant BF, Hasin DS. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805–15. https://doi.org/10.1001/archpsyc.65.7.805.
Hung CI, Liu CY, Yang CH. Untreated duration predicted the severity of depression at the two-year follow-up point. PLoS One. 2017;12(9):e0185119. https://doi.org/10.1371/journal.pone.0185119.
Earls MF. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126(5):1032–9. https://doi.org/10.1542/peds.2010-2348.
ACOG. The American College of Obstetricians and Gynecologists Committee opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268–71. https://doi.org/10.1097/01.aog.0000465192.34779.dc.
O’Connor E, Rossom RC, Henninger M, Groom HC, Burda BU. Primary care acreening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(4):388–406. https://doi.org/10.1001/jama.2015.18948.
Dindo L, Elmore A, O’Hara M, Stuart S. The comorbidity of Axis I disorders in depressed pregnant women. Arch Womens Ment Health. 2017;20(6):757–64. https://doi.org/10.1007/s00737-017-0769-y.
Hirschfeld RM. The comorbidity of major depression and anxiety disorders: recognition and management in primary care. Prim Care Companion J Clin Psychiatry. 2001;3(6):244–54.
Newport DJ, Levey LC, Pennell PB, Ragan K, Stowe ZN. Suicidal ideation in pregnancy: assessment and clinical implications. Arch Womens Ment Health. 2007;10(5):181–7. https://doi.org/10.1007/s00737-007-0192-x.
Dennis CL, Brown HK, Wanigaratne S, Fung K, Vigod SN, Grigoriadis S, et al. Prevalence, incidence, and persistence of postpartum depression, anxiety, and comorbidity among Chinese immigrant and nonimmigrant women: a longitudinal cohort study. Can J Psychiatry. 2018;63(1):44–53. https://doi.org/10.1177/0706743717720689.
Putnam K, Robertson-Blackmore E, Sharkey K, Payne J, Bergink V, Munk-Olsen T, et al. Heterogeneity of postpartum depression: a latent class analysis. Lancet Psychiatry. 2015;2(1):59–67. https://doi.org/10.1016/s2215-0366(14)00055-8.
Putnam KT, Wilcox M, Robertson-Blackmore E, Sharkey K, Bergink V, Munk-Olsen T, et al. Clinical phenotypes of perinatal depression and time of symptom onset: analysis of data from an international consortium. Lancet Psychiatry. 2017;4(6):477–85. https://doi.org/10.1016/S2215-0366(17)30136-0.
Bernstein IH, Rush AJ, Yonkers K, Carmody TJ, Woo A, McConnell K, et al. Symptom features of postpartum depression: are they distinct? Depress Anxiety. 2008;25(1):20–6. https://doi.org/10.1002/da.20276.
Pope CJ, Xie B, Sharma V, Campbell MK. A prospective study of thoughts of self-harm and suicidal ideation during the postpartum period in women with mood disorders. Arch Womens Ment Health. 2013;16(6):483–8. https://doi.org/10.1007/s00737-013-0370-y.
Plant DT, Pariante CM, Sharp D, Pawlby S. Maternal depression during pregnancy and offspring depression in adulthood: role of child maltreatment. Br J Psychiatry. 2015;207(3):213–20. https://doi.org/10.1192/bjp.bp.114.156620.
Spinelli MG. Maternal infanticide associated with mental illness: prevention and the promise of saved lives. Am J Psychiatry. 2004;161(9):1548–57. https://doi.org/10.1176/appi.ajp.161.9.1548.
Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health. 2005;8(2):77–87. https://doi.org/10.1007/s00737-005-0080-1.
Draper ES, Gallimore ID, Kurinczuk JJ, Smith PW, Boby T, Smith LK, et al. MBRACE-UK perinatal mortality surveillance report, UK perinatal deaths for births from January to December 2016. In: DoHS, editor. The infant mortality and morbidity studies. Leicester: University of Leicester; 2018.
Wisner KL, Sit DK, McShea MC, Rizzo DM, Zoretich RA, Hughes CL, et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013;70(5):490–8. https://doi.org/10.1001/jamapsychiatry.2013.87.
Segre LS, O’Hara MW, Arndt S, Stuart S. The prevalence of postpartum depression: the relative significance of three social status indices. Soc Psychiatry Psychiatr Epidemiol. 2007;42(4):316–21. https://doi.org/10.1007/s00127-007-0168-1.
Mersky JP, Janczewski CE. Adverse childhood experiences and postpartum depression in home visiting programs: prevalence, association, and mediating mechanisms. Matern Child Health J. 2018;22(7):1051–8. https://doi.org/10.1007/s10995-018-2488-z.
Homish GG, Cornelius JR, Richardson GA, Day NL. Antenatal risk factors associated with postpartum comorbid alcohol use and depressive symptomatology. Alcohol Clin Exp Res. 2004;28(8):1242–8.
Hu R, Li Y, Zhang Z, Yan W. Antenatal depressive symptoms and the risk of preeclampsia or operative deliveries: a meta-analysis. PLoS One. 2015;10(3):e0119018. https://doi.org/10.1371/journal.pone.0119018.
Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–24. https://doi.org/10.1001/archgenpsychiatry.2010.111.
Yedid Sion M, Harlev A, Weintraub AY, Sergienko R, Sheiner E. Is antenatal depression associated with adverse obstetric and perinatal outcomes? J Matern Fetal Neonatal Med. 2016;29(6):863–7. https://doi.org/10.3109/14767058.2015.1023708.
Badr LK, Ayvazian N, Lameh S, Charafeddine L. Is the effect of postpartum depression on mother-infant bonding universal? Infant Behav Dev. 2018;51:15–23. https://doi.org/10.1016/j.infbeh.2018.02.003.
Smith-Nielsen J, Tharner A, Krogh MT, Vaever MS. Effects of maternal postpartum depression in a well-resourced sample: Early concurrent and long-term effects on infant cognitive, language, and motor development. Scand J Psychol. 2016;57(6):571–83. https://doi.org/10.1111/sjop.12321.
