Highlights
-
A series of 10 imidazolium-based ionic liquids (IILs) with different cations and anions have applied to unravel the passivation mechanism of the IILs on CsPbI2Br inorganic perovskites.
-
It is found that anions of IILs play a more important role in passivation of lead- and cesium-related defects in inorganic perovskite compared with imidazole cations because they can form strong ionic interactions (Pb-F, Cs-F).
-
A high-power conversion efficiency of 17.02% is obtained, which is among the highest values of CsPbI2Br-based perovskite solar cells.
Abstract
The application of ionic liquids in perovskite has attracted wide-spread attention for its astounding performance improvement of perovskite solar cells (PSCs). However, the detailed mechanisms behind the improvement remain mysterious. Herein, a series of imidazolium-based ionic liquids (IILs) with different cations and anions is systematically investigated to elucidate the passivation mechanism of IILs on inorganic perovskites. It is found that IILs display the following advantages: (1) They form ionic bonds with Cs+ and Pb2+ cations on the surface and at the grain boundaries of perovskite films, which could effectively heal/reduce the Cs+/I− vacancies and Pb-related defects; (2) They serve as a bridge between the perovskite and the hole-transport-layer for effective charge extraction and transfer; and (3) They increase the hydrophobicity of the perovskite surface to further improve the stability of the CsPbI2Br PSCs. The combination of the above effects results in suppressed non-radiative recombination loss in CsPbI2Br PSCs and an impressive power conversion efficiency of 17.02%. Additionally, the CsPbI2Br PSCs with IILs surface modification exhibited improved ambient and light illumination stability. Our results provide guidance for an in-depth understanding of the passivation mechanism of IILs in inorganic perovskites.
Similar content being viewed by others
1 Introduction
Cesium-based all-inorganic halide perovskites (CsPbX3, X = I, Br, Cl, or their mixtures) have recently attracted great attention because of their excellent light stability, heat stability and optoelectronic properties [1,2,3]. Although great progress has been made in inorganic perovskite solar cells (PSCs) in past two years [1, 4], two core issues limit their future commercialization. On one hand, due to the solution fabrication process and ionic nature of inorganic perovskite, a large number of defects are inevitably formed at the surface and grain boundaries (GBs) of polycrystalline inorganic perovskite film, which serve as non-radiative recombination centers and cause open-circuit voltage (Voc) and power conversion efficiency (PCE) loss for inorganic PSCs [5]. For example, Huang et al. have demonstrated that the defect density at the interface between the perovskite and hole–transport-layer (HTL) is two orders of magnitude higher than that in the perovskite bulk [6]. These interfacial defects bring in deep electronic states as non-radiative recombination centers, thus restricting the photovoltaic parameters, especially the Voc [7,8,9]. In addition to the efficiency, the inferior phase stability of inorganic perovskite compared to organic–inorganic hybrid perovskites is another serious issue. Researchers also found that defects and water are often involved in the phase transition process of inorganic perovskite film, leading to the instability issue of inorganic PSCs [1, 4]. Therefore, the effective passivation of surface defects and blocking water penetration are especially paramount for simultaneously enhancing the efficiency and prolonging the lifetime of inorganic PSCs.
Surface passivation, for eliminating defects (lattice vacancies, undercoordinated ions and interstitial species) and improving charge transport/hydrophobicity, is one of the most prominent strategies to promote the efficiency and stability of PSCs [10]. Recently, common surface passivators including Lewis bases/acids, organic halide salts, polymers, fullerenes, and their derivatives have been used to passivate the defects of perovskite film [11,12,13,14,15]. Among them, most Lewis bases/acids, organic halide salts and polymers possess insulating properties and could hinder efficient carrier extraction at the interface owing to their poor conductivity, and negatively affect the reproducibility of PSCs due to a distribution in molecular weights [16]. The fullerenes and their derivatives such as PCBM require many steps for synthesis, are expensive, and exhibit low yields [17, 18]. Therefore, the exploration of new passivation materials has become the main topic in research for achieving stable and efficient inorganic PSCs [19].
