Abstract
The efficacy of standard antidepressants is limited for many patients with mood disorders such as major depressive disorder (MDD) and bipolar depression, underscoring the urgent need to develop novel therapeutics. Both clinical and preclinical studies have implicated glutamatergic system dysfunction in the pathophysiology of mood disorders. In particular, rapid reductions in depressive symptoms have been observed in response to subanesthetic doses of the glutamatergic modulator racemic (R,S)-ketamine in individuals with mood disorders. These results have prompted investigation into other glutamatergic modulators for depression, both as monotherapy and adjunctively. Several glutamate receptor-modulating agents have been tested in proof-of-concept studies for mood disorders. This manuscript gives a brief overview of the glutamate system and its relevance to rapid antidepressant response and discusses the existing clinical evidence for glutamate receptor-modulating agents, including (1) broad glutamatergic modulators ((R,S)-ketamine, esketamine, (R)-ketamine, (2R,6R)-hydroxynorketamine [HNK], dextromethorphan, Nuedexta [a combination of dextromethorphan and quinidine], deudextromethorphan [AVP-786], axsome [AXS-05], dextromethadone [REL-1017], nitrous oxide, AZD6765, CLE100, AGN-241751); (2) glycine site modulators (d-cycloserine [DCS], NRX-101, rapastinel [GLYX-13], apimostinel [NRX-1074], sarcosine, 4-chlorokynurenine [4-Cl-KYN/AV-101]); (3) subunit (NR2B)-specific N-methyl-d-aspartate (NMDA) receptor antagonists (eliprodil [EVT-101], traxoprodil [CP-101,606], rislenemdaz [MK-0657/CERC-301]); (4) metabotropic glutamate receptor (mGluR) modulators (basimglurant, AZD2066, RG1578, TS-161); and (5) mammalian target of rapamycin complex 1 (mTORC1) activators (NV-5138). Many of these agents are still in the preliminary stages of development. Furthermore, to date, most have demonstrated relatively modest effects compared with (R,S)-ketamine and esketamine, though some have shown more favorable characteristics. Of these novel agents, the most promising, and the ones for which the most evidence exists, appear to be those targeting ionotropic glutamate receptors.

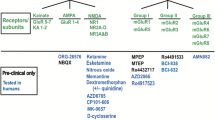
Adapted with permission from Kadriu et al. [5]
Similar content being viewed by others
References
World Health Organization. Depression Fact Sheet. [Website] 2018 [cited 2019 January 8]; https://www.who.int/news-room/fact-sheets/detail/depression. Accessed 31 July 2020.
Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439–45.
Machado-Vieira R, Salvadore G, Luckenbaugh D, Manji HK, Zarate CAJ. Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. J Clin Psychiatry. 2008;69:946–58.
Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry. 2004;9:984–7.
Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA Jr. Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int J Neuropsychopharmacol. 2019;22(2):119–35.
Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87:1186–93.
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–4.
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–64.
Iadarola ND, Niciu MJ, Richards EM, Vande Voort JL, Ballard ED, Lundin NB, et al. Ketamine and other N-methyl-d-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6:97–114.
aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67(2):139–45.
Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry. 2019;176:401–9.
Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74(4):250–6.
Park M, Niciu MJ, Zarate CAJ. Novel glutamatergic treatments for severe mood disorders. Curr Behav Neurosci Rep. 2015;2:198–208.
Niciu MJ, Henter ID, Luckenbaugh DA, Zarate CAJ, Charney DS. Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annu Rev Pharmacol Toxicol. 2014;54:119–39.
Lener M, Iosifescu DV. In pursuit of neuroimaging biomarkers to guide treatment selection in major depressive disorder: a review of the literature. Ann N Y Acad Sci. 2015;1344:50–65.
Kadriu B, Ballard ED, Henter ID, Murata S, Gerlus N, Zarate CAJ. Neurobiological biomarkers of response to ketamine. Adv Pharmacol. 2020;89:195–235.
