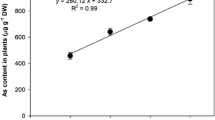
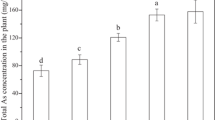
Abstract
Antioxidant enzymes are important components in the defense against arsenic (As) stress in plants. Here, we tested the hypothesis that Salvinia molesta, an aquatic fern, counteracts the harmful arsenite (AsIII) effects by activating scavenging reactive oxygen species (ROS) enzymes. Thus, our objective was to investigate the role of the superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), and ascorbate peroxidase (APX) in S. molesta tolerance to AsIII and indicate the use of this plant in remediation of contaminated water. Plants were grown in nutrient solution at pH 6.5 and exposed to 0, 5, 10, or 20 µM AsIII for 96 h (analyses of As absorption, mineral nutrient content, and relative growth rate) and for 24 h (analyses of oxidative stress indicators and enzymatic antioxidant defenses). In the floating leaves, there was a greater basal activity of the antioxidant enzymes and less accumulation of As than in submerged leaves. The submerged leaves, which function as roots in S. molesta, accumulated more As than floating leaves, and SOD and CAT activities were inhibited. Thus, there was a greater production of ROS and oxidative stress. Our results show that S. molesta presents enzymatic antioxidant defenses to alleviate AsIII toxicity and are more effectives in the floating leaves. These results are important to elucidate the AsIII tolerance mechanisms in S. molesta and the possibility of their use in contamined water phytoremediation. Additional studies exposing plants to more prolonged stress and using AsIII concentrations closer to those found in contaminated environments will confirm this claim.

Similar content being viewed by others
References
Afzal Z, Howton TC, Sun Y, Mukhtar MS (2016) The roles of aquaporins in plant stress responses. J Dev Biol. https://doi.org/10.3390/jdb4010009
Ahammed GJ, Choudhary SP, Chen S, Xia X, Shi K, Zhou Y, Yu J (2013) Role of brassinosteroids in alleviation of phenanthrene–cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J Exp Bot 64:199–213
Ahmad P, Sarwat M, Bhat NA, Wani MR, Kazi AG, LSP Tran (2015) Alleviation of cadmium toxicity in Brassica juncea L.(Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS ONE. https://doi.org/10.1371/journal.pone.0114571
Andrade HM, Oliveira JA, Farnese FS, Ribeiro C, Silva AA, Campos FV, Neto JL (2016) Arsenic toxicity: cell signalling and the attenuating effect of nitric oxide in Eichhornia crassipes. Biol Plant 60:173–180
Anjum NA, Sofo A, Scopa A et al (2015) Lipids and proteins–major targets of oxidative modifications in abiotic stressed plants. Environ Sci Pollut Res 22:4099–4121
Anjum NA, Sharma P, Gill SS et al (2016) Catalase and ascorbate peroxidase-representative H2O2-detoxifying heme enzymes in plants. Environ Sci Pollut Res 23:19002–19029
Barros JPA, Henares MNP (2015) Biomass reduction of Salvinia molesta exposed to copper sulfate pentahydrate (CuSO4.5H2O). Rev Ambient Água. https://doi.org/10.4136/ambi-agua.1633
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay aplicable to acrylamide gels. Anal Biochem 44:276–287
Begum MC, Islam MS, Islam M, Amin R, Parvez MS, Kabir AH (2016) Biochemical and molecular responses underlying differential arsenic tolerance in rice (Oryza sativa L.). Plant Physiol Biochem 104:266–277
Boveris A, Alvarez S, Bustamante J, Valdez L (2002) Measurement of superoxide radical and hydrogen peroxide production in isolated cells and subcellular organelles. Methods Enzymol 349:280–287
Chakraborti D, Rahmana MM, Chatterjeea A et al (2016) Fate of over 480 million inhabitants living in arsenic and fluoride endemic Indian districts: magnitude, health, socio-economic effects and mitigation approaches. J Trace Elem Med Biol 38:33–45
Chance B, Maehley AC (1955) Assay of catalase and peroxidase. Methods Enzymol 2:755–764
Chen Z, Zhu YG, Liu WJ, Meharg AA (2005) Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots. New Phytol 165:91–97
Clark RB (1975) Characterization of phosphatase of intact maize roots. J Agric Food Chem 23:458–460
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci. https://doi.org/10.3389/fenvs.2014.00053
da-Silva CJ, Canatto RA, Cardoso AA, Ribeiro C, de Oliveira JA (2018) Oxidative stress triggered by arsenic in a tropical macrophyte is alleviated by endogenous and exogenous nitric oxide. Braz J Bot 41:21–28
