Abstract
Background and Objective
Since the initial coronavirus disease outbreak in late 2019 (COVID-19), reverse-transcription real-time polymerase chain reaction (RT-qPCR) has become the gold standard test to detect severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2). However, a more sensitive and accurate diagnostic tool was required. Therefore, droplet digital polymerase chain reaction (ddPCR) was suggested as an alternative method. Here, we evaluated the performance of ddPCR to detect SARS-CoV-2 and compared it to the performance of RT-qPCR.
Methods
The analytical performances, including limit of blank and limit of detection, were established using positive and negative SARS-CoV-2 reference materials. A total of 366 RNA extracts (173 positive and 193 negative by RT-qPCR) were collected from four institutions and tested with a Bio-Rad SARS-CoV-2 ddPCR kit that detects the SARS-CoV-2 genome using primers for N1 and N2.
Results
Limit of blank was set at 0, and the limits of detection of N1 and N2 were 1.99 copies/μL and 5.18 copies/μL, respectively. Linearity was evaluated using serial dilution samples, which demonstrated good results (R2: 0.999, linear range: 5.88–6825.25 copies/μL for N1 and R2: 0.999, 5.53–5855.47 copies/μL for N2). The results of ddPCR and RT-qPCR revealed substantial agreement (Cohen’s kappa: 0.639, p < 0.01). The 63 samples with positive ddPCR but negative RT-qPCR showed low copy numbers, and 55% of them had COVID-19-related symptoms.
Conclusions
Droplet digital polymerase chain reaction demonstrated excellent sensitivity for SARS-Cov-2 detection and consistently agreed with the results from conventional RT-qPCR. Furthermore, ddPCR provided quantitative data that can be used to monitor changes in the viral load of patients with COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Since severe acute respiratory syndrome-related coronavirus 2 was spread globally, reverse-transcription real-time polymerase chain reaction became a common diagnostic tool for diagnosing coronavirus disease 2019 in many countries. |
However, some issues such as false-negative results by reverse-transcription real-time polymerase chain reaction necessitated a more sensitive method. |
A droplet digital polymerase chain reaction, which has progressed and become available recently, was highlighted as an alternative method by some studies because of its superior analytical sensitivity and quantification performance. |
1 Introduction
Coronavirus disease appeared in late 2019 (COVID-19) and spread globally to become a pandemic by 2020. COVID-19 is the disease caused by infection with severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2), which belongs to the genus Betacoronavirus. This infectious disease is characterized by typical symptoms of other coronavirus infections, such as fever, cough, fatigue, pneumonia, and diarrhea. COVID-19 also often causes neurologic symptoms including the loss of taste or smell, headache, and muscle or joint pain. COVID-19 is mainly spread through breathing, and is very highly infective [1,2,3,4]. Therefore, rapid diagnosis and patient isolation are both very important to control the pandemic.
The current diagnosis of COVID-19 depends on nucleic acid amplification tests, and recently, many new methods such as loop-mediated isothermal amplification or CRISPR-based assays were developed. The serologic tests using the antigen/antibody of SARS-CoV-2 was also introduced and used for specific purpose or limited circumstance [5,6,7]. In particular, reverse-transcription quantitative real-time polymerase chain reaction (RT-qPCR) to identify the target gene of SARS-CoV-2 is a common diagnostic method performed from the early stage of the pandemic in many clinical laboratories. SARS-CoV-2 is a single positive-strand RNA virus consisting of ten open reading frames (ORFs) [8]. There are many types of RT-qPCR targeting the ORF1ab, RNA-dependent RNA polymerase (RdRp), Spike (S), Envelope (E), and/or Nucleocapsid (N) genes of SARS-CoV-2 [9,10,11,12]. Conventionally, RT-qPCR is a validated, sensitive molecular diagnostic tool that is used to detect infectious pathogens. However, during massive testing for COVID-19, several studies discussed the concern of false-negative results using RT-qPCR [5, 13,14,15,16]. Therefore, more sensitive and accurate diagnostic tools are required. A few studies have suggested the use of droplet digital polymerase chain reaction (ddPCR) as an alternative diagnostic method to RT-qPCR [17, 18]. Droplet digital polymerase chain reaction is a precise molecular diagnostic method that can quantify nucleic acid without a standard curve, which is necessary for quantification with RT-qPCR. In the past, ddPCR was evaluated for detecting viral pathogens such as cytomegalovirus, hepatitis B virus, or mutant influenza virus [19,20,21]. More recently, ddPCR has been found to be useful for detecting SARS-CoV-2 in various conditions and situations [22,23,24,25,26].
In this study, we evaluated the analytical and clinical performance of ddPCR for detecting SARS-CoV-2. We analyzed the detection capability, precision, and linearity of ddPCR, and compared the results of clinical samples with those from RT-qPCR. We also obtained quantitative results from ddPCR and studied the changes in the viral load of patients with COVID-19 during treatment.