Shen H, Magnusson C, Rai D, Lundberg M, Le-Scherban F, Dalman C, et al. Associations of parental depression with child school performance at age 16 years in Sweden. JAMA Psychiatry. 2016;73(3):239–46. https://doi.org/10.1001/jamapsychiatry.2015.2917.
Kawai E, Takagai S, Takei N, Itoh H, Kanayama N, Tsuchiya KJ. Maternal postpartum depressive symptoms predict delay in non-verbal communication in 14-month-old infants. Infant Behav Dev. 2017;46:33–45. https://doi.org/10.1016/j.infbeh.2016.11.006.
Martini J, Petzoldt J, Knappe S, Garthus-Niegel S, Asselmann E, Wittchen HU. Infant, maternal, and familial predictors and correlates of regulatory problems in early infancy: the differential role of infant temperament and maternal anxiety and depression. Early Hum Dev. 2017;115:23–31. https://doi.org/10.1016/j.earlhumdev.2017.08.005.
Meiser S, Zietlow AL, Reck C, Trauble B. The impact of postpartum depression and anxiety disorders on children’s processing of facial emotional expressions at pre-school age. Arch Womens Ment Health. 2015;18(5):707–16. https://doi.org/10.1007/s00737-015-0519-y.
Kersten-Alvarez LE, Hosman CM, Riksen-Walraven JM, van Doesum KT, Smeekens S, Hoefnagels C. Early school outcomes for children of postpartum depressed mothers: comparison with a community sample. Child Psychiatry Hum Dev. 2012;43(2):201–18. https://doi.org/10.1007/s10578-011-0257-y.
Klier CM, Rosenblum KL, Zeller M, Steinhardt K, Bergemann N, Muzik M. A multirisk approach to predicting chronicity of postpartum depression symptoms. Depress Anxiety. 2008;25(8):718–24. https://doi.org/10.1002/da.20419.
Netsi E, Pearson RM, Murray L, Cooper P, Craske MG, Stein A. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry. 2018;75(3):247–53. https://doi.org/10.1001/jamapsychiatry.2017.4363.
Letourneau NL, Tramonte L, Willms JD. Maternal depression, family functioning and children’s longitudinal development. J Pediatr Nurs. 2013;28(3):223–34. https://doi.org/10.1016/j.pedn.2012.07.014.
Kimmel MC, Cox E, Schiller C, Gettes E, Meltzer-Brody S. Pharmacologic treatment of perinatal depression. Obstet Gynecol Clin N Am. 2018;45(3):419–40. https://doi.org/10.1016/j.ogc.2018.04.007.
Roy A, Cole K, Goldman Z, Barris M. Fluoxetine treatment of postpartum depression. Am J Psychiatry. 1993;150(8):1273.
Stowe ZN, Casarella J, Landry J, Nemeroff CB. Sertraline in the treatment of women with postpartum major depression. Depression. 1995;3(1–2):49–55. https://doi.org/10.1002/depr.3050030109.
Suri R, Burt VK, Altshuler LL, Zuckerbrow-Miller J, Fairbanks L. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739–40. https://doi.org/10.1176/appi.ajp.158.10.1739.
Misri S, Abizadeh J, Albert G, Carter D, Ryan D. Restoration of functionality in postpartum depressed mothers: an open-label study with escitalopram. J Clin Psychopharmacol. 2012;32(5):729–32. https://doi.org/10.1097/JCP.0b013e31826867c9.
Milgrom J, Gemmill AW, Ericksen J, Burrows G, Buist A, Reece J. Treatment of postnatal depression with cognitive behavioural therapy, sertraline and combination therapy: a randomised controlled trial. Aust N Z J Psychiatry. 2015;49(3):236–45. https://doi.org/10.1177/0004867414565474.
Hantsoo L, Ward-O’Brien D, Czarkowski KA, Gueorguieva R, Price LH, Epperson CN. A randomized, placebo-controlled, double-blind trial of sertraline for postpartum depression. Psychopharmacology (Berl). 2014;231(5):939–48. https://doi.org/10.1007/s00213-013-3316-1.
Bloch M, Meiboom H, Lorberblatt M, Bluvstein I, Aharonov I, Schreiber S. The effect of sertraline add-on to brief dynamic psychotherapy for the treatment of postpartum depression: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(2):235–41. https://doi.org/10.4088/JCP.11m07117.
Sharp DJ, Chew-Graham C, Tylee A, Lewis G, Howard L, Anderson I, et al. A pragmatic randomised controlled trial to compare antidepressants with a community-based psychosocial interven Assess. 2010;14(43):iii–iv, ix–xi, 1–153. https://doi.org/10.3310/hta14430.
Yonkers KA, Lin H, Howell HB, Heath AC, Cohen LS. Pharmacologic treatment of postpartum women with new-onset major depressive disorder: a randomized controlled trial with paroxetine. J Clin Psychiatry. 2008;69(4):659–65.
Wisner KL, Hanusa BH, Perel JM, Peindl KS, Piontek CM, Sit DK, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26(4):353–60. https://doi.org/10.1097/01.jcp.0000227706.56870.dd.
Misri S, Reebye P, Corral M, Milis L. The use of paroxetine and cognitive-behavioral therapy in postpartum depression and anxiety: a randomized controlled trial. J Clin Psychiatry. 2004;65(9):1236–41.
Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. 1997;314(7085):932–6.
Molyneaux E, Howard LM, McGeown HR, Karia AM, Trevillion K. Antidepressant treatment for postnatal depression. CochraneDatabase Syst Rev. 2014;9:C002018. https://doi.org/10.1002/14651858.cd002018.pub2.
De Crescenzo F, Perelli F, Armando M, Vicari S. Selective serotonin reuptake inhibitors (SSRIs) for post-partum depression (PPD): a systematic review of randomized clinical trials. J Affect Disord. 2014;152–154:39–44. https://doi.org/10.1016/j.jad.2013.09.019.