Dave R, Singh PK, Tripathi P et al (2013) Arsenite tolerance is related to proportional thiolic metabolite synthesis in rice (Oryza sativa L.). Arch Environ Contam Toxicol 64:235–242
Del Río LA, López-Huertas E (2016) ROS generation in peroxisomes and its role in cell signaling. Plant Cell Physiol 57:1364–1376
Demidchik V (2015) Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Exp Bot 109:212–228
Dixit G, Singh AP, Kumar A et al (2015) Sulfur mediated reduction of arsenic toxicity involves efficient thiol metabolism and the antioxidant defense system in rice. J Hazard Mater 298:241–251
Du L, Xia X, Lan X, Liu M, Zhao L, Zhang P, Wu Y (2017) Influence of arsenic stress on physiological, biochemical, and morphological characteristics in seedlings of two cultivars of maize (Zea mays L.). Water Air Soil Pollut 228:255
Edel KH, Marchadier E, Brownlee C, Kudla J, Hetherington AM (2017) The evolution of calcium-based signalling in plants. Curr Biol 27:667–679
Faria AP, Lemos-Filho JP, Modolo LV, França MGC (2013) Electrolyte leakage and chlorophyll a fluorescence among castor bean cultivars under induced water deficit. Acta Physiol Plant 35:119–128
Farnese FS, Oliveira JA, Lima F, Leão GA, Gusman GS, Silva LC (2014) Evaluation of the potential of Pistia stratiotes L. (water lettuce) for bioindication and phytoremediation of aquatic environments contaminated with arsenic. Braz J Biol 74:108–112
Farooq MA, Li L, Ali B et al (2015) Oxidative injury and antioxidant enzymes regulation in arsenic-exposed seedlings of four Brassica napus L. cultivars. Environ Sci Pollut Res 22:10699–10712
Farooq AM, Islam F, Ali B (2016a) Arsenic toxicity in plants: cellular and molecular mechanisms of its transport and metabolism. Environ Exp Bot. https://doi.org/10.1016/j.envexpbot.2016.08.004
Farooq MA, Gill RA, Ali B, Wang J, Islam F, Ali S, Zhou W (2016b) Subcellular distribution, modulation of antioxidant and stress-related genes response to arsenic in Brassica napus L. Ecotoxicology 25:350–366
Fayiga AO, Saha KU (2016) Arsenic hyperaccumulating fern: implications for remediation of arsenic contaminated soils. Geoderma 284:132–143
Fazi S, Amalfitano S, Casentini B et al (2016) Arsenic removal from naturally contaminated waters: a review of methods combining chemical and biological treatments. Rend Fis Acc Lincei 27:51–58
Finnegan PM, Chen W (2012) Arsenic toxicity: the effects on plant metabolism. Front Physiol. https://doi.org/10.3389/fphys.2012.00182
Freitas-Silva LD, de Araújo TO, da Silva LC, Oliveira JA, Araújo JM (2016) Arsenic accumulation in Brassicaceae seedlings and its effects on growth and plant anatomy. Ecotoxicol Environ Saf 124:1–9
Fresno T, Peñalosa JM, Santner J, Puschenreiter M, Prohaska T, Moreno-Jiménez E (2016) Iron plaque formed under aerobic conditions efficiently immobilizes arsenic in Lupinus albus L roots. Environ Pollut 216:215–222
Gay C, Gebicki JM (2000) A critical evaluation of the effect of sorbitol on the ferric-xylenol orange hydroperoxide assay. Anal Biochem 284:217–220
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314
Gill SS, Anjum NA, Gill R (2015) Superoxide dismutase–mentor of abiotic stress tolerance in crop plants. Environ Sci Pollut Res Int 22:10375–10394
Gomes MP, Carvalho M, Marques TCLLSM, Duarte DM, Nogueira COG, Soares AM, Garcia QS (2012) Arsenic-sensitivity in Anadenanthera peregrina due to arsenic-induced lipid peroxidation. Int J Appl Sci Technol 2:55–63
Gupta DK, Inouhe M, Rodríguez-Serrano M, Romero-Puertas MC, Sandalio LM (2013) Oxidative stress and arsenic toxicity: role of NADPH oxidases. Chemosphere 90:1987–1996
Gusman GS, Oliveira JA, Farnese FS, Cambraia J (2013) Arsenate and arsenite: the toxic effects on photosynthesis and growth of lettuce plants. Acta Physiol Plant 35:1201–1209
Hajiboland R (2014) Reactive oxygen species and photosynthesis. In: Ahmad P (ed) Oxidative damage to plants, 3rd edn. Academic Press, India, pp 1–63
Halliwell B, Gutteridge JMC (2015) Free radicals in biology and medicine. Oxford University Press, Oxford
Han YH, Fu JW, Chen Y, Rathinasabapathi B, Ma LQ (2016) Arsenic uptake, arsenite efflux and plant growth in hyperaccumulator Pteris vittata: role of arsenic-resistant bacteria. Chemosphere 144:1937–1942