2 Methods and Materials
2.1 Clinical Sample Collection and Preparation
A total of 366 clinical samples were used from four medical institutions including Seoul St. Mary’s hospital (CMC1), Incheon St. Mary’s hospital (CMC2), Uijeongbu St. Mary’s Hospital (CMC3), and Samkwang Medical Laboratories. The extracted RNA samples were collected after a COVID-19 RT-qPCR test from 330 nasopharyngeal (NP) swabs, 28 sputum samples, and eight blood samples between March 2020 and June 2020 (Table 1). Patient medical records, including their clinical findings and subsequent laboratory and radiologic results, were reviewed in cases in which there were discrepant results between RT-qPCR and ddPCR. This study was approved by the Institutional Review Board (IRB) of the Catholic Medical Center, Seoul, Republic of Korea, and by the IRB of Samkwang Medical Laboratories. The requirement for informed consent was waived (IRB No. XC20SIDE0109, IRB No. S-IRB-2020-016-06-30).
2.2 Materials for Performance Evaluation
According to the CLSI document EP17-A2 [27], limit of blank (LOB) was defined as the highest observable measurement result assumed to be found when reproductions of a blank sample are examined with a probability of 0.05 of falsely claiming its presence. Limit of detection (LOD) was defined as the lowest concentration of analyte that can be consistently detected in ≥ 95% of samples tested. AccuPlex™ SARS-CoV-2 reference material (Cat. No. 0505-0126, Batch Number 10483977; Seracare, Milford, MA, USA) was used to evaluate these detection performances of ddPCR. It contains positive reference material with 5203 copies/mL of SARS-CoV-2 genomic RNA including ORF1a, RdRp, E and N genes, and negative reference material with 3900 copies/mL of human RNase P. The LOB was determined after measuring the negative reference material 20 times. For the LOD analysis, we prepared six concentrations of each 120 μL through a serial four-fold dilution of the positive reference material with the negative reference material (1:1, 1:4, 1:16, 1:32, 1:256, 1:1024). The LOD was calculated with a probit analysis by measuring the diluted materials 10–13 times. Linearity and reproducibility were evaluated using pooled clinical samples. We selected five positive samples with high copy values (34,465.88–44,269.68 copies/μL of N1, 31,222.2–41,391.68 copies/μL of N2). After pooling the samples, we diluted these with the negative samples serially at five concentrations (1:10, 1:100, 1:1,000, 1:10,000, 1:100,000). Each dilution was prepared and stored as ten aliquots initially, and two tests were performed every day for each concentration.
2.3 RT-qPCR for SARS-CoV-2
Four types of RT-qPCR were used at each institution (Table 1). CMC1 used the alphaPrep™ Viral DNA/RNA Extraction Mini-Kit (Cat. No. VID-C110, VID-C120; AlphaGene, Seongnam-si, Republic of Korea) on a NC15-PLUS instrument (Cat. No. HWTD-01-32, HWTD-01-48; Hanwool TPC Co., Bucheon-si, Republic of Korea) for RNA extraction. These were tested using the PowerChek™ 2019-nCoV Real-Time PCR Kit (Cat. No. R6900TD; KogeneBiotech, Seoul, Republic of Korea; PowerChek kit), Allplex™ 2019-nCoV Assay (Cat. No. RP10243X/RP10252W; Seegene, Seoul, Republic of Korea; Allplex Kit), or Real-Q 2019-nCoV Detection Kit (Cat. No. BS7nCoV; BioSewoom, Seoul, Republic of Korea; Real-Q kit). CMC2 and CMC3 used the AdvanSure Nucleic Acid R Kit on the AdvanSure E3 System (Cat. No. YETS0001EG; Cat. No. YSTP0500KG [Strip]; LG Chem, Republic of Seoul, Korea) for RNA extraction, and were tested using the PowerChek Kit (KogeneBiotech) or STANDARD M nCoV Real-Time Detection Kit (Cat. No. M-NCOV-01; SD Biosensor Inc., Suwon-si, Republic of Korea; STANDARD M kit). Samkwang Medical Laboratories used the NX-48 Viral NA Kit (Cat. No. VN101, VN111, VN121; Genolution, Seoul, Republic of Korea) on a Nextractor NX-48 instrument (Cat. No. NX-48; Genolution), and were tested using the PowerChek Kit (KogeneBiotech). The PowerChek, Real-Q, and STANDARD M Kits target the RdRp and E genes of SARS-CoV-2. The Allplex Kit also targets the RdRp, E, and N genes. All RT-qPCR tests were performed according to the manufacturer’s instructions and analyzed on a CFX96 Real-Time PCR with a C1000 thermal cycler (Cat. No. 185-5096; Bio-Rad, Hercules, CA, USA). The collected RT-qPCR results included qualitative results as positive, indeterminate, or negative with a quantification cycle (Cq) value for each target gene. The detail protocols were described in Table S1 of the Electronic Supplementary Material (ESM).