Cohen LS, Viguera AC, Bouffard SM, Nonacs RM, Morabito C, Collins MH, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592–6.
Misri S, Swift E, Abizadeh J, Shankar R. Overcoming functional impairment in postpartum depressed or anxious women: a pilot trial of desvenlafaxine with flexible dosing. Ther Adv Psychopharmacol. 2016;6(4):269–76. https://doi.org/10.1177/2045125316656297.
Nonacs RM, Soares CN, Viguera AC, Pearson K, Poitras JR, Cohen LS. Bupropion SR for the treatment of postpartum depression: a pilot study. Int J Neuropsychopharmacol. 2005;8(3):445–9. https://doi.org/10.1017/s1461145705005079.
Suri R, Burt VK, Altshuler LL. Nefazodone for the treatment of postpartum depression. Arch Womens Ment Health. 2005;8(1):55–6. https://doi.org/10.1007/s00737-005-0071-2.
Marrocco J, McEwen BS. Sex in the brain: hormones and sex differences. Dialogues Clin Neurosci. 2016;18(4):373–83.
Rubinow DR, Schmidt PJ. Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology. 2019;44(1):111–28. https://doi.org/10.1038/s41386-018-0148-z.
Gregoire AJ, Kumar R, Everitt B, Henderson AF, Studd JW. Transdermal oestrogen for treatment of severe postnatal depression. Lancet. 1996;347(9006):930–3.
Wisner KL, Sit DK, Moses-Kolko EL, Driscoll KE, Prairie BA, Stika CS, et al. Transdermal estradiol treatment for postpartum depression: a pilot, randomized trial. J Clin Psychopharmacol. 2015;35(4):389–95. https://doi.org/10.1097/jcp.0000000000000351.
Moses-Kolko EL, Berga SL, Kalro B, Sit DK, Wisner KL. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52(3):516–29. https://doi.org/10.1097/GRF.0b013e3181b5a395.
Lawrie TA, Hofmeyr GJ, De Jager M, Berk M, Paiker J, Viljoen E. A double-blind randomised placebo controlled trial of postnatal norethisterone enanthate: the effect on postnatal depression and serum hormones. Br J Obstet Gynaecol. 1998;105(10):1082–90.
Singata-Madliki M, Hofmeyr GJ, Lawrie TA. The effect of depot medroxyprogesterone acetate on postnatal depression: a randomised controlled trial. J Fam Plann Reprod Health Care. 2016;42(3):171–6. https://doi.org/10.1136/jfprhc-2015-101334.
Tsai R, Schaffir J. Effect of depot medroxyprogesterone acetate on postpartum depression. Contraception. 2010;82(2):174–7. https://doi.org/10.1016/j.contraception.2010.03.004.
Dennis CL, Ross LE, Herxheimer A. Oestrogens and progestins for preventing and treating postpartum depression. Cochrane Database Syst Rev. 2008;4:CD001690. https://doi.org/10.1002/14651858.cd001690.pub2.
Wisner KL, Perel JM, Peindl KS, Hanusa BH, Findling RL, Rapport D. Prevention of recurrent postpartum depression: a randomized clinical trial. J Clin Psychiatry. 2001;62(2):82–6.
Yonkers KA, Vigod S, Ross LE. Diagnosis, pathophysiology, and management of mood disorders in pregnant and postpartum women. Obstet Gynecol. 2011;117(4):961–77. https://doi.org/10.1097/AOG.0b013e31821187a7.
Wisner KL, Perel JM, Peindl KS, Hanusa BH, Piontek CM, Findling RL. Prevention of postpartum depression: a pilot randomized clinical trial. Am J Psychiatry. 2004;161(7):1290–2. https://doi.org/10.1176/appi.ajp.161.7.1290.
Khazaie H, Ghadami MR, Knight DC, Emamian F, Tahmasian M. Insomnia treatment in the third trimester of pregnancy reduces postpartum depression symptoms: a randomized clinical trial. Psychiatry Res. 2013;210(3):901–5. https://doi.org/10.1016/j.psychres.2013.08.017.
Molyneaux E, Telesia LA, Henshaw C, Boath E, Bradley E, Howard LM. Antidepressants for preventing postnatal depression. Cochrane Database Syst Rev. 2018;4:CD004363. https://doi.org/10.1002/14651858.cd004363.pub3.
Walton GD, Ross LE, Stewart DE, Grigoriadis S, Dennis CL, Vigod S. Decisional conflict among women considering antidepressant medication use in pregnancy. Arch Womens Ment Health. 2014;17(6):493–501. https://doi.org/10.1007/s00737-014-0448-1.
Goodman JH. Women’s attitudes, preferences, and perceived barriers to treatment for perinatal depression. Birth. 2009;36(1):60–9. https://doi.org/10.1111/j.1523-536X.2008.00296.x.
Hall HR, Jolly K. Women’s use of complementary and alternative medicines during pregnancy: a cross-sectional study. Midwifery. 2014;30(5):499–505. https://doi.org/10.1016/j.midw.2013.06.001.
Wu P, Fuller C, Liu X, Lee HC, Fan B, Hoven CW, et al. Use of complementary and alternative medicine among women with depression: results of a national survey. Psychiatr Serv. 2007;58(3):349–56. https://doi.org/10.1176/ps.2007.58.3.349.
Stuart S, Koleva H. Psychological treatments for perinatal depression. Best Pract Res Clin Obstet Gynaecol. 2014;28(1):61–70. https://doi.org/10.1016/j.bpobgyn.2013.09.004.
Dennis CL, Dowswell T. Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database Syst Rev. 2013;2:CD001134. https://doi.org/10.1002/14651858.cd001134.pub3.
Reza N, Deligiannidis KM, Eustis EH, Battle CL. Complementary health practices for treating perinatal depression. Obstet Gynecol Clin North Am. 2018;45(3):441–54. https://doi.org/10.1016/j.ogc.2018.04.002.
