Abstract
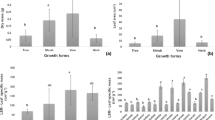
Lianescent aroids grow horizontally while on the ground (shaded, humid habitats), but switch to vertical growth when they reach a host (exposed, desiccant habitats). There are three morphotypes with respect to leaf adjustment to canopy conditions: isomorphic, allomorphic, and heteromorphic. Allomorphs and heteromorphs exhibit marked leaf modifications (e.g., leaf area) that have a strategic effect on the plant’s carbon balance, since they increase its light foraging capacity in the canopy. Isomorphs do not show external leaf morphology modifications. Therefore, we hypothesized that leaf anatomy and physiology change instead, allowing their light foraging capacity to improve while they grow towards the canopy. We evaluated leaf area, lamina-specific mass, mesophyll anatomy, venation density and thickness, stomatal density, chlorophyll concentration and fluorescence in leaves of the isomorph Philodendron hederaceum, grown to three heights (terrestrial, 1.5 and 3 m). Stomatal density, chlorophyll a content, and electron transport rate were significantly higher in the canopy leaves (60, 54, and 50%, respectively). Thus, P. hederaceum exhibits leaf adjustments that enhance its light foraging capacity. However, these changes are relatively less marked than those exhibited by sympatric heteromorphs and allomorphs. Although the canopy leaves of P. hederaceum exhibited a limited improvement in light foraging capacity, the species’ construction and maintenance costs are expected to be lower than those of its sympatric heteromorphs and allomorphs. Future studies that consider leaf lifespan will add another perspective for understanding the costs and benefits of these growth habits in aroid vines.




Similar content being viewed by others
References
Ackerly DD (1992) Light, leaf age, and leaf nitrogen concentration in a tropical vine. Oecologia 89:596–600
Avalos G, Mulkey SS (1999) Photosynthetic acclimation of the liana Stimaphyllon lindenianum to light changes in a tropical dry forest canopy. Oecologia 120:475–484.
BFG—The Brazil Flora Group (2015) Growing knowledge: an overview of seed plant diversity in Brazil. Rodriguésia 66:1085–1113
Box GEP, Lucas HL (1959) Design of experiments in non-linear situations. Biometrika 46:77–90
Brodribb TJ, Feild TS, Jordan GJ (2007) Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Phys 144:1890–1898
Carins Murphy MR, Jordan GJ, Brodribb TJ (2012) Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant Cell Environ 35:1407–1418
Carins Murphy MR, Jordan GJ, Brodribb TJ (2014) Acclimation to humidity modifies the link between leaf size and the density of veins and stomata. Plant Cell Environ 37:124–131
Coelho MAN (2010) A família Araceae na Reserva Natural Vale, Linhares, Espírito Santo, Brasil. Bol Museu Biol Mello Leitão 28:41–87
Day JS (1998) Light conditions and the evolution of heteroblasty (and the divaricate form) in New Zealand. N Z J Ecol 22:43–54
Fetene M, Nauke P, Lüttge U, Beck E (1997) Photosynthesis and photoinhibition in a tropical alpine giant rosette plant, Lobelia rhychopetalum. New Phytol 137:453–461
Filartiga AL, Vieira RC, Mantovani A (2014) Size-correlated morpho-physiology of the aroid vine Rhodospatha oblongata along a vertical gradient in a Brazilian rain forest. Plant Biol 16:155–165
Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H (2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ 31:602–621
Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriquí M, Díaz-Espejo A, Douthe C, Dreyec E, Ferrio JP, Gago J, Gallé A, Galmés J, Kodama N, Medrano H, Niinemets U, Peguero-Pina JJ, Pou A, Ribas-Carbó M, Tomás M, Tosens T, Warren CR (2012) Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci 193–194:70–84
Freiberg M (1997) Spatial and temporal pattern of temperature and humidity of a tropical premontane rain forest tree in Costa Rica. Selbyana 18:77–84
Gamage HK, Jesson L (2007) Leaf heteroblasty is not an adaptation to shade: seedling anatomical and physiological responses to light. N Z J Ecol 31:245–254
Genty B, Briantais JM, Baker NR (1989) The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Gitz DC, Baker JT (2009) Methods for creating stomatal impressions directly onto archivable slides. Am Soc Agron 101:232–236.