2.4 ddPCR for SARS-CoV-2
We described the details and protocol of ddPCR according to the dMIQE2020 guideline [28]. Droplet digital polymerase chain reaction for SARS-CoV-2 was performed using the Bio-Rad SARS-CoV-2 ddPCR Kit (Cat. No. 12008202, Cat. No. 1864021, Cat. No. COV019, and Cat. No. COV000) and the QX200 ddPCR system (Bio-Rad). The kit includes two primer sets (N1, N2) reported by the Centers for Disease Control and Prevention [29] for SARS-CoV-2 detection, and one primer set for the human RNase P gene as the internal control. The tests were performed according to the manufacturer’s instructions for use as detailed: based on one sample, 16.5 μL of the RT Master Mix (which consists of 5.5 μL of Supermix, 2.2 μL of reverse transcriptase, 1.1 μL of 300 mM dithiothreitol, 1.1 μL of 2019-nCoV CDC ddPCR Triplex Probe Assay, and 6.6 μL of RNase/DNase free water) was prepared. Next, the 5.5-μL template (RNA extract or reference material) was added to 16.5 μL of thr Master Mix in a well on a 96-well plate. The droplets were then generated by the QX200 Automated Droplet Generator. After droplet generation was completed, PCR amplification was performed using a GeneAmp™ PCR System 9700 (Cat. No. 4413750; Thermo Fisher Scientific, Waltham, MA, USA) as follows: 50 °C for 60 minutes, 95 °C for 10 minutes, 94 °C for 30 seconds, and 55 °C for 60 seconds repeated over 40 cycles, then 98 °C for 10 minutes, and 4 °C for 30 minutes. At every run, the ExactDx Standard and ExactDx Negative (Exact Diagnostics, Fort Worth, TX, USA) materials were included as external positive and negative controls.
Next, the amplified droplet plate was read using the QX200 Droplet Reader and analyzed with the QuantaSoft™ Analysis Pro 1.0.596.0525 (Bio-Rad). The data analysis process using QuantaSoft™ Analysis Pro is described in Fig. S1 of the ESM. The ddPCR results were interpreted as “positive” in cases of more than one positive droplet in either N1 or N2, according to the instructions for use. The quantitative result was presented initially as the copy value (copies/μL) of the reaction volume (22 μL), which was total volume of the Master Mix (16.5 μL) and template (5.5 μL). This initial copy value was automatically calculated from the proportion of positive droplets showing N1 or N2 probe fluorescence intensity over the threshold compared to the number of total counted droplets by QuantaSoft™ Analysis Pro software. This value was then multiplied by four to be converted to the copy value (copies/μL) of the template (5.5 μL), which reflects the nucleic acid concentration of the template, or RNA extract. This template copy value was used in this study.
2.5 Statistics
Linearity was evaluated using polynomial and linear regression methods based on four replicated test results from each concentrate, as the log transformation of the copy value. Precision was analyzed by calculating the mean, standard deviation, and percent coefficient of variation of the copy value of ten replicates in each concentrate. Before the analysis, the results that were 1.5 interquartile range above the third quartile or 1.5 interquartile range below the first quartile in each dilution group were recognized as outliers using the box-whiskers plot and excluded. In addition, we compared results from the ddPCR with those from RT-qPCR. The agreement and Cohen’s kappa were evaluated for qualitative results. Pearson’s correlation analysis and linear regression were performed and the Bland–Altman plot was presented to compare the quantitative results of the Cq value of RT-qPCR and the copy value of ddPCR. All of the statistical analyses were performed using SPSS Statistics 20 (IBM, Armonk, NY, USA) and Microsoft Excel 2019 (Microsoft, Redmond, WA, USA) software.
3 Results
3.1 Analytical Performance of ddPCR for SARS-CoV-2
Nineteen of 20 negative reference materials showed 0 copies/μL in both N1 and N2, except one that showed 0.09 copies/μL by one positive droplet in N1. Therefore, the LOBs of ddPCR for N1 and N2 were evaluated as 0 with 95% probability. The LODs were obtained from probit analysis by repeated tests of six serial dilutions. The LODs for N1 and N2 in the RNA extract were evaluated as 1.99 copies/μL (95% confidence interval [CI] 1.38–3.98 copies/μL) and 5.18 copies/μL (95% CI 3.33–12.93 copies/μL), respectively.
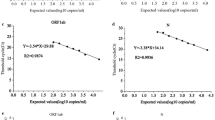
During linearity evaluation, the results of the first dilution (1:10) with very high copies were excluded because they could not be adjusted to the linear model (Fig. 1). Polynomial regression analysis demonstrated good correlation in both linear and cubic curve models for N1 (R2 = 0.9994 and 0.9995, p-value: 4.30 × 10-24 and 4.52 × 10-20, respectively) and N2 (R2 = 0.9986 and 0.9986, p-value: 2.22 × 10-21 and 2.02 × 10-17, respectively). Based on these results, the linearity ranges of the ddPCR copy values for measuring the N1 and N2 genomes were established as 5.88–6825.25 copies/μL for N1 and 5.53–5855.47 copies/μL for N2 in this study. The precision was evaluated by repeated tests of five serial dilutions and revealed acceptable percent coefficients of variation of the N1 and N2 copy values as 4.47–15.94% and 9.1–38.84%, respectively (Table S2 of the ESM).