Rundgren S, Brus O, Bave U, Landen M, Lundberg J, Nordanskog P, et al. Improvement of postpartum depression and psychosis after electroconvulsive therapy: a population-based study with a matched comparison group. J Affect Disord. 2018;235:258–64. https://doi.org/10.1016/j.jad.2018.04.043.
Ward HB, Fromson JA, Cooper JJ, De Oliveira G, Almeida M. Recommendations for the use of ECT in pregnancy: literature review and proposed clinical protocol. Arch Womens Ment Health. 2018;21(6):715–22. https://doi.org/10.1007/s00737-018-0851-0.
Myczkowski ML, Dias AM, Luvisotto T, Arnaut D, Bellini BB, Mansur CG, et al. Effects of repetitive transcranial magnetic stimulation on clinical, social, and cognitive performance in postpartum depression. Neuropsychiatr Dis Treat. 2012;8:491–500. https://doi.org/10.2147/ndt.s33851.
Garcia KS, Flynn P, Pierce KJ, Caudle M. Repetitive transcranial magnetic stimulation treats postpartum depression. Brain Stimul. 2010;3(1):36–41. https://doi.org/10.1016/j.brs.2009.06.001.
Andriotti T, Stavale R, Nafee T, Fakhry S, Mohamed MMA, Sofiyeva N, et al. ASSERT trial: how to assess the safety and efficacy of a high frequency rTMS in postpartum depression ? A multicenter, double blinded, randomized, placebo-controlled clinical trial. Contemp Clin Trials Commun. 2017;5:86–91. https://doi.org/10.1016/j.conctc.2017.01.004.
Vigod S, Dennis CL, Daskalakis Z, Murphy K, Ray J, Oberlander T, et al. Transcranial direct current stimulation (tDCS) for treatment of major depression during pregnancy: study protocol for a pilot randomized controlled trial. Trials. 2014;15:366. https://doi.org/10.1186/1745-6215-15-366.
Kim DR, Snell JL, Ewing GC, O’Reardon J. Neuromodulation and antenatal depression: a review. Neuropsychiatr Dis Treat. 2015;11:975–82. https://doi.org/10.2147/ndt.s80480.
Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151(7):979–86.
Bloch M, Rubinow DR, Schmidt PJ, Lotsikas A, Chrousos GP, Cizza G. Cortisol response to ovine corticotropin-releasing hormone in a model of pregnancy and parturition in euthymic women with and without a history of postpartum depression. J Clin Endocrinol Metab. 2005;90(2):695–9. https://doi.org/10.1210/jc.2004-1388.
Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157(6):924–30.
Deligiannidis KM, Sikoglu EM, Shaffer SA, Frederick B, Svenson AE, Kopoyan A, et al. GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: a preliminary study. J Psychiatr Res. 2013;47(6):816–28. https://doi.org/10.1016/j.jpsychires.2013.02.010.
Harris B, Lovett L, Newcombe RG, Read GF, Walker R, Riad-Fahmy D. Maternity blues and major endocrine changes: Cardiff puerperal mood and hormone study II. BMJ. 1994;308(6934):949–53.
Magiakou MA, Mastorakos G, Rabin D, Dubbert B, Gold PW, Chrousos GP. Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab. 1996;81(5):1912–7.
Stuebe AM, Grewen K, Pedersen CA, Propper C, Meltzer-Brody S. Failed lactation and perinatal depression: common problems with shared neuroendocrine mechanisms? J Womens Health (Larchmt). 2012;21(3):264–72. https://doi.org/10.1089/jwh.2011.3083.
Hellgren C, Akerud H, Skalkidou A, Sundstrom-Poromaa I. Cortisol awakening response in late pregnancy in women with previous or ongoing depression. Psychoneuroendocrinology. 2013;38(12):3150–4. https://doi.org/10.1016/j.psyneuen.2013.08.007.
Meinlschmidt G, Martin C, Neumann ID, Heinrichs M. Maternal cortisol in late pregnancy and hypothalamic-pituitary-adrenal reactivity to psychosocial stress postpartum in women. Stress. 2010;13(2):163–71. https://doi.org/10.3109/10253890903128632.
Nierop A, Bratsikas A, Zimmermann R, Ehlert U. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms? Psychosom Med. 2006;68(6):931–7. https://doi.org/10.1097/01.psy.0000244385.93141.3b.
Corwin EJ, Pajer K, Paul S, Lowe N, Weber M, McCarthy DO. Bidirectional psychoneuroimmune interactions in the early postpartum period influence risk of postpartum depression. Brain Behav Immun. 2015;49:86–93. https://doi.org/10.1016/j.bbi.2015.04.012.
Boufidou F, Lambrinoudaki I, Argeitis J, Zervas IM, Pliatsika P, Leonardou AA, et al. CSF and plasma cytokines at delivery and postpartum mood disturbances. J Affect Disord. 2009;115(1–2):287–92. https://doi.org/10.1016/j.jad.2008.07.008.
Sharkey KM, Pearlstein TB, Carskadon MA. Circadian phase shifts and mood across the perinatal period in women with a history of major depressive disorder: a preliminary communication. J Affect Disord. 2013;150(3):1103–8. https://doi.org/10.1016/j.jad.2013.04.046.
Parry BL, Meliska CJ, Sorenson DL, Lopez AM, Martinez LF, Nowakowski S, et al. Plasma melatonin circadian rhythm disturbances during pregnancy and postpartum in depressed women and women with personal or family histories of depression. Am J Psychiatry. 2008;165(12):1551–8. https://doi.org/10.1176/appi.ajp.2008.08050709.
Costas J, Gratacos M, Escaramis G, Martin-Santos R, de Diego Y, Baca-Garcia E, et al. Association study of 44 candidate genes with depressive and anxiety symptoms in post-partum women. J Psychiatr Res. 2010;44(11):717–24. https://doi.org/10.1016/j.jpsychires.2009.12.012.
Guintivano J, Arad M, Gould TD, Payne JL, Kaminsky ZA. Antenatal prediction of postpartum depression with blood DNA methylation biomarkers. Mol Psychiatry. 2014;19(5):560–7. https://doi.org/10.1038/mp.2013.62.