As a class of molten salts, ionic liquids (ILs) have recently become hot candidates to passivate/modify perovskites and/or charge transport layers for realizing stable and efficient PSCs due to their diverse structures and unique properties, such as wide liquid temperature range, low toxicity, non-volatility, strong conductivity and good stability [20, 21]. Imidazolium ILs (IILs) have unique physicochemical properties, such as versatile functional ligands, large electrochemical window, high thermal stability, and nonhazardous nature, and they have been widely used to passivate the defects both in the interior and at surface grain boundaries of perovskites via additive-assisted techniques or surface-antisolvent methods [22,23,24]. Bai et al. reported the use of a small amount of the BMIMBF4 additive in the perovskite precursor solution [25], where BMIM+ in the BMIMBF4 can accumulate at the top surface of the perovskite film. The formed BMIMBF4 layer could modify the surface dipole and improve the energetic alignment at the top interface of the perovskite film, resulting in reduced voltage loss and improved charge extraction at the interface. In addition, IILs surface passivation of the charge transport layer and perovskite layer has recently emerged as an efficacious strategy to suppress interface defects and reduce interface energy loss in hybrid perovskites [26,27,28]. For example, Noel et al. recently deposited BMIMBF4 on a SnO2 electron transport layer (ETL), and it was found that BMIMBF4 can simultaneously reduce the work functions of SnO2 and perovskite film, thus reducing charge recombination loss and improving the charge extraction and transport at the SnO2/perovskite interface [29]. Compared with bottom-surface modification of the ETL, an IIL-based upper-perovskite modification can passivate the bulk defects due to the permeation of IILs into the perovskite layer. Zhang et al. introduced an IIL (1-butyl-2,3-dimethylimidazolium chloride: BMMIMCl) for surface modification of a CsPbBr3 perovskite film[30]. It was found that BMMIMCl can passivate the defects (unsaturated Pb2+ and Cs+) on the surface of the CsPbBr3 perovskite film to obtain a PCE of 9.92% in an HTL-free CsPbBr3 PSC. Nonetheless, IILs have an electron-rich nitrogen atom, an alkyl side chain and an anion, the crucial interactions between IILs and perovskite precursors are still unclear, and the mechanism of IILs passivation of the inorganic perovskite layer has been rarely investigated. Therefore, further studies are needed to better understand the interaction between the IILs and perovskite and to provide a principle for rational design of the IIL molecules.
Herein, we report a series of IILs as surface passivators for efficient and stable inorganic CsPbI2Br PSCs. These IILs possess imidazolium cations with different side chains and different anions. First-principle calculations reveal that anions of the IILs play a more important role in passivation of lead- and cesium-related defects in inorganic perovskite compared with imidazole cations. The anions cause simultaneous ionic bonding of the IILs with Cs+ and Pb2+ cations on the surface and at the grain boundaries (GBs) of perovskite films, which could effectively heal/reduce Cs+/I− vacancies and Pb-related defects. Meanwhile, the IILs could improve the energy-level alignment between the perovskite and Spiro-OMeTAD for promoting hole extraction and reducing electron–hole recombination at the perovskite/Spiro-OMeTAD interface, eventually leading to an increase of Voc of 50 mV for the photovoltaic device. Profiting from superior IILs passivation, the efficiency of CsPbI2Br PSCs has been elevated from 15.62 to 17.02% with a high VOC of 1.33 V. Furthermore, the exposed hydrophobic alkyl component protects the perovskite against detrimental environmental factors. The unencapsulated device modified with BMMIMBF4 presents outstanding long-term stability when stored in ambient air at 25 °C with a relative humidity (RH) of 25% or under continuous illumination for 100 h. Our work provides a complete set of characterization methods to elucidate the passivation mechanism of IILs, which provides guidelines for the design of new ionic liquids to improve the performance of inorganic PSCs.