Linearity evaluation of droplet digital polymerase chain reaction performance to measure N1 and N2. The horizontal axis is the dilution ratio of the pooled sample (1:x). The vertical axis is the log-scaled copy value. Regression equation was denoted with y, 10 log-scaled copy value (copies/mL), and x, dilution ratio. The linear model and cubic model of N1 (a) and N2 (b) was evaluated with four serial dilutions. The differences between the measured and predicted values in N1 (c) and N2 (d) was presented as the deviation plot with the log-scaled copy value unit (an allowable deviation from linearity was shown as 1% in a dotted line)
Because ddPCR presented the absolute copy value of SARS-CoV-2, we performed Pearson correlation and polynomial regression analyses to compare the quantitative results. The copies of N1 and N2 were excellently correlated (R: 0.966, p < 0.01), but differed within each sample. When comparing the agreement between N1 and N2, the positive percent agreement, negative percent agreement, and overall percent agreement were 90.86%, 74.44%, and 82.79%, respectively. Then, ten log-scaled copy values of N1 and N2 were compared with the Cq values of each target gene from RT-qPCR. Negative results (0 copies/μL, which could not be log-scale transformed) and outliers, including unmeasurable copy values (> 4,000,000 copies/μL) and results out of the linear range of ddPCR, were excluded from the analyses. The total counts of samples included in analysis and counts sorted by the original specimen type as NP swab/sputum were 67 (60/7), 65 (59/6), and 22 (17/5) in N1 to RdRp, E, and N, and 66 (59/7), 69 (62/7), and 24 (19/5) in N2.
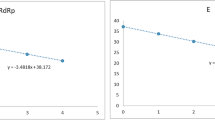
When comparing with results from RT-qPCR, the N1 and N2 copies showed a better correlation with the Cq of the N gene (R: − 964 and − 0.891) than they did with those of RdRp (R: − 0.941 and − 0.821) and E (R: − 0.931 and − 0.811) genes. According to the RT-qPCR kits, the PowerChek and Allplex kits were significantly correlated with N1 and N2 copies. In particular, the N2 copy was strongly correlated with the Cq values of the Allplex kit (− 0.975, − 0.940, and − 0.946 with RdRp, E, and N, respectively). The regression analysis also showed good linear relation between the Cq and copies, showing significant R2 to RdRp, E, and N (0.886, 0.866, and 0.928 with N1; 0.674, 0.658, and 0.794 with N2, p < 0.01 in all pairs). Bland–Altman plots with predicted Cq values from a regression equation using the copy value of ddPCR compared to a measured Cq value of RT-qPCR are presented in Fig. 2, where no markable systemic bias or difference was observed. Furthermore, most results were within the limit of agreement in both N1 and N2. However, plots of N2 showed a relatively wider range of limit of agreement (upper limit: 3.78–4.00, lower limit: − 4.00 ~ − 3.78) than that of N1 (upper limit: 1.78–2.22, lower limit: − 2.22 to − 1.78).
Scatter plot and linear regression of the copy value of droplet digital polymerase chain reaction (ddPCR) for the quantification cycle (Cq) of reverse-transcription real-time polymerase chain reaction (RT-qPCR) and Bland–Altman plots of the difference against the mean for measured and predicted Cq values. All positive RNA extract samples with definite Cq values and positive copy values within a linear range of ddPCR were analyzed. Log-scaled copy values (log copies/mL) of N1 (a–c) and N2 (d–f) were dotted for the Cq of each RT-qPCR target gene, RdRp, E, and N with paired Bland–Altman plots. The linear regression line, equation, and R2 are displayed on each graph. All pairs showed significant linear correlation (p < 0.01). In each Bland–Altman plots, the x-axis was the average of measured Cq values by RT-qPCR and predicted Cq values using linear regression function with the log-scaled copy value, and the y-axis was the difference of two values. The upper and lower limit of agreement was displayed with a horizontal dotted line, with the 95% confidence interval presented in square brackets. SD standard deviation
3.2 Clinical Performance of ddPCR for SARS-CoV-2
All of the clinical samples demonstrated positive results for the RNase P gene, which is an internal control of the ddPCR test. A total of 299 samples showed consistent results with RT-qPCR and ddPCR, as follows: positive RT-qPCR and ddPCR (RT(+)dd(+), n = 169) and negative RT-qPCR and ddPCR (RT(–)dd(–), n = 130). In contrast, there were discrepant results in 67 samples, as follows: positive RT-qPCR and negative ddPCR (RT(+)dd(–), (n = 4); and negative RT-qPCR and positive ddPCR (RT(–)dd(+), (n = 63). The positive percent agreement, negative percent agreement, and overall percent agreement, which were calculated between RT-qPCR and ddPCR, were 97.7%, 67.4%, and 81.7%, respectively. Cohen’s kappa coefficient (κ) was 0.639 (95% CI 0.601–0.677), which suggests substantial agreement. The detailed description with the sample distribution and interpretation in the subgroups is summarized in Table S3 of the ESM.