Malhi GS, Morris G, Bell E, Hamilton A. A new paradigm for achieving a rapid antidepressant response. Drugs. 2020;80:755–64.
Niciu MJ, Kelmendi B, Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav. 2012;100(4):656–64.
Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res. 2006;147(1):1–25.
Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64(2):193–200.
Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–13.
Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–13.
Moriguchi S, Takamiya A, Noda Y, Horita N, Wada M, Tsugawa S, et al. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic responance spectroscopy studies. Mol Psychiatry. 2019;24:952–64.
Milak MS, Rashid R, Dong Z, Kegeles LS, Grunebaum MF, Ogden RT, et al. Assessment of relationship of ketamine dose with magnetic resonance spectroscopy of Glx and GABA responses in adults with major depression: a andomized clinical trial. JAMA Netw Open. 2020;3:
Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185(1):1–10.
Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–11.
Fogaça MV, Wu M, Li C, Li X-Y, Picciotto MR, Duman RS. Inhibition of GABA interneurons in the mPFC is sufficient and necessary for rapid antidepressant responses. Mol Psychiatry. 2020;Oct 17 [epub ahead of print].
Moghadam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2912–7.
Suzuki K, Monteggia LM. The role of eEF2 kinase in the rapid antidepressant actions of ketamine. Adv Pharmacol. 2020;89:79–99.
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–6.
Taha E, Gildish I, Gal-Ben-Ari S, Rosenblum K. The role of eEF2 pathway in learning and synaptic plasticity. Neurobiol Learn Mem. 2013;105:100–6.
Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64.
Aguilar-Valles A, De Gregorio D, Matta-Camacho E, Eslamizade MJ, Khlaifia A, Skaleka A, et al. Antidepressant actions of ketamine engage cell-specific translation via eIF4E. Nature. 2020;December 16 [epub ahead of print].
Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021;184:1299–313.
Zarate CAJ, Machado-Vieira R. Ketamine: translating mechanistic discoveries into the next generation of glutamate modulators for mood disorders. Mol Psychiatry. 2017;22:324–7.
Maeng S, Zarate CA, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–52.
Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. 2019;364(6436).
Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175:1205–15.
Riggs LM, Gould TD. Ketamine and the future of rapid-acting antidepressants. Annu Rev Clin Psychol. 2021;Feb 9 [epub ahead of print].
Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134–42.
Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793–802.
Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71(11):939–46.
Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016;46(7):1459–72.
Caddy C, Giaroli G, White TP, Shergill SS, Tracy DK. Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and meta-analysis of efficacy. Ther Adv Psychopharmacol. 2014;4(2):75–99.
McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693–704.
Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950–66.
Romeo B, Choucha W, Fossati P, Rotge JY. Meta-analysis of short- and mid-term efficacy of ketamine in unipolar and bipolar depression. Psychiatry Res. 2015;230(2):682–8.
Diazgranados N, Ibrahim L, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71(12):1605–11.
Murrough JW, Soleimani L, DeWilde KE, Collins KA, Lapidus KA, Iacoviello BM, et al. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol Med. 2015;45(16):3571–80.
Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66(5):522–6.
Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;3(175):150–8.
Grunebaum MF, Galalvy HC, Choo TH, Keilp JG, Moitra VK, Parris MS, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175:327–35.
Grunebaum MF, Ellis SP, Keilp JG, Moitra VK, Cooper TB, Marver JE, et al. Ketamine versus midazolam in bipolar depression with suicidal thoughts: a pilot midazolam-controlled randomized clinical trial. Bipolar Disord. 2017;19:176–83.
Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;14(4):
Mkrtchian A, Evans JW, Kraus C, Yuan P, Kadriu B, Nugent AC, et al. Ketamine modulates fronto-striatal circuitry in depressed and healthy individuals. Mol Psychiatry. 2020;Sept 14 [epub ahead of print].
Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71(6):681–8.
Feder A, Costi S, Rutter SB, Collins AB, Govindarajulu U, Jha MK, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178:193–202.
Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38:2475–83.
Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;16(175):620–30.
Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139–48.
Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76:893–903.
U.S. Food & Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression. 2019. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm632761.htm. Accessed 12 June 2020.
Kraus C, Rabl U, Vanicek T, Carlberg L, Popovic A, Spies M, et al. Administration of ketamine for unipolar and bipolar depression. Int J Psychiatry Clin Pract. 2017;18:1–12.
Acevedo-Diaz EE, Cavanaugh GW, Greenstein D, Kraus C, Kadriu B, Zarate CAJ, et al. Comprehensive assessment of side effects associated with a single dose of ketamine in treatment-resistant depression. J Affect Disord. 2020;263:568–75.
Swainson J, McGirr A, Blier P, Brietzke E, Richard-Devantoy S, Ravindran N, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) Task Force Recommendations for the Use of Racemic Ketamine in Adults with Major Depressive Disorder: Recommandations Du Groupe De Travail Du Réseau Canadien Pour Les Traitements De L’humeur Et De L’anxiété (Canmat) Concernant L’utilisation De La Kétamine Racémique Chez Les Adultes Souffrant De Trouble Dépressif Majeur. Can J Psychiatry. 2020;Nov 11 [epub ahead of print].
Wajs E, Aluisio L, Holder R, Daly EJ, Lane R, Lim P, et al. Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a Phase 3, open-label study (SUSTAIN-2). J Clin Psychiatry. 2020;81:19m12891.
Correia-Melo FS, Leal GC, Vieira F, Jesus-Nunes AP, Mello RP, Magnavita G, et al. Efficacy and safety of adjunctive therapy using esketamine or racemic ketamine for adult treatment-resistant depression: a randomized, double-blind, non-inferiority study. J Affect Disord. 2020;264:527–34.
Bahji A, Vazquez GH. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2020;278:542–55.
Sanacora G, Frye MA, McDonald WM, Mathew SJ, Turner MS, Schatzberg AF, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74:399–405.
McIntyre RS, Rosenblat JD, Nemeroff CB, Sanacora G, Murrough JW, Berk M, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry. 2021;Mar 17 [epub ahead of print].
Zarate CAJ. Commentary on the Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the use of racemic ketamine in adults with major depressive disorder. Can J Psychiatry. 2021;March 19 [epub ahead of print].
Morris PJ, Moaddel R, Zanos P, Moore CE, Gould TD, Zarate CAJ, et al. Synthesis and N-methyl-d-aspartate (NMDA) receptor activity of ketamine metabolites. Org Lett. 2017;19:4572–5.
Zhang JC, Li SX, Hashimoto K. R(-)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–41.
Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:
Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi J-I, Hashimoto K, et al. Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther. 2017;361:9–16.
Leal GC, Bandeira I, Correia-Melo FS, Telles M, Mello RP, Vieira F, et al. Intravenous arketamine for treatment resistant depression: open-label pilot study. Eur Arch Psychiatry Clin Neurosci. 2021;271:577–82.
Zanos P, Thompson SM, Duman RS, Zarate CA Jr, Gould TD. Convergent mechanisms underlying rapid antidepressant action. CNS Drugs. 2018;32(3):197–227.
Zanos P, Highland JN, Stewart BW, Georgiou P, Jenne CE, Lovett J, et al. (2R,6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions. Proc Natl Acad Sci USA. 2019;116:6441–50.
Lumsden EW, Troppoli TA, Myers SJ, Zanos P, Aracava Y, Kehr J, et al. Antidepressant-relevant concentrations of the ketamine metabolite (2R,6R)-hydroxynorketamine do not block NMDA receptor function. Proc Natl Acad Sci USA. 2019;116:5160–9.
Lauterbach EC. Dextromethorphan as a potential rapid-acting antidepressant. Med Hypotheses. 2011;76(5):717–9.
Lauterbach EC. An extension of hypotheses regarding rapid-acting, treatment-refractory, and conventional antidepressant activity of dextromethorphan and dextrorphan. Med Hypotheses. 2012;78(6):693–702.