2 Experimental Section
2.1 Materials
Cesium iodide (CsI, 99.99%), lead bromide (PbBr2, 99.99%), lead iodide (PbI2, 99.99%), and lead acetate (PbAc2, 99.5%) were purchased from Xi’an Polymer Light Technology Corp. PbI2(DMSO), and PbBr2(DMSO) were prepared by the antisolvent method. The ionic liquids (ILs) 1-butyl-2,3-dimethylimidazolium tetrafluoroborate (BMMIMBF4, 99%), 1-butyl-3-methylimidazolium tetrafluoroborate (BMIMBF4, 99%), 1-propyl-3-methylimidazolium tetrafluoroborate (PMIMBF4, 99%), 1-hexyl-3-methylimidazolium tetrafluoroborate (HMIMBF4, C10H19BF4N2, 99%), and 1-hexyl-2,3-dimethylimidazolium tetrafluoroborate (HMMIMBF4, 99%) were purchased from Shanghai Chengjie Chemical Co., Ltd. N,N-dimethylformamide (DMF) and dimethylsulfoxide (DMSO) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.
2.2 Device Fabrication
The fluorine-doped tin oxide (FTO) glass substrates were sequentially cleaned with ethanol, acetone, and isopropanol in an ultrasonic bath for 30 min and then dried with N2. The cleaned FTO glass substrates were then treated with UV-Ozone for 10 min prior to the deposition of TiO2. The TiO2 layer was deposited by immersing FTO glass substrates in 200 mL aqueous solution with 4.5 mL titanium tetrachloride for 60 min at 70 °C, then rinsed with distilled water and annealed at 200 °C for 30 min. The CsPbI2Br precursor solution was prepared by dissolving PbBr2(DMSO), PbI2(DMSO), CsI, and PbAc2 (molar ratio = 1:1:2:0.023) in DMF and DMSO (17:3 v/v). The resulting perovskite precursor solution was spin-coated at 1000 rpm for 10 s, followed by 4000 rpm for 40 s. Afterward, the film was annealed at 35 °C for 6 min, 120 °C for 10 min, and 180 °C for 4 min to obtain the perovskite layer. The IILs were dissolved in isopropanol with different concentrations (0.01, 0.02, 0.03, and 0.04 wt%) and spin-coated onto the CsPbI2Br perovskite film at 5000 rpm for 45 s; then the films were thermally annealed at 100 °C for 10 min to form the TiO2/perovskite/IIL structure. The hole-transport layer was prepared by spin-coating Spiro-OMeTAD solution (90 mg mL−1) doped with 36 µL t-BP and 22 µL Li-TFSI (520 mg mL−1) solution in acetonitrile at 5000 rpm for 30 s onto CsPbI2Br films to produce a TiO2/perovskite/IIL/Spiro-OMeTAD architecture. Finally, an 80-nm Au electrode was deposited by thermal evaporation through a shadow mask to form a device with an active area of 0.09 cm2.
2.3 Density Function Theory (DFT) Calculation
The calculation was performed using VASP code in the framework of the PBE approximation. The van der Waals interaction was treated by the Tkatchenko-Scheffler method. The lattice constant of CsPbI3 perovskite was set to 6.16 Å, which was obtained by optimizing a cubic CsPbI3 unit cell. The interaction energies of the anions in this study with an I vacancy on the film surface were calculated by comparing the energies of a CsPbI3 slab (2 × 2 × 3 unit cells in the supercell) with one I vacancy and that of the slab with the I vacancy saturated with the anions. The dimension of the calculation was set to 12.32 × 12.32 × 50 Å3. The k-mesh was set to 10 × 10 × 1. In the calculations, the first layer of CsPbI3 perovskite was fully relaxed. The interaction energies of the ions of interest with PbI2 were calculated in a volume of 20 × 20 × 20 Å3. The k-mesh was set to 1 × 1 × 1. In all the calculations, the cut-off energy was set to 500 eV, and the convergence condition was set to 10–6 eV to ensure convergence of the system to a stable state.