Next, we investigated the samples with discrepant results. The Cq values of RT-qPCR were high in the RT(+)dd(–) samples, referring to a low viral burden or indeterminate result. Likewise, the RT(–)dd(+) samples showed low copies of N1 and N2. The copies of the RT(–)dd(+) samples were not significantly different with RT(+)dd(–) (Table 2, Fig. S2 in the ESM9. Six RT(–)dd(+) samples came from COVID-19-infected patients after post-admission day #7. We reviewed the available medical records from 49 patients with RT(–)dd(+) who were screened for COVID-19 infection (Table S4 of the ESM). Twenty-seven patients (55.1%) presented with COVID-19-related symptoms [30] including fever, myalgia, sore throat, headache, vomiting and/or diarrhea. Thirty-nine patients (79.6%) were followed, and none was diagnosed or treated for COVID-19 infection.
3.3 Monitoring Viral Load by ddPCR
We monitored the viral load from eight patients who were treated for COVID-19 infection in CMC1. Most patients improved during treatment, except one whose symptoms worsened requiring treatment in the COVID-19 intensive care system. We found that the viral loads changed variably in each patient during follow-up (Fig. 3). In improved patients, the median viral loads of N1 and N2 in the NP swabs on the day of admission day were 1684.5 copies/μL and 1600.6 copies/μL, respectively. The copy values of the NP swabs decreased with each admission day. The median viral load of N1 and N2 at the last follow-up (post-admission day #12 to #27) before transfer to a residential treatment center were 0.8158 copies/μL and 1.4 copies/μL, respectively. However, the pattern of viral load improvement varied among patients. In contrast, the viral load did not decrease in patients with clinical progression. Results from sputum samples showed similar copies to those from NP swabs; however, they were not statistically analyzed because of the small number of tests.
Severe acute respiratory syndrome-related coronavirus 2 copy value changes of confirmed patients in follow-up test samples. The vertical axis reflects the copy value (copies/μL) and the horizontal axis reflects the post-admission days. In each graph, the droplet digital polymerase chain reaction test results were presented by full or empty dots (nasopharyngeal [NP] swab, N1, and N2) and squares (sputum, N1 and N2). Three patients, SCMC116, SCMC117, and SCMC118 (a–c) were improved and transferred to residential treatment centers. However, one patient, SCMC122 (d) worsened during hospital admission and was transferred to the COVID-19 intensive care system
4 Discussion
Droplet digital polymerase chain reaction is an ultra-sensitive quantitative molecular technique that is advantageous to other PCR-based methods given its resilience to inhibitors and its precision [31,32,33]. There were many studies for ddPCR to various targets, such as cytomegalovirus, hepatitis B virus, or norovirus quantification [19, 20, 32] and detecting a subpopulation of oseltamivir-resistant influenza A virus [21]. Human genome-like somatic mutation, copy number variation, or microRNA are also candidate targets of ddPCR [33,34,35,36], and in the field of detection and monitoring of environmental pathogens, ddPCR is getting attention from researchers [37]. Recently, SARS-CoV-2 has been studied as a target molecule of ddPCR. Studies using laboratory-developed primers and experimental protocols optimized for ddPCR have shown high sensitivity and specificity [17, 18, 22,23,24,25,26]. However, most studies have been conducted using a small number of clinical samples or reference materials. Thus, we tried to evaluate and compare based on large samples taken from several centers to obtain relatively statistically robust results.
In this study, the Bio-Rad SARS-CoV-2 ddPCR kit demonstrated excellent analytical performance including precision, linearity, and detection capability. This method is very highly specific and sensitive based on a definite LOB of 0 copies/μL and LODs of N1 and N2 of 2 and 5 copies/μL, respectively. These LOD values are also comparable to those of RT-qPCR kits (4.03–4.81 copies/μL) [38]. We also observed significant quantification results showing superior precision within linearity ranges of N1 and N2 as 6–6825 and 6–5855 copies/μL, respectively.
Droplet digital polymerase chain reaction and RT-qPCR showed substantial agreement. The copies of N1 and N2 were well correlated with each other, and with the Cq from RT-qPCR. We observed several cases in which there were divergent results between N1 and N2. This difference seemed to reflect various amounts of subgenomic RNA of SARS-Cov-2 according to the position on the viral genome, which was suggested by one SARS-CoV-2 transcriptome study [8]. The quantitative difference in the SARS-CoV-2 RNA expression by positions and out-of-frame recombinations of viral subgenomic RNA was revealed. These issues suggest that multiple genomic targets are recommended in nucleic acid amplification tests for SARS-CoV-2 to improve the analytical sensitivity and prevent false-negative results.