Deligiannidis KM, Fales CL, Kroll-Desrosiers AR, Shaffer SA, Villamarin V, Tan Y, et al. Intrinsic resting-state functional connectivity, cortical γ-aminobutyric acid and peripheral neuroactive steroids in peripartum and peripartum depressed women: a functional magnetic imaging and spectroscopy study. Neuropsychopharmacol. 2019;44(3):546–54. https://doi.org/10.1038/s41386-018-0242-2.
Silver M, Moore CM, Villamarin V, Jaitly N, Hall JE, Rothschild AJ, et al. White matter integrity in medication-free women with peripartum depression: a tract-based spatial statistics study. Neuropsychopharmacology. 2018;43(7):1573–80. https://doi.org/10.1038/s41386-018-0023-y.
Blackburn ST. Reproductive and developmental processes. In: Jackson C, editor. Maternal, fetal and neonatal physiology. Philadelphia (PAO: Elsevier Health Sciences; 2007. p. 100–2.
Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol. 1972;112(8):1095–100.
Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137(3):293–8.
Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal-fetal attachment. Peptides. 2007;28(6):1162–9. https://doi.org/10.1016/j.peptides.2007.04.016.
Hill M, Parizek A, Klak J, Hampl R, Sulcova J, Havlikova H, et al. Neuroactive steroids, their precursors and polar conjugates during parturition and postpartum in maternal and umbilical blood: 3.3beta-hydroxy-5-ene steroids. J Steroid Biochem Mol Biol. 2002;82(2–3):241–50.
Hill M, Cibula D, Havlikova H, Kancheva L, Fait T, Kancheva R, et al. Circulating levels of pregnanolone isomers during the third trimester of human pregnancy. J Steroid Biochem Mol Biol. 2007;105(1–5):166–75. https://doi.org/10.1016/j.jsbmb.2006.10.010.
Deligiannidis KM, Kroll-Desrosiers AR, Svenson A, Jaitly N, Barton BA, Hall JE, et al. Cortisol response to the Trier Social Stress Test in pregnant women at risk for postpartum depression. Arch Womens Ment Health. 2016;19(5):789–97. https://doi.org/10.1007/s00737-016-0615-7.
Halligan SL, Herbert J, Goodyer I, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biol Psychiatry. 2007;62(1):40–6. https://doi.org/10.1016/j.biopsych.2006.09.011.
Nierop A, Bratsikas A, Klinkenberg A, Nater UM, Zimmermann R, Ehlert U. Prolonged salivary cortisol recovery in second-trimester pregnant women and attenuated salivary alpha-amylase responses to psychosocial stress in human pregnancy. J Clin Endocrinol Metab. 2006;91(4):1329–35. https://doi.org/10.1210/jc.2005-1816.
O’Connor TG, Tang W, Gilchrist MA, Moynihan JA, Pressman EK, Blackmore ER. Diurnal cortisol patterns and psychiatric symptoms in pregnancy: short-term longitudinal study. Biol Psychol. 2014;96:35–41. https://doi.org/10.1016/j.biopsycho.2013.11.002.
Meltzer-Brody S, Stuebe A, Dole N, Savitz D, Rubinow D, Thorp J. Elevated corticotropin releasing hormone (CRH) during pregnancy and risk of postpartum depression (PPD). J Clin Endocrinol Metab. 2011;96(1):E40–7. https://doi.org/10.1210/jc.2010-0978.
Zaconeta AM, Amato AA, Barra GB, Casulari da Motta LD, de Souza VC, Karnikowski MG, et al. Cerebrospinal fluid CRH levels in late pregnancy are not associated with new-onset postpartum depressive symptoms. J Clin Endocrinol Metab. 2015;100(8):3159–64. https://doi.org/10.1210/jc.2014-4503.
Glynn LM, Sandman CA. Evaluation of the association between placental corticotrophin-releasing hormone and postpartum depressive symptoms. Psychosom Med. 2014;76(5):355–62. https://doi.org/10.1097/psy.0000000000000066.
Yim IS, Glynn LM, Dunkel-Schetter C, Hobel CJ, Chicz-DeMet A, Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry. 2009;66(2):162–9. https://doi.org/10.1001/archgenpsychiatry.2008.533.
Ferguson EH, Di Florio A, Pearson B, Putnam KT, Girdler S, Rubinow DR, et al. HPA axis reactivity to pharmacologic and psychological stressors in euthymic women with histories of postpartum versus major depression. Arch Womens Ment Health. 2017;20(3):411–20. https://doi.org/10.1007/s00737-017-0716-y.
Crowley SK, O’Buckley TK, Schiller CE, Stuebe A, Morrow AL, Girdler SS. Blunted neuroactive steroid and HPA axis responses to stress are associated with reduced sleep quality and negative affect in pregnancy: a pilot study. Psychopharmacology (Berl). 2016;233(7):1299–310. https://doi.org/10.1007/s00213-016-4217-x.
Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology. 2011;36(9):1886–93. https://doi.org/10.1038/npp.2011.74.
King L, Robins S, Chen G, Yerko V, Zhou Y, Nagy C, et al. Perinatal depression and DNA methylation of oxytocin-related genes: a study of mothers and their children. Horm Behav. 2017;96:84–94. https://doi.org/10.1016/j.yhbeh.2017.09.006.
Kroll-Desrosiers AR, Nephew BC, Babb JA, Guilarte-Walker Y, Moore Simas TA, Deligiannidis KM. Association of peripartum synthetic oxytocin administration and depressive and anxiety disorders within the first postpartum year. Depress Anxiety. 2017;34(2):137–46. https://doi.org/10.1002/da.22599.
Okun ML, Luther J, Prather AA, Perel JM, Wisniewski S, Wisner KL. Changes in sleep quality, but not hormones predict time to postpartum depression recurrence. J Affect Disord. 2011;130(3):378–84. https://doi.org/10.1016/j.jad.2010.07.015.