2.4 Characterization
X-ray diffraction (XRD) patterns of the perovskite films were acquired on a D/MAX 2400 diffractometer. The absorption spectra of perovskite films were measured using a UV–vis NIR spectrophotometer (PerkinElmer, Lambda 950). Photoluminescence (PL) (excitation at 510 nm) spectra were measured using a FLS980 spectrometer (Edinburgh Instruments Ltd), and TRPL spectra were measured with a PicoQuant FluoQuant 300. The scanning electron microscopy (SEM) images of perovskite films were obtained by field-emission scanning electron microscopy (HITACHI, SU-8020). X-ray photoelectron spectroscopy (XPS) and ultraviolet photoelectron spectroscopy (UPS) of the perovskite films were carried out using a photoelectron spectrometer (ESCALAB250Xi, Thermo Fisher Scientific). Fourier-transform infrared spectroscopy (FTIR) were performed with a Bruker Vertex 70. Nuclear magnetic resonance spectroscopy (NMR) was performed using a JNM-ECZ400S/L1 with a frequency of 400 MHz, and deuterated DMSO was used as the solvent to dissolve BMMIMBF4 and PbI2 with BMMIMBF4. The J-V curves of the inorganic PSCs were measured using a Keithley 2400 SourceMeter under AM1.5G illumination at 100 mW cm−2. The external quantum efficiencies (EQEs) of the PSCs were recorded using a QTest Station 2000ADI system (Crowntech Inc.). EIS analysis was performed on a Zahner Electrochemical Workstation. Water contact angles were measured using a DataPhysics OCA 20.
3 Results and Discussion
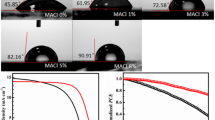
The chemical structures of IILs with the same anions and different imidazolyl and alkyl groups are presented in Fig. S1. The IILs passivation layer was prepared by solution coating the IILs isopropanol solution onto perovskite film and annealing at 100 °C for 10 min. During the annealing process, the IILs could anchor to the CsPbI2Br through the coordination of N/F-atoms in the imidazolyl/anion and Pb in the perovskite [30]. Simultaneously, the hydrophobic alkyl chains of the IILs are arranged along the perovskite surface, which provides hydrophobicity to increase the moisture-resistance of the CsPbI2Br film [31]. In order to explore the effects of the IILs treatment on the crystallinity or orientation of the perovskite crystal structure, the XRD patterns of bare perovskite and perovskites modified by different IILs are characterized. As presented in Fig. 1a, only two main diffraction peaks from the (100) and (200) planes of the perovskite crystal structure can be observed, which is consistent with our previous reports [32, 33]. All the diffraction intensities of the (100) and (200) peaks were slightly enhanced in perovskite treated by IILs, while the peak positions have no obvious shift compared to bare perovskite film, indicating that the IILs could enhance the (100) orientation of perovskite but weren ‘t incorporated into the perovskite crystal lattice. According to the ultraviolet–visible (UV–vis) spectra of the perovskites (Fig. 1b), the absorption intensity and edge of the IILs-treated perovskite films have no discernible change, confirming that the crystal structure of the perovskite remains largely unchanged. The XRD and UV–vis results suggest that the IILs treatment does not change the cubic perovskite structure, and the IILs only remain at the surface and/or GBs of the perovskite film [34].
a XRD patterns and (200) diffraction peaks and b UV–vis absorption spectra of perovskite films with different cation-based IILs treatments. c ToF–SIMS depth profile of the CsPbI2Br/BMMIMBF4 perovskite film. d Top-view SEM images of the CsPbI2Br films with different cation-based IILs treatments. e PL and f TRPL spectra of CsPbI2Br perovskite films with different cation-based IILs treatments
Next, time-of-flight secondary-ion mass spectrometry (ToF–SIMS) was performed under negative polarity to check the chemical distribution within the perovskite film with BMMIMBF4 treatment. As illustrated in Fig. 1c, it is found that the anions and cations of the IIL (BMMIMBF4) are not identically distributed in the perovskite film. Specifically, BMMIM+ is found to be distributed throughout the bulk film, while BF4− is located mainly at the surface of the perovskite film. As shown in Fig. S2, the chemical distributions of N from BMMIM+ and F from BF4− can be visualized and confirmed in the elemental 3D maps in the depth profile.