Lee SY, Chen SL, Chang YH, Chen SH, Chu CH, Huang SY, et al. The DRD2/ANKK1 gene is associated with response to add-on dextromethorphan treatment in bipolar disorder. J Affect Disord. 2012;138:295–300.
Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR. Pharmacology of dextromethorphan: relevance to dextromethorphan/quinidine (Nuedexta) clinical use. Pharmacol Ther. 2016;164:170–82.
Messias E, Everett B. Dextromethorphan and quinidine combination in emotional lability associated with depression: a case report. Prim Care Companion CNS Disord. 2012;14:PCC.12I01400.
Kelly TF, Lieberman DZ. The utility of the combination of dextromethorphan and quinidine in the treatment of bipolar II and bipolar NOS. J Affect Disord. 2014;167:333–5.
Murrough JW, Wade E, Sayed S, Ahle G, Kiraly DD, Welch A, et al. Dextromethorphan/quinidine pharmacotherapy in patients with treatment-resistant depression: a proof of concept trial. J Affect Disord. 2017;218:277–83.
Axsome Therapeutics. Axsome Therapeutics announces AXS-05 achieves primary endpoint in Phase 2 trial in major depressive disorder. January 7, 2019. 2019 [cited; https://www.globenewswire.com/news-release/2019/01/07/1681055/0/en/Axsome-Therapeutics-Announces-AXS-05-Achieves-Primary-Endpoint-in-Phase-2-Trial-in-Major-Depressive-Disorder.html. Accessed 31 July 2020.
The Pharmaletter. Axsome sees mixed top-line Ph III results with AXS-05 in TR depression. March 31, 2020. https://www.thepharmaletter.com/article/axsome-sees-mixed-top-line-ph-iii-results-with-axs-05-in-tr-depression. Accessed 31 July 2020.
Bernstein G, Davis K, Mills C, Wang L, McDonnell M, Oldenhof J, et al. Characterization of the safety and pharmacokinetic profile of D-methadone, a novel N-methyl-d-aspartate receptor antagonist in healthy, opioid-naive subjects: results of two Phase 1 studies. J Clin Psychopharmacol. 2019;39:226–37.
Fogaca MV, Fukumoto K, Franklin T, Liu R-J, Duman CH, Vitolo OV, et al. N-methyl-d-aspartate receptor antagonist d-methadone produces rapid, mTORC1-dependent antidepressant effects. Neuropsychopharmacology. 2019;44:2230–8.
Moryl N, Tamasdan C, Tarcatu D, Thaler HT, Correa D, Steingart R, et al. A phase I study of d-methadone in patients with chronic pain. J Opioid Manag. 2016;12:47–55.
Hagler G. BioSpace: Relmada Therapeutics developing REL-1017 to treat major depression. April 21, 2020. https://www.biospace.com/article/relmada-developing-rel-1017-to-treat-major-depression/. Accessed 31 July 2020.
Relmada Therapeutics I. Relmada Therapeutics announces top-line results from REL-1017 Phase 2 study in individual with treatment resistant deprssion. October 15, 2019. https://www.prnewswire.com/news-releases/relmada-therapeutics-announces-top-line-results-from-rel-1017-phase-2-study-in-individuals-with-treatment-resistant-depression-300938577.html. Accessed 31 July 2020.
Nagele P, Duma A, Kopec M, Gebara MA, Parsoei A, Walker M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. 2015;78:10–8.
Zarate CA, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, et al. A randomized trial of a low-trapping nonselective N-methyl-d-aspartate channel blocker in major depression. Biol Psychiatry. 2013;74:257–64.
Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ, et al. Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry. 2014;19(9):978–85.
Sanacora G, Johnson MR, Khan A, Atkinson SD, Riesenberg RR, Schronen JP, et al. Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and history of inadequate response to antidepressants: a randomized, placebo-controlled study. Neuropsychopharmacology. 2017;42(4):844–53.