O’Hara MW, Schlechte JA, Lewis DA, Wright EJ. Prospective study of postpartum blues. Biologic and psychosocial factors. Arch Gen Psychiatry. 1991;48(9):801–6.
Parizek A, Mikesova M, Jirak R, Hill M, Koucky M, Paskova A, et al. Steroid hormones in the development of postpartum depression. Physiol Res. 2014;63(Suppl. 2):S277–82.
Hohlagschwandtner M, Husslein P, Klier C, Ulm B. Correlation between serum testosterone levels and peripartal mood states. Acta Obstet Gynecol Scand. 2001;80(4):326–30.
Aswathi A, Rajendiren S, Nimesh A, Philip RR, Kattimani S, Jayalakshmi D, et al. High serum testosterone levels during postpartum period are associated with postpartum depression. Asian J Psychiatry. 2015;17:85–8. https://doi.org/10.1016/j.ajp.2015.08.008.
Deligiannidis KM, Kroll-Desrosiers AR, Mo S, Nguyen HP, Svenson A, Jaitly N, et al. Peripartum neuroactive steroid and gamma-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology. 2016;70:98–107. https://doi.org/10.1016/j.psyneuen.2016.05.010.
Groer MW, Vaughan JH. Positive thyroid peroxidase antibody titer is associated with dysphoric moods during pregnancy and postpartum. J Obstet Gynecol Neonatal Nursing. 2013;42(1):E26–32. https://doi.org/10.1111/j.1552-6909.2012.01425.x.
Albacar G, Sans T, Martin-Santos R, Garcia-Esteve L, Guillamat R, Sanjuan J, et al. Thyroid function 48h after delivery as a marker for subsequent postpartum depression. Psychoneuroendocrinology. 2010;35(5):738–42. https://doi.org/10.1016/j.psyneuen.2009.10.015.
Pedersen CA, Johnson JL, Silva S, Bunevicius R, Meltzer-Brody S, Hamer RM, et al. Antenatal thyroid correlates of postpartum depression. Psychoneuroendocrinology. 2007;32(3):235–45. https://doi.org/10.1016/j.psyneuen.2006.12.010.
Sylven SM, Elenis E, Michelakos T, Larsson A, Olovsson M, Poromaa IS, et al. Thyroid function tests at delivery and risk for postpartum depressive symptoms. Psychoneuroendocrinology. 2013;38(7):1007–13. https://doi.org/10.1016/j.psyneuen.2012.10.004.
Sandman CA, Glynn LM. Corticotropin-releasing hormone (CRH) programs the fetal and maternal brain. Future Neurol. 2009;4(3):257–61.
Duthie L, Reynolds RM. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology. 2013;98(2):106–15. https://doi.org/10.1159/000354702.
McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1(5):460–3.
Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27(6):1457–63. https://doi.org/10.1016/j.peptides.2005.10.002.
Sasaki A, Liotta AS, Luckey MM, Margioris AN, Suda T, Krieger DT. Immunoreactive corticotropin-releasing factor is present in human maternal plasma during the third trimester of pregnancy. J Clin Endocrinol Metab. 1984;59(4):812–4. https://doi.org/10.1210/jcem-59-4-812.
Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. 2003;997:136–49.
Nolten WE, Lindheimer MD, Rueckert PA, Oparil S, Ehrlich EN. Diurnal patterns and regulation of cortisol secretion in pregnancy. J Clin Endocrinol Metab. 1980;51(3):466–72.
Jung C, Ho JT, Torpy DJ, Rogers A, Doogue M, Lewis JG, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96(5):1533–40. https://doi.org/10.1210/jc.2010-2395.
O’Keane V, Lightman S, Patrick K, Marsh M, Papadopoulos AS, Pawlby S, et al. Changes in the maternal hypothalamic-pituitary-adrenal axis during the early puerperium may be related to the postpartum ‘blues’. J Neuroendocrinol. 2011;23(11):1149–55. https://doi.org/10.1111/j.1365-2826.2011.02139.x.
Buss C, Entringer S, Reyes JF, Chicz-DeMet A, Sandman CA, Waffarn F, et al. The maternal cortisol awakening response in human pregnancy is associated with the length of gestation. Am J Obstet Gynecol. 2009;201(4):398.e1–8. https://doi.org/10.1016/j.ajog.2009.06.063.
Entringer S, Buss C, Shirtcliff EA, Cammack AL, Yim IS, Chicz-DeMet A, et al. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress. 2010;13(3):258–68. https://doi.org/10.3109/10253890903349501.
Schulte HM, Weisner D, Allolio B. The corticotrophin releasing hormone test in late pregnancy: lack of adrenocorticotrophin and cortisol response. Clin Endocrinol. 1990;33(1):99–106.
de Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy: a review. Neurosci Biobehav Rev. 2005;29(2):295–312. https://doi.org/10.1016/j.neubiorev.2004.10.005.
Giesbrecht GF, Campbell T, Letourneau N, Kaplan BJ. Advancing gestation does not attenuate biobehavioural coherence between psychological distress and cortisol. Biol Psychol. 2013;93(1):45–51. https://doi.org/10.1016/j.biopsycho.2013.01.019.
Brunton PJ. Neuroactive steroids and stress axis regulation: pregnancy and beyond. J Steroid Biochem Mol Biol. 2016;160:160–8. https://doi.org/10.1016/j.jsbmb.2015.08.003.
Butterfield MI, Stechuchak KM, Connor KM, Davidson JR, Wang C, MacKuen CL, et al. Neuroactive steroids and suicidality in posttraumatic stress disorder. Am J Psychiatry. 2005;162(2):380–2. https://doi.org/10.1176/appi.ajp.162.2.380.
Lopez-Rodriguez AB, Acaz-Fonseca E, Giatti S, Caruso D, Viveros MP, Melcangi RC, et al. Correlation of brain levels of progesterone and dehydroepiandrosterone with neurological recovery after traumatic brain injury in female mice. Psychoneuroendocrinology. 2015;56:1–11. https://doi.org/10.1016/j.psyneuen.2015.02.018.