We also identified a considerable number of samples with discrepant qualitative results. These samples demonstrated a very low viral burden. Three of four RT(+)dd(−) samples were interpretated as “negative” in ddPCR according to the criteria of instructions for use, but one positive droplet was found in two samples (SC_185, SC_193) for N2, and in one sample (SC_205) for both N1 and N2. Because these four samples were taken from patients with confirmed COVID-19 during admission, it is reasonable that they were thought as true-positives with a low viral burden. One sample (SC_130) with no positive droplet in ddPCR was tested by the Real-Q RT-qPCR Kit, which targets RdRp and E genes, not the N gene. As a result, presence of the N gene was not confirmed by RT-qPCR, thus there were three possible explanations: no N gene component in the sample, or ddPCR failing to detect the low burden of the N gene, or RNA degradation due to the wrong storage condition. We investigated the data set and discovered two samples (SC_131, SC_132) of the same patient (SCMC117, described in Fig. 3b), taken on the same day (PAD#4), showed positive results in both RT-qPCR and ddPCR. These two samples were originated from sputum, unlike a previous RT(+)/dd(−) sample extracted from a NP swab. Three samples showed similar Cq value patterns, thus containing similar viral burdens was suggested; however, RNA degradation could not be excluded for a relatively low RP gene copy value of SC_130 (31.84 copies/μL) compared with SC_131 (357.04 copies/μL) and SC132 (316.08 copies/μL). This extreme difference of the RP gene copy value between the NP swab and sputum sample of the same day was not observed in samples of other days. However, no further investigation was made. Meanwhile, some RT(−)dd(+) samples were taken from patients with COVID-19 after improvement, while others were samples from patients who were screened for COVID-19 infection. Because none of these patients was diagnosed with COVID-19, we could not provide confident evidence of true COVID-19 infection. Nonetheless, considering the excellent sensitivity of ddPCR, we suspected that some of these cases reflected asymptomatic carrier status with a very low viral burden rather than false-positives. Previous studies have demonstrated that asymptomatic carriers with COVID-19 have a lower viral load than do symptomatic patients, even though they still have a certain period of viral shedding [39,40,41]. Further long-term surveillance is needed in these asymptomatic cases using virus nucleic acid testing. The characteristics of the N gene, which is the most abundantly expressed gene in SARS-Cov-2 transcription [8] that persists for the longest period of time (compared with other genes in patients with COVID-19) [42], enhanced the possibility of true-positive results in this study.
In this study, we monitored the viral load of eight patients with COVID-19 and found that various changes occurred with declining viral loads during improvement. Reverse-transcription real-time polymerase chain reaction did not provide quantitative results. Therefore, these variably declining viral load patterns are not very informative during treatment. Sometimes, the results are converted to positive after becoming negative. The quantitative ddPCR data can provide serial changes in the viral burden to monitor patients with COVID-19. We expect that further studies that accumulate monitoring data will provide a guide for optimal follow-up.
4.1 Limitations of this Study
Although we analyzed a large number of clinical samples, this study has several limitations. First, although this study was a focused comparison of RT-qPCR and ddPCR, we used commercial kits so we could not customize primers or other reagents, and necessary information about the experimental materials provided by kits was not obtained enough, such as primer sequences. Furthermore, because of the different input template volumes (4.5–10 µL) of each method, it was impossible to fairly compare detection performance, including LOD, between each method. Second, the influence of the nucleic acid extraction process by different kits and instruments and long-term preservation of the RNA extract on ddPCR could not be fully evaluated because we used remaining RNA samples. Additionally, quantification of RT-qPCR results using known standards simultaneously with experiments to compare directly with the results of ddPCR was impossible because of the limitation of a retrospective study design. Third, we did not have access to all of the follow-up data from patients with COVID-19 because they were transferred to residential treatment centers after hospital discharge. A fourth limitation is that the significance of RT(−)dd(+) cases was not fully elucidated. The question of whether these results were false-positives or true-positives from asymptomatic carriers must be addressed.
5 Conclusions
Droplet digital polymerase chain reaction demonstrated excellent sensitivity for SARS-Cov-2 detection and sustained agreement with results from conventional RT-qPCR. Furthermore, ddPCR provided quantitative data, which can be used to monitor changes in the viral load over time in patients with COVID-19. Our data support the use of ddPCR for early viral detection from close contact individuals, massive screening, and environmental sample inspection, and to confirm indeterminate results from RT-qPCR. Nevertheless, further studies are needed to evaluate the utility of this test in determining pre-symptomatic or asymptomatic states, and assessing their clinical significance.
Change history
18 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s40291-021-00557-z
References
Tsatsakis A, Calina D, Falzone L, Petrakis D, Mitrut R, Siokas V, et al. SARS-CoV-2 pathophysiology and its clinical implications: an integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem Toxicol. 2020;146:111769.
Pennisi M, Lanza G, Falzone L, Fisicaro F, Ferri R, Bella R. SARS-CoV-2 and the nervous system: from clinical features to molecular mechanisms. Int J Mol Sci. 2020;21(15):5475.
Vallamkondu J, John A, Wani WY, Ramadevi SP, Jella KK, Reddy PH, et al. SARS-CoV-2 pathophysiology and assessment of coronaviruses in CNS diseases with a focus on therapeutic targets. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10):165889.