Hariyadi, Yanuwiadi B, Polii B, Soemarno (2013) Phytoremediation of arsenic from geothermal power plant waste water using Monochoria vaginalis, Salvinia molesta and Colocasia esculenta. Int J Biosci 3:104–111
Havir EA, Mchale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol 84:450–455
Hepler PK (2005) Calcium: a central regulator of plant growth and development. Plant Cell 17:2142–2155
Hettick BE, Cañas-Carrell JE, French AD, Klein DM (2015) Arsenic: a review of the element’s toxicity, plant interactions, and potential methods of remediation. J Agric Food Chem 19:7097–7107
Hofffman H, Schenk M (2011) Arsenite toxicity and uptake rate of rice (Oryza sativa L.) in vivo. Environ Pollut 159:2398–2404
Hu M, Li F, Liu C, Wu W (2015) The diversity and abundance of As(III) oxidizers on root iron plaque is critical for arsenic bioavailability to rice. Sci Rep 5:13611. https://doi.org/10.1038/srep13611
Hunt R (1978) Plant growth analysis (Studies in Biology). Edward Arnold Ltd., London
Jasrotia S, Kansal A, Mehra A (2017) Performance of aquatic plant species for phytoremediation of arsenic-contaminated water. Appl Water Sci. 7:889–896
Kanwar MK, Poonam, Bhardwaj R (2015) Arsenic induced modulation of antioxidative defense system and brassinosteroids in Brassica juncea L. Ecotoxicol Environ Saf 115:119–125
Khang VH, Hatayama M, Inoue C (2012) Arsenic accumulation by aquatic macrophyte coontail (Ceratophyllum demersum L.) exposed to arsenite, and the effect of iron on the uptakeof arsenite and arsenate. Environ Exp Bot 83:47–52
Kumar S, Dubey RS, Tripathi RD, Chakrabarty D, Trivedi PK (2015) Omics and biotechnology of arsenic stress and detoxification in plants: current updates and prospective. Environ Int 74:221–230
Kumar M, Rahman MM, Ramanathan AL, Naidu R (2016) Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: health risk index. Sci Total Environ 539:125–134
Kumari S, Kumar B, Sheel R (2017) Biological control of heavy metal pollutants in water by Salvinia molesta. Int J Curr Microbiol App Sci 6:2838–2843
Leão GA, Oliveira JA, Felipe RTA, Farnese FS (2017) Phytoremediation of arsenic-contaminated water: the role of antioxidant metabolism of Azolla caroliniana Willd. (Salviniales). Acta Bot Bras 31:161–168
LeBlanc MS, McKinney EC, Meagher RB, Smith AP (2013) Hijacking membrane transporters for arsenic phytoextraction. J Biotechnol 163:1–9
Li N, Wang J, Song WY (2016) Arsenic uptake and translocation in plants. Plant Cell Physiol 57:4–13
Liu WJ, Wood BA, Raab A, McGrath SP, Zhao FJ, Feldmann J (2010) Complexation of arsenite with phytochelatins reduces arsenite efflux and translocation from roots to shoots in Arabidopsis thaliana. Plant Physiol 152:2211–2221
Luque GM, Bellard C, Bertelsmeier C (2014) The 100th of the world’s worst invasive alien species. Biol Invasions 16:981–985
Marin AR, Pezeshki SR, Masscheleyn PH, Choi HS (1993) Effect of dimethylarsenic acid (DMAA) on growth, tissue arsenic and photosynthesis in rice plants. J Plant Nut 16:865–880
Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61:4197–4220
Miranda CV, Schwartsburd PB (2016) Aquatic ferns from Viçosa (MG, Brazil): Salviniales (Filicopsida; Tracheophyta). Braz J Bot 39:935–942
Mishra S, Jha AB, Dubey RS (2011) Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma 248:565–577
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19
Mohammadi M, Karr AL (2001) Superoxide anion generation in effective and ineffective soybean root nodules. J Plant Physiol 158:1023–1029
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-especific peroxidase en spinach chloroplasts. Plant Cell Physiol 22:867–880
Newete SW, Byrne MJ (2016) The capacity of aquatic macrophytes for phytoremediation and their disposal with specific reference to water hyacinth. Environ Sci Pollut Res Int 23:10630–10643
Nicomel NR, Leus K, Folens K, Voort PV, Du Laing GD (2016) Technologies for arsenic removal from water: current status and future perspectives. Int J Environ Res Public Health 13:62. https://doi.org/10.3390/ijerph13010062
Ozturk F, Duman F, Leblebici Z, Temizgul R (2010) Arsenic accumulation and biological responses of watercress (Nasturtium officinale R. Br.) exposed to arsenite. Environ Exp Bot 69:167–174