Guille C, Spencer S, Cavus I, Epperson CN. The role of sex steroids in catamenial epilepsy and premenstrual dysphoric disorder: implications for diagnosis and treatment. Epilepsy Behav. 2008;13(1):12–24. https://doi.org/10.1016/j.yebeh.2008.02.004.
Backstrom T, Haage D, Lofgren M, Johansson IM, Stromberg J, Nyberg S, et al. Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons. Neuroscience. 2011;191:46–54. https://doi.org/10.1016/j.neuroscience.2011.03.061.
Luchetti S, Bossers K, Van de Bilt S, Agrapart V, Morales RR, Frajese GV, et al. Neurosteroid biosynthetic pathways changes in prefrontal cortex in Alzheimer’s disease. Neurobiol Aging. 2011;32(11):1964–76. https://doi.org/10.1016/j.neurobiolaging.2009.12.014.
Schiller CE, Meltzer-Brody S, Rubinow DR. The role of reproductive hormones in postpartum depression. CNS Spectr. 2015;20(1):48–59. https://doi.org/10.1017/s1092852914000480.
Schiller CE, Schmidt PJ, Rubinow DR. Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology (Berl). 2014;231(17):3557–67. https://doi.org/10.1007/s00213-014-3599-x.
Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6(6):2311–22.
Baulieu EE. Steroid hormones in the brain: several mechanisms? In: Fuxe F, Gustaffson JA, Wetterberg L, editors. Steroid hormone regulation of the brain. Oxford: Pergamon Press; 1981. p. 3–14.
Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28(2):139–68.
Avoli M, Krnjevic K. The long and winding road to gamma-amino-butyric acid as neurotransmitter. Can J Neurol Sci. 2016;43(2):219–26. https://doi.org/10.1017/cjn.2015.333.
Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci. 1987;231(1264):359–69.
Lambert JJ, Peters JA, Sturgess NC, Hales TG. Steroid modulation of the GABAA receptor complex: electrophysiological studies. Ciba Found Symp. 1990;153:56–71 (discussion 71–82).
Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60(3):243–60. https://doi.org/10.1124/pr.108.00505.
Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6(5):484–90. https://doi.org/10.1038/nn1043.
Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci USA. 2003;100(24):14439–44. https://doi.org/10.1073/pnas.2435457100.
Parizek A, Hill M, Kancheva R, Havlikova H, Kancheva L, Cindr J, et al. Neuroactive pregnanolone isomers during pregnancy. J Clin Endocrinol Metab. 2005;90(1):395–403. https://doi.org/10.1210/jc.2004-0444.
Mostallino MC, Sanna E, Concas A, Biggio G, Follesa P. Plasticity and function of extrasynaptic GABA(A) receptors during pregnancy and after delivery. Psychoneuroendocrinology. 2009;34(Suppl. 1):S74–83. https://doi.org/10.1016/j.psyneuen.2009.06.013.
Zhao C, Gammie SC. Glutamate, GABA, and glutamine are synchronously upregulated in the mouse lateral septum during the postpartum period. Brain Res. 2014;1591:53–62. https://doi.org/10.1016/j.brainres.2014.10.023.
Smolen A, Smolen TN, Han PC. Alterations in regional brain GABA concentration and turnover during pregnancy. Pharmacol Biochem Behav. 1993;44(1):63–9.
Maguire J, Mody I. Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology. 2009;34(Suppl. 1):S84–90. https://doi.org/10.1016/j.psyneuen.2009.06.019.
Maguire J, Ferando I, Simonsen C, Mody I. Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci. 2009;29(30):9592–601. https://doi.org/10.1523/JNEUROSCI.2162-09.2009.
Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59(2):207–13. https://doi.org/10.1016/j.neuron.2008.06.019.
Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, Krystal JH, et al. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology (Berl). 2006;186(3):425–33. https://doi.org/10.1007/s00213-006-0313-7.
Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, Genazzani AR. Serum allopregnanolone in women with postpartum “blues”. Obstet Gynecol. 2001;97(1):77–80.
Osborne LM, Gispen F, Sanyal A, Yenokyan G, Meilman S, Payne JL. Lower allopregnanolone during pregnancy predicts postpartum depression: an exploratory study. Psychoneuroendocrinology. 2017;79:116–21. https://doi.org/10.1016/j.psyneuen.2017.02.012.
Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45(8):742–7.
Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56(11):1043–7.
Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851–8.
Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159(4):663–5.
Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61(7):705–13. https://doi.org/10.1001/archpsyc.61.7.705.
Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, et al. Anterior cingulate cortex gamma-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry. 2012;69(2):139–49. https://doi.org/10.1001/archgenpsychiatry.2011.131.
Gabbay V, Bradley KA, Mao X, Ostrover R, Kang G, Shungu DC. Anterior cingulate cortex gamma-aminobutyric acid deficits in youth with depression. Transl Psychiatry. 2017;7(8):e1216. https://doi.org/10.1038/tp.2017.187.
van Broekhoven F, Bäckström T, van Luijtelaar G, Buitelaar JK, Smits P, Verkes RJ. Effects of allopregnanolone on sedation in men, and in women on oral contraceptives. Psychoneuroendocrinology. 2007;32(5):555–64. https://doi.org/10.1016/j.psyneuen.2007.03.009.
Timby E, Balgard M, Nyberg S, Spigset O, Andersson A, Porankiewicz-Asplund J, et al. Pharmacokinetic and behavioral effects of allopregnanolone in healthy women. Psychopharmacology (Berl). 2006;186(3):414–24. https://doi.org/10.1007/s00213-005-0148-7.
Kask K, Bäckström T, Nilsson L-G, Sundström-Poromaa I. Allopregnanolone impairs episodic memory in healthy women. Psychopharmacology. 2008;199(2):161. https://doi.org/10.1007/s00213-008-1150-7.