Wilkinson ST, Sanacora G. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov Today. 2019;24:606–15.
Millan MJ. N-methyl-d-aspartate receptor-coupled glycineB receptors in the pathogenesis and treatment of schizophrenia: a critical review. Curr Drug Targets CNS Neurol Disord. 2002;1(2):191–213.
Kantrowitz JT, Milak MS, Mao X, Shungu DC, Mann JJ. d-Cycloserine, an NMDA glutamate receptor glycine site partial agonist, induces acute increases in brain glutamate plus glutamine and GABA comparable to ketamine. Am J Psychiatry. 2016;173:1241–2.
Heresco-Levy U, Javitt DC, Gelfin Y, Gorelik E, Bar M, Blanaru M, et al. Controlled trial of d-cycloserine adjuvant therapy for treatment-resistant major depressive disorder. J Affect Disord. 2006;93(1–3):239–43.
Heresco-Levy U, Gelfin G, Bloch B, Levin R, Edelman S, Javitt DC, et al. A randomized add-on trial of high-dose d-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol. 2013;16(3):501–6.
Kantrowitz JT, Halberstam B, Gangwisch J. Single-dose ketamine followed by daily d-Cycloserine in treatment-resistant bipolar depression. J Clin Psychiatry. 2015;76(6):737–8.
NeuroRx. NeuroRx: Phase 3 drug for suicidal bipolar depression to present at BIO CEO conference. February 10, 2020. 2020. https://www.prnewswire.com/news-releases/neurorx-phase-3-drug-for-suicidal-bipolar-depression-to-present-at-bio-ceo-conference-301001882.html. Accessed 12 Aug 2020.
Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM. Randomized proof of concept trial of GLYX-13, an N-methyl-d-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015;21(2):140–9.
Allergan. Allergan announces Phase 3 results for rapastinel as an adjunctive treatment of major depressive disorder (MDD). 2019.
Fasipe OJ. The emergence of new antidepressants for clinical use: agomelatine paradox versus other novel agents. IBRO Reports. 2019;6:95–110.
Huang C-C, Hua Wei I, Huang C-L, Chen K-T, Tsai M-H, Tsai P, et al. Inhibition of glycine transporter-I as a novel mechanism for the treatment of depression. Biol Psychiatry. 2013;74:734–41.
Park LT, Kadriu B, Gould TD, Zanos P, Greenstein D, Evans JW, et al. A randomized trial of the N-methyl-d-aspartate receptor glycine site antagonist prodrug 4-chlorokynurenine in treatment-resistant depression. Int J Neuropsychopharmacol. 2020;Mar 31 [epub ahead of print].
Patel W, Rimmer L, Smith M, Moss L, Smith MA, Snodgrass HR, et al. Probenecid increases the concentration of 7-chlorokynurenic acid derived from the prodrug 4-chlorokynurenine within the prefrontal cortex. Mol Pharm. 2021;18:113–23.
Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-d-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–7.
Ibrahim L, Diazgranados N, Jolkovsky L, Brutsche N, Luckenbaugh D, Herring WJ, et al. A randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol. 2012;32:551–7.
Paterson B, Fraser H, Wang C, Marcus R. A randomized, double-blind, placebo-controlled, sequential parallel study of CERC-301 in the adjunctive treatment of subjects with severe depression and recent active suicidal ideation despite antidepressant treatment. National Network of Depression Centers Annual Conference; 2015 November 5-6; Ann Arbor, Michigan; 2015. http://c.eqcdn.com/_2a1b537df6a6e1398b6806c9b30635ad/cerecor/db/322/603/file/Poster+-+Study+Clin301-201_FINAL.pdf. Accessed 12 June 2020.
Quiroz JA, Tamburri P, Deptula D, Banken L, Beyer U, Rabbia M, et al. Efficacy and safety of basimglurant as adjunctive therapy for major depression: a randomized clinical trial. JAMA Psychiatry. 2016;73:675–84.