As shown in Fig. 1d, the surface morphologies of perovskite films with different IILs treatments were characterized with SEM measurements. After treatment with very dilute IILs solution, there was no significant change to the surface morphology of the perovskite film. However, the hydrophobic feature of the perovskite film was obviously improved, which is due to the hydrophobic alkyl chains and fluorinated anions. The hydrophobic feature can also be adjusted by the alkyl length and added side chains. The hydrophobic IIL layer deposited on the surface of the perovskite could prevent moisture and oxygen from infiltrating the perovskite layer, thereby improving device performance stability.
To obtain more information regarding the effect of IILs modification on the charge transfer process of CsPbI2Br film, steady-state PL measurements were conducted. As shown in Fig. 1e, all the PL intensities of the perovskite films with IILs modification are obviously quenched compared to that of the control film, mainly due to the quenching effect induced by charge transfer from the perovskite to the IILs [35]. In addition, when the spiro-OMeTAD layer was introduced, the PL quenching occurs more effectively in the IILs-treated perovskite films (Fig. S3). Further, time-resolved photoluminescence (TRPL) measurements were carried out to study the photogenerated carrier lifetime, as shown in Fig. 1f. Table S1 lists the fitting parameters of the TRPL curves fitted by a biexponential function. All the perovskite films with IILs treatment exhibit shorter average lifetimes than the bare perovskite film, which indicates the existence of accelerated charge transfer from the perovskite to the IILs [26]. As show in Fig. 1e, f and Table S1, the perovskite film with BMMIMBF4 treatment shows the most effective quenching efficiency and shortest PL decay time, indicating efficient charge transfer that would reduce the interfacial non-radiative recombination.
In order to clarify the possibility of charge transfer between the perovskite and IILs, UPS and electrochemical cyclic voltammetry (CV) characterization of perovskite and BMMIMBF4 IIL were conducted. As shown in Fig. S4, the Fermi level (EF) of the perovskite is close to the conduction band, indicating it is n-type, and the valence band maximum (VBM) is located at − 6.0 eV, while the highest occupied molecular orbital (HOMO) of BMMIMBF4 is at − 5.54 eV. When the n-type perovskite contacts the BMMIMBF4 under illumination, the holes will transfer from the perovskite to the BMMIMBF4, which results in the quenching of PL and decreased carrier lifetime. Therefore, IILs treatment leads to a faster charge transport and extraction at the perovskite surface, which means there is a possibility that charge-carrier recombination could be reduced [36] with potential benefit to the performance of the derived solar cells. To confirm this hypothesis, PSCs (FTO/TiO2/Perovskite/IILs/Au) without HTLs are fabricated (Fig. S5). Compared to the device without treatment, the IILs-treated device shows a remarkable increase in PCE from 5.16 to 9.86%, mainly driven by enhanced Voc and fill factor (FF). This is indicative of reduced recombination and enhanced charge extraction at the perovskite/IILs interface, which is consistent with the PL and TRPL results (Fig. 1e, f) [12, 37,38,39].
In order to further investigate, the interaction between the IIL (BMMIMBF4) and CsPbI2Br perovskite, FTIR, XPS, and 1H, 19F, 11B NMR were conducted. Figure 2a shows the FTIR spectra of bare BMMIMBF4, BMMIMBF4 + PbI2, and BMMIMBF4 + CsI mixtures. The bond positions in the different mixtures are listed in Table S2. It is found that the stretching vibration peaks at 1252 and 1466 cm−1 assigned to the C–N and C=N bonds, respectively, in the pure BMMIMBF4 are shifted to lower wavenumbers of 1240 and 1457 cm−1 in BMMIMBF4 + PbI2. This indicates that the uncoordinated Pb2+ can form coordination bonds with C–N and C=N groups in BMMIMBF4. In contrast, the peaks for C–N and C=N bonds show almost no shift in BMMIMBF4 + CsI, indicating that there was no interaction between Cs+ and C–N/C=N bonds [40]. Meanwhile, the B-F peaks in the BMMIMBF4 + PbI2 and BMMIMBF4 + CsI mixtures show large red-shifts from 1056 cm−1 to 1025 and 1039 cm−1, respectively. This indicates that the uncoordinated Pb2+ and Cs+ can be effectively passivated by BF4− through formation of ionic bonds. In addition, the bonding relationship between Pb2+(Cs+) and the IIL was also validated by the XPS spectra. As shown in Fig. 2b-e, when the IIL was mixed with CsI or PbI2, the binding energies of Cs 3d, Pb 4f, and N 1 s are all obviously shifted to lower position, while that of F 1 s is shifted to a higher position, which was attributed to the formation of strong bonding between the Cs+/Pb2+ and F atom in BF4− and/or between Cs+ (Pb2+) and the electron-rich N atom in the alkyl chains. The formation of bonds increases the electron cloud density and decreases the electron affinity of Cs+ and Pb2+ ions. As shown in the inserts of Fig. 2b-d, all the Cs, F, and Pb peaks in the mixture split into two peaks, indicating that new ionic bonds (Cs-F and Pb-F) are formed between the CsI/PbI2 and BF4− in the BMMIMBF4. These results imply that the strong bonding interaction between the BMMIMBF4 and perovskite is favorable and could provide passivation of uncoordinated Cs+/Pb2+ defects and the deep-level Pb-I antisite defects in perovskite through Cs-F and Pb-F bonds and suppress both the diffusion of inorganic cations and the phase transition of the inorganic perovskite crystal, thus significantly improving the stability of inorganic PSCs.