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–93.
Jing R, Kudinha T, Zhou ML, Xiao M, Wang H, Yang WH, et al. Laboratory diagnosis of COVID-19 in China: a review of challenging cases and analysis. J Microbiol Immunol Infect. 2021;54(1):17–26.
Lai CKC, Lam W. Laboratory testing for the diagnosis of COVID-19. Biochem Biophys Res Commun. 2021;538:226–30.
Falzone L, Gattuso G, Tsatsakis A, Spandidos DA, Libra M. Current and innovative methods for the diagnosis of COVID-19 infection (review). Int J Mol Med. 2021;47(6):100.
Kim D, Lee JY, Yang JS, Kim JW, Kim VN, Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181(4):914–21.
Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–9 ([published correction appears in Nature. 2020 Dec;588(7839):E35]).
Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR [published correction appears in Euro Surveill. Euro Surveill. 2020;25(3):45 ([published correction appears in Euro Surveill. 2020 Jul;25(30):]).
Nalla AK, Casto AM, Huang MW, Perchetti GA, Sampoleo R, Shrestha L, et al. Comparative performance of SARS-CoV-2 detection assays using seven different primer-probe sets and one assay kit. J Clin Microbiol. 2020;58(6):e00557-e620.
Chan JF, Yip CC, To KK, Tang TH, Wong SC, Leung K, et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020;58(5):e00310-e320.
Wang X, Yao H, Xu X, Zhang P, Zhang M, Shao J, et al. Limits of detection of 6 approved RT-PCR kits for the novel SARS-coronavirus-2 (SARS-CoV-2). Clin Chem. 2020;66(7):977–9.
Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20(5):453–4.
Chen L, Lou J, Bai Y, Wang M. COVID-19 disease with positive fecal and negative pharyngeal and sputum viral tests. Am J Gastroenterol. 2020;115(5):790.
Li D, Wang D, Dong J, Wang N, Huang H, Xu H, et al. False-negative results of real-time reverse-transcriptase polymerase chain reaction for severe acute respiratory syndrome coronavirus 2: role of deep-learning-based CT diagnosis and insights from two cases. Korean J Radiol. 2020;21(4):505–8.
Suo T, Liu X, Feng J, Guo M, Hu W, Guo D, et al. ddPCR: a more accurate tool for SARS-CoV-2 detection in low viral load specimens. Emerg Microbes Infect. 2020;9(1):1259–68.
Alteri C, Cento V, Antonello M, Colagrossi L, Merli M, Ughi N, et al. Detection and quantification of SARS-CoV-2 by droplet digital PCR in real-time PCR negative nasopharyngeal swabs from suspected COVID-19 patients. PLoS ONE. 2020;15(9):e0236311.
Hayden RT, Gu Z, Ingersoll J, Abdul-Ali D, Shi L, Pounds S, et al. Comparison of droplet digital PCR to real-time PCR for quantitative detection of cytomegalovirus. J Clin Microbiol. 2013;51(2):540–6.
Tang H, Cai Q, Li H, Hu P. Comparison of droplet digital PCR to real-time PCR for quantification of hepatitis B virus DNA. Biosci Biotechnol Biochem. 2016;80(11):2159–64.
Taylor SC, Carbonneau J, Shelton DN, Boivin G. Optimization of Droplet Digital PCR from RNA and DNA extracts with direct comparison to RT-qPCR: clinical implications for quantification of oseltamivir-resistant subpopulations. J Virol Methods. 2015;224:58–66.
Yu F, Yan L, Wang N, Yang S, Wang L, Tang Y, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71(15):793–8.
Falzone L, Musso N, Gattuso G, Bongiorno D, Palermo CI, Scalia G, et al. Sensitivity assessment of droplet digital PCR for SARS-CoV-2 detection. Int J Mol Med. 2020;46(3):957–64.
de Kock R, Baselmans M, Scharnhorst V, Deiman B. Sensitive detection and quantification of SARS-CoV-2 by multiplex droplet digital RT-PCR. Eur J Clin Microbiol Infect Dis. 2021;40(4):807–13.
Jiang Y, Wang H, Hao S, Chen Y, He J, Liu Y, et al. Digital PCR is a sensitive new technique for SARS-CoV-2 detection in clinical applications. Clin Chim Acta. 2020;511:346–51.
Vasudevan HN, Xu P, Servellita V, Miller S, Liu L, Gopz A, et al. Digital droplet PCR accurately quantifies SARS-CoV-2 viral load from crude lysate without nucleic acid purification. Sci Rep. 2021;11(1):780.
CLSI. Evaluation of detection capability for clinical laboratory measurement procedures; approved guideline. 2nd ed. CLSI document EP17-A2. Wayne: Clinical and Laboratory Standard Institute; 2012.
Huggett JF, dMIQE Group. The digital MIQE guidelines update: minimum information for publication of quantitative digital PCR experiments for 2020. Clin Chem. 2020;66(8):1012–29.