Chaki S. mGlu2/3 receptor as a novel target for rapid acting antidepressants. Adv Pharmacol. 2020;89:289–309.
Umbricht D, Niggli M, Sanwald-Ducray P, Deptula D, Moore R, Grünbauer W, et al. Randomized, double-blind, placebo-controlled trial of the mGlu2/3 negative allosteric modulator decoglurant in partically refractory major depressive disorder. J Clin Psychiatry. 2020;81:18m12470.
Campo B, Kalinichev M, Lambeng N, El Yacoubi M, Royer-Urios I, Schneider M, et al. Characterization of an mGluR2/3 negative allosteric modulator in rodent models of depression. J Neurogenet. 2011;25:152–66.
Pothula S, Liu R-J, Wu M, Sliby AN, Picciotto MR, Banerjee P, et al. Positive modulation of NMDA receptors by AGN-241751 exerts rapid antidepressant-like effects via excitatory neurons. Neuropsychopharmacology. 2020;Oct 15 [online ahead of print].
Sengupta S, Giaime E, Narayan S, Hahm S, Howell J, O’Neill D, et al. Discovery of NV-5138, the first selective Brain mTORC1 activator. Sci Rep. 2019;9:4107.
Hasegawa Y, Zhu X, Kamiya A. NV-5138 as a fast-acting antidepressant via direct activation of mTORC1 signaling. J Clin Invest. 2019;129:2207–9.
Kato T, Pothula S, Liu R-J, Duman CH, Terwilliger R, Vlasuk GP, et al. Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mTORC1 activation. J Clin Invest. 2019;129:2542–54.
Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232(4753):1004–7.
Kleinman RA, Schatzberg AF. Understanding the clinical effects and mechanisms of action of neurosteroids. Am J Psychiatry. 2021;178:221–3.
Frieder A, Fersh M, Hainline R, Deligiannidis KM. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33:265–82.
Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480–9.
Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058–70.
Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, Riesenberg R, Sankoh AJ, et al. Trial of SAGE-217 in patients with major depressive disorder. N Engl J Med. 2019;381:903–11.
Sage Therapeutics. Sage announces pivotal Phase 3 trial status for SAGE-217 in major depressive disorder and postpartum depression based on FDA breakthrough therapy meeting. 2018. https://investor.sagerx.com/news-releases/news-release-details/sage-announces-pivotal-phase-3-trial-status-sage-217-major. Accessed 12 June 2020.
Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, et al. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry. 2017;81:886–97.
Fogaca MV, Duman RS. Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interverntions. Front Cell Neurosci. 2019;13:87.
Ballard ED, Yarrington JS, Farmer CA, Lener MS, Kadriu B, Lally N, et al. Parsing the heterogeneity of depression: an exploratory factor analysis across commonly used depression rating scales. J Affect Disord. 2018;231:51–7.
Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–7.
Williams AV, Trainor BC. The impact of sex as a biological variable in the search for novel antidepressants. Front Neuroendocrinol. 2018;50:107–17.
Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF, et al. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs. 2014;23:243–54.
Acknowledgements
The authors thank the 7SE research unit and staff for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Funding for this work was provided by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIAMH002857). The work was completed as part of the authors’ official duties as Government employees. The views expressed do not necessarily reflect the views of the NIH, the Department of Health and Human Services, or the United States Government.
Conflicts of interest
Dr Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the US government but will share a percentage of any royalties that may be received by the government. All other authors have no conflict of interest to disclose, financial or otherwise.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Authors’ contributions
All authors contributed equally to the literature search, generation of the table and figure, writing, and revision of this manuscript. All authors approved the final version of the paper.
Rights and permissions
About this article
Cite this article
Henter, I.D., Park, L.T. & Zarate, C.A. Novel Glutamatergic Modulators for the Treatment of Mood Disorders: Current Status. CNS Drugs 35, 527–543 (2021). https://doi.org/10.1007/s40263-021-00816-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-021-00816-x