a FTIR spectra of BMMIMBF4 solution with or without PbI2/CsI additive. XPS spectra of b Cs 3d, c F 1 s of BMMIMBF4 solution with or without CsI additive. XPS spectra of d Pb 4f, and e N 1 s of BMMIMBF4 solution with or without PbI2 additive. f 19F NMR spectra of BMMIMBF4 solution with or without CsI additive. g 19F NMR spectra of BMMIMBF4 solution with or without PbI2 additive
In order to gain deeper insights into the chemical interaction between the perovskite precursor and BMMIMBF4, a series of liquid state 1H, 19F, and 11B NMR spectra of BMMIMBF4 with PbI2 or CsI are presented in Figs. 2f, g, S6 and S7. With the addition of PbI2 or CsI into the BMMIMBF4 solution, as shown in Tables S3 and S4 in the Supporting Information, all the 1H peaks in the mixture had weak shifts compared with bare BMMIMBF4, suggesting that the coordinative bonds between N atoms in the alkyl chains and Pb (Cs) atoms in the PbI2 (CsI) are not so strong to affect adjacent CH3 or CH2 groups in the alkyl chains. In contrast, the characteristic F and B peaks of BF4− show obvious shifts when mixed with PbI2 or CsI, due to the formation of Cs-F and Pb-F ionic bonds. From the aforementioned results, the interaction between BMMIMBF4 and inorganic perovskite CsPbI2Br most likely originates from the ionic bonds between BF4− and Pb2+/Cs− ions. The long alkyl chains and side methyl groups on the imidazole group lead to steric hindrance effects, hampering the interaction of the nitrogen atom on the imidazole with the PbI2 or CsI [41]. From examination of the results of NMR, FTIR, and XPS, it is concluded that the BMMIM+ cation in BMMIMBF4 could form a coordinative bond with uncoordinated Pb2+, while the anion (BF4−) could bond with Pb2+ and Cs+ through ionic bonding, which are favorable for inhibiting non-radiative recombination in CsPbI2Br perovskite.
To comprehensively evaluate the effect of the IILs treatment on the photovoltaic performance of CsPbI2Br PSCs, as shown in Fig. 3a, an n-i-p device with the planar structure glass/FTO/TiO2/CsPbI2Br(IILs)/Spiro-OMeTAD/Au was fabricated, where the IILs with different cations were coated onto the perovskite layers as surface passivators. Figure 3b and Table 1 present the current density–voltage (J-V) curves and the corresponding photovoltaic parameters of optimized devices. The device with BMMIMBF4 treatment delivers a champion PCE as high as 17.02%, with short-circuit current density (Jsc) of 15.96 mA cm−2, Voc of 1.33 V, and FF of 80.08%. Meanwhile, the best control device shows an inferior PCE of 15.62%, with Jsc of 15.81 mA cm−2, Voc of 1.28 V, and FF of 77.10%. As shown in Fig. S8