Haigh A, Clark B, Reynolds L, Mayo SJ, Croat TB, Lay L, Boyce PC, Mora M, Bogner J, Sellaro M, Wong SY, Kostelac C, Grayum MH, Keating RC, Ruckert G, Naylor MF and Hay A (2011) CATE Araceae. http://araceae.e-monocot.org
Kitajima K, Hogan KP (2003) Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ 26:857–865
Lee DW, Richards JH (1991) Heteroblastic development in vines. In: Putz FE, Mooney HA (eds) The biology of vines. Cambridge University Press, Cambridge, pp 205–224
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 603:591–592
Lopez-Portillo J, Ewers FW, Angeles G, Fisher JB (2000) Hydraulic architecture of Monstera acuminata: evolutionary consequences of the hemiepiphytic growth form. New Phytol 145:289–299
Mantovani A (1999) A method to improve leaf succulence quantification. Braz Arch Biol Technol 42:9–14
Mantovani A, Pereira TE (2005) Comparative anatomy of leaf and spathe of nine species of Anthurium (section Urospadix; sub-section Flavescentiviridia) (Araceae) and their diagnostic potential for taxonomy. Rodriguésia 56:146–160
Mantovani A, Pereira TE, Mantuano D (2017a) Allomorphic growth of Epipremnum aureum (Araceae) as characterized by changes in leaf morphophysiology during the transition from ground to canopy. Braz J Bot 40:177–191
Mantovani A, Mantuano D, de Mattos EA (2017b) Relationship between nitrogen resorption and leaf size in the aroid vine Rhodospatha oblongata (Araceae). Aust J Bot 65:431–437
Niinemets U, Tenhunen JD (1997) A model separating structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ 20:845–866
Niklas KJ (1994) Plant allometry: the scaling of form and process. University of Chicago Press, Chicago
Nobel PS (2005) Physicochemical and environmental plant physiology, 4th edn. Elsevier Academic Press, Cambridge
O’Brien TP, McCully ME (1981) The study of plants structure, principles and selected methods. Termarcarphi Pty, Melbourne
Oguchi R, Hikosaka K, Hirose T (2003) Does the photosynthetic light acclimation need change in leaf anatomy? Plant Cell Environ 26:505–512
Oguchi R, Hikosaka K, Hirose T (2005) Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ 28:916–927
Oguchi R, Hikosaka K, Hiura T, Hirose T (2006) Leaf anatomy and light acclimation in woody seedling after gap formation in a cool-temperate deciduous forest. Oecologia 149:571–582
Pontes TA, Alves M (2011) Padrões de distribuição geográfica das espécies de Araceae ocorrentes em fragmentos de floresta atlântica em Pernambuco, Brasil. Rev Bras Bioc 9:444–454
Potvin C, Lechowicz MJ, Tardif S (1990) The statistical analysis of ecophysiological response curves obtained from experiments involving repeated measures. Ecology 71:1389–1400
Ray TS (1990) Metamorphosis in the Araceae. Am J Bot 77:1599–1609
Ray TS (1992) Foraging behavior in tropical herbaceous climbers (Araceae). J Ecol 80:189–203
Ruzin S (1999) Plant microthechnique and microscopy. Oxford University Press, New York
Schnitzer SA, Bongers F (2002) The ecology of lianas and their role in forests. Trends Ecol Evol 17:223–230
Shobe WR, Lersten NR (1967) A technique for clearing and staining gymnosperm leaves. Bot Gaz 127:150–152
Steinitz B, Hagiladi A, Anav D (1992) Thigmomorphogenesis and its interaction with gravity in climbing plants of Epipremnum aureum. J Plant Physiol 140:571–574
Strittmatter CGD (1973) Nueva técnica de diafanización. Bol Soc Argent Bot 15:126–129
Terashima I, Hanba YT, Tholen D, Niinemets U (2011) Leaf functional anatomy in relation to photosynthesis. Plant Phys 155:108–116
White AJ, Critchley C (1999) Rapid light curves: a new fluorescence method to assess the state of photosynthetic apparatus. Photosynth Res 59:63–72
Witkowski ETF, Lamont BB (1991) Leaf specific mass confounds leaf density and thickness. Oecologia 88:486–493
Zar J (1996) Biostatistical analysis, 2nd edn. Prentice Hall, New Jersey
Zotz G (2013) ‘‘Hemiepiphyte’’: a confusing term and its history. Ann Bot 111:1015–1020
Zotz G, Wilhelm K, Becker A (2011) Heteroblasty: a review. Bot Rev 77:109–151
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mantovani, A., Brito, C. & Mantuano, D. Does the same morphology mean the same physiology? Morphophysiological adjustments of Philodendron hederaceum (Jacq.) Schott, an isomorphic aroid, to ground-canopy transition. Theor. Exp. Plant Physiol. 30, 89–101 (2018). https://doi.org/10.1007/s40626-018-0105-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-018-0105-6