Centers for Disease Control and Prevention. Research use only 2019: novel coronavirus (2019-nCoV) real-time RT-PCR primers and probes. 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html. Accessed 16 Jul 2021.
World Health Organization. WHO COVID-19: case definitions: updated in public health surveillance for COVID-19, published 16 December 2020. License: CC BY-NC-SA 3.0 IGO. Available from: https://apps.who.int/iris/handle/10665/337834. Accessed 16 Jul 2021.
Quan PL, Sauzade M, Brouzes E. dPCR: a technology review. Sensors (Basel). 2018;18(4):1271.
Persson S, Eriksson R, Lowther J, Ellström P, Simonsson M. Comparison between RT droplet digital PCR and RT real-time PCR for quantification of noroviruses in oysters. Int J Food Microbiol. 2018;284:73–83.
Hindson CM, Chevillet JR, Briggs HA, Gallichotte EN, Ruf IK, Hindson BJ, et al. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat Methods. 2013;10(10):1003–5.
Crimi S, Falzone L, Gattuso G, Grillo CM, Candido S, Bianchi A, et al. Droplet digital PCR analysis of liquid biopsy samples unveils the diagnostic role of hsa-miR-133a-3p and hsa-miR-375-3p in oral cancer. Biology (Basel). 2020;9(11):379.
Miotke L, Lau BT, Rumma RT, Ji HP. High sensitivity detection and quantitation of DNA copy number and single nucleotide variants with single color droplet digital PCR. Anal Chem. 2014;86(5):2618–24.
Uchiyama Y, Nakashima M, Watanabe S, Miyajima M, Taguri M, Miyatake S, et al. Ultra-sensitive droplet digital PCR for detecting a low-prevalence somatic GNAQ mutation in Sturge-Weber syndrome [published correction appears in Sci Rep. 2017 Jan 12;7:39897]. Sci Rep. 2016;6:22985.
Falzone L, Gattuso G, Lombardo C, Lupo G, Grillo CM, Spandidos DA, et al. Droplet digital PCR for the detection and monitoring of Legionella pneumophila. Int J Mol Med. 2020;46(5):1777–82.
Hur KH, Park K, Lim Y, Jeong YS, Sung H, Kim MN. Evaluation of four commercial kits for SARS-CoV-2 real-time reverse-transcription polymerase chain reaction approved by emergency-use-authorization in Korea. Front Med (Lausanne). 2020;7:521.
Duan S, Zhou M, Zhang W, Shen J, Qi R, Qin X, et al. Seroprevalence and asymptomatic carrier status of SARS-CoV-2 in Wuhan City and other places of China. PLoS Negl Trop Dis. 2021;15(1):e0008975.
Sohn Y, Jeong SJ, Chung WS, Hyun JH, Baek YJ, Cho Y, et al. Assessing viral shedding and infectivity of asymptomatic or mildly symptomatic patients with COVID-19 in a later phase. J Clin Med. 2020;9(9):2924.
Zhou R, Li F, Chen F, Liu H, Zheng J, Lei C, et al. Viral dynamics in asymptomatic patients with COVID-19. Int J Infect Dis. 2020;96:288–90.
Sung H, Roh KH, Hong KH, Seong M, Ryoo N, Kim HS, et al. COVID-19 Molecular testing in Korea: practical essentials and answers from experts based on experiences of emergency use authorization assays [published correction appears. Ann Lab Med. 2021;41(1):126–7 (Yoo, Sookwon [corrected to Ryu, Sook Won]]. Ann Lab Med. 2020;40(6):439–47).
Acknowledgments
We thank the members of Samkwang Medical Laboratories for supplying samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no specific funding for this work.
Conflicts of Interest/Competing Interests
Kyoung Bo Kim, Hayoung Choi, Gun Dong Lee, Jaewoong Lee, Seungok Lee, Yonggoo Kim, Sung-Yeon Cho, Dong-Gun Lee, and Myungshin Kim have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
This study was approved by the IRB of the Catholic Medical Center, Seoul, Korea, and by the IRB of Samkwang Medical Laboratories. The requirement for informed consent was waived (IRB No. XC20SIDE0109, IRB No. S-IRB-2020-016-06-30).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of data and material
The data that support the findings of this study are available on request from the corresponding author.
Code availability
Not applicable.
Authors’ contributions
KBK designed the study, performed the ddPCR tests, interpreted the results, analyzed the data statistically, and wrote the manuscript. HC designed the study, performed the ddPCR tests, and interpreted the results. GDL, JL, and SL designed the study and performed the RT-PCR tests, interpreted the results, collected the samples, and reviewed cases. MK designed the study, analyzed the data statistically, reviewed the cases, and wrote the manuscript.
Additional information
The original online version of this article was revised due to a retrospective Open Access order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kim, K.B., Choi, H., Lee, G.D. et al. Analytical and Clinical Performance of Droplet Digital PCR in the Detection and Quantification of SARS-CoV-2. Mol Diagn Ther 25, 617–628 (2021). https://doi.org/10.1007/s40291-021-00547-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-021-00547-1