Parrotta L, Guerriero G, Sergeant K, Cai G, Hausman JF (2015) Target or barrier? The cell wall of early and later-diverging plants vs cadmium toxicity: differences in the response mechanisms. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00133
Pommerrenig B, Diehn TA, Bienert GP (2015) Metalloido-porins: essentiality of Nodulin 26-like intrinsic proteins in metalloid transport. Plant Sci 238:212–227
Rahman MA, Hasegawa H (2011) Aquatic arsenic: phytoremediation using floating macrophytes. Chemosphere 83:633–646
Rahman A, Mostofa MG, Alam MM, Nahar K, Hasanuzzaman M, Fujita M (2015) Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidante defense and glyoxalase systems and stress markers. BioMed Res Int. https://doi.org/10.1155/2015/340812
Reed ST, Ayala-Silva T, Dunn CB, Gordon GG (2015) Effects of arsenic on nutrient accumulation and distribution in selected ornamental plants. Agric Sci 6:1513–1531
Rezania S, Taib SM, Md Din MF, Dahalan FA, Kamyab H (2016) Comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater. J Hazard Mater 318:587–599
Rout JR, Sahoo SL (2013) Antioxidant enzyme gene expression in response to copper stress in Withania somnifera L. Plant Growth Regul 71:95–99
Sarwar N, Imran M, Shaheen MR, Ishaque W, Kamran MA, Matloob A, Hussain S (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721
Shaibur MR, Kawai S (2009) Effect of arsenic on visible symptom and arsenic concentration in hydroponic Japanese mustard spinach. Environ Exp Bot 67:65–70
Sharma I (2012) Arsenic induced oxidative stress in plants. Biologia 67:447–453
Sharma I (2013) Arsenic-induced oxidative stress and antioxidant defense system of Pisum sativum and Pennisetum typhoides: a comparative study. Res J Biotech 8:48–56
Shen S, Li XF, Cullen WR, Weinfeld M, Le XC (2013) Arsenic binding to proteins. Chem Rev 113:7769–7792
Shen J, Song L, Müller K et al (2016) Magnesium alleviates adverse effects of lead on growth, photosynthesis, and ultrastructural alterations of Torreya grandis seedlings. Front Plant Sci. https://doi.org/10.3389/fpls.2016.01819
Silva AA, Oliveira JA, Campos FV, Ribeiro C, Farnese FS (2017) Role of glutathione in tolerance to arsenite in Salvinia molesta, an aquatic fern. Acta Bot Bras. https://doi.org/10.1590/0102-33062017abb0087
Singh AP, Dixit G, Kumar A (2015a) Nitric oxide alleviated arsenic toxicity by modulation of antioxidants and thiol metabolism in rice (Oryza sativa L.). Front Plant Sci. https://doi.org/10.3389/fpls.2015.01272
Singh VP, Singh S, Kumar J, Prasad SM (2015b) Investigating the roles of ascorbate-glutathione cycle and thiol metabolism in arsenate tolerance in ridged Luffa seedlings. Protoplasma 252:1217–1229
Sofo A, Scopa A, Nuzzaci M, Vitti A (2015) Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci 16:13561–13578
Talukdar D (2013) Arsenic-induced oxidative stress in the common bean legume, Phaseolus vulgaris L. seedlings and its amelioration by exogenous nitric oxide. Physiol Mol Biol Plants 19:69–79
Talukdar D, Talukdar T (2013) Superoxide-dismutase deficient mutants in common beans (Phaseolus vulgaris L.): genetic control, differential expressions of isozymes, and sensitivity to arsenic. BioMed Res Int. https://doi.org/10.1155/2013/782450
Upadhyaya H, Shome S, Roy D, Bhattacharya MK (2014) Arsenic induced changes in growth and physiological responses in Vigna radiata seedling: effect of curcumin interaction. Am J Plant Sci 5:3609–3618
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92:487–511
Xu W, Dai W, Yan H et al (2015) Arabidopsis NIP3;1 plays an important role in arsenic uptake and root-to-shoot translocation under arsenite stress conditions. Mol Plant 8:722–733
Yamaguchi N, Ohkura T, Takahashi Y, Maejima Y, Arao T (2014) Arsenic distribution and speciation near rice roots influenced by iron plaques and redox conditions of the soil matrix. Environ Sci Technol 48:1549–1556
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Acknowledgements
The authors acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Federal University of Viçosa for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, A.A., de Oliveira, J.A., de Campos, F.V. et al. Phytoremediation potential of Salvinia molesta for arsenite contaminated water: role of antioxidant enzymes. Theor. Exp. Plant Physiol. 30, 275–286 (2018). https://doi.org/10.1007/s40626-018-0121-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-018-0121-6