Kask K, Backstrom T, Lundgren P, Sundstrom Poromaa I. Allopregnanolone has no effect on startle response and prepulse inhibition of startle response in patients with premenstrual dysphoric disorder or healthy controls. Pharmacol Biochem Behav. 2009;92(4):608–13. https://doi.org/10.1016/j.pbb.2009.02.014.
Timby E, Hedstrom H, Backstrom T, Sundstrom-Poromaa I, Nyberg S, Bixo M. Allopregnanolone, a GABAA receptor agonist, decreases gonadotropin levels in women: a preliminary study. Gynecol Endocrinol. 2011;27(12):1087–93. https://doi.org/10.3109/09513590.2010.540603.
Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS. Progress report on new antiepileptic drugs: a summary of the Twelfth Eilat Conference (EILAT XII). Epilepsy Res. 2015;111:85–141. https://doi.org/10.1016/j.eplepsyres.2015.01.001.
Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480–9. https://doi.org/10.1016/S0140-6736(17)31264-3.
Kanes SJ, Colquhoun H, Doherty J, Raines S, Hoffmann E, Rubinow DR, et al. Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Hum Psychopharmacol. 2017. https://doi.org/10.1002/hup.2576.
Luisi S, Petraglia F, Benedetto C, Nappi RE, Bernardi F, Fadalti M, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429–33. https://doi.org/10.1210/jcem.85.7.6675.
Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, et al. Efficacy and safety of brexanolone iv, a GABAA receptor modulator, in women with postpartum depression: results from two double-blind, randomised, placebo-controlled phase 3 studies. Lancet. 2018. https://doi.org/10.1016/s0140-6736(18)31551-4.
US Food and Drug Administration. 2018 meeting materials, Psychopharmacologic Drugs Advisory Committee Meeting. 2018. https://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PsychopharmacologicDrugsAdvisoryCommittee/ucm598677.htm?fbclid=IwAR2bq2yaYOqzn0u-p0yAFjsgVWNwalq87l7ZzQO8cuJ3SQ8eLIfgrewIdj4. Accessed 30 Nov 2018.
Sage Therapeutics. Sage Therapeutics receives notification of PDUFA extension for ZULRESSO™ (brexanolone) injection. 2018. https://investor.sagerx.com/news-releases/news-release-details/sage-therapeutics-receives-notification-pdufa-extension. Accessed 30 Nov 2018.
Newton ER, Hale TW. Drugs in breast milk. Clin Obstet Gynecol. 2015;58(4):868–84. https://doi.org/10.1097/grf.0000000000000142.
Martinez Botella G, Salituro FG, Harrison BL, Beresis RT, Bai Z, Blanco MJ, et al. Neuroactive steroids 2. 3Alpha-hydroxy-3betanmethyl-21-(4-cyano-1H-pyrazol-1’-yl)-19-nor-5beta-pregnan-20 -one (SAGE-217): a clinical next generation neuroactive steroid positive allosteric modulator of the (gamma-aminobutyric acid)A receptor. J Med Chem. 2017;60(18):7810–9. https://doi.org/10.1021/acs.jmedchem.7b00846.
Sage Therapeutics. Sage Therapeutics announces SAGE-217 meets primary and secondary endpoints in phase 3 clinical trial in postpartum depression. 2019. https://investor.sagerx.com/news-releases/news-release-details/sage-therapeutics-announces-sage-217-meets-primary-and-secondary. Accessed 7 Jan 2019.
Marinus Pharmaceuticals. Marinus Pharmaceuticals announces positive ganaxolone data in women with postpartum depression. 2018. https://globenewswire.com/news-release/2018/12/10/1664282/0/en/Marinus-Pharmaceuticals-Announces-Positive-Ganaxolone-Data-in-Women-With-Postpartum-Depression.html. Accessed 7 Jan 2019.
Osborne LM, Hermann A, Burt V, Driscoll K, Fitelson E, Meltzer-Brody S, et al. Reproductive psychiatry: the gap between clinical need and education. Am J Psychiatry. 2015;172(10):946–8. https://doi.org/10.1176/appi.ajp.2015.15060837.
Kashani L, Eslatmanesh S, Saedi N, Niroomand N, Ebrahimi M, Hosseinian M, et al. Comparison of saffron versus fluoxetine in treatment of mild to moderate postpartum depression: a double-blind, randomized clinical trial. Pharmacopsychiatry. 2017;50(2):64–8. https://doi.org/10.1055/s-0042-115306.
Yonkers KA, Gotman N, Smith MV, Forray A, Belanger K, Brunetto WL, et al. Does antidepressant use attenuate the risk of a major depressive episode in pregnancy? Epidemiology. 2011;22(6):848–54. https://doi.org/10.1097/EDE.0b013e3182306847.
Xu Y, Li Y, Huang X, Chen D, She B, Ma D. Single bolus low-dose of ketamine does not prevent postpartum depression: a randomized, double-blind, placebo-controlled, prospective clinical trial. Arch Gynecol Obst. 2017;295(5):1167–74. https://doi.org/10.1007/s00404-017-4334-8.
Acknowledgments
We acknowledge Janice Lester, MLS, the Reference and Education Librarian at the Health Science Library of Long Island Jewish Medical Center at Northwell Health for her assistance with the literature review searches.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This article was supported by a National Institutes of Health Grant (No. 5K23MH097794). Kristina M. Deligiannidis currently receives royalties from an National Institutes of Health Employee Invention.
Conflict of Interest
Kristina M. Deligiannidis has received research grant support as a site for the clinical trials of brexanolone and SAGE-217 and serves as a consultant for Sage Therapeutics. Ariela Frieder, Madeleine Fersh, and Rachel Hainline have no conflicts of interest that are directly relevant to the contents of this article. The views expressed in this article are those of the authors and do not necessarily reflect the position of the National Institutes of Health.
Rights and permissions
About this article
Cite this article
Frieder, A., Fersh, M., Hainline, R. et al. Pharmacotherapy of Postpartum Depression: Current Approaches and Novel Drug Development. CNS Drugs 33, 265–282 (2019). https://doi.org/10.1007/s40263-019-00605-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-019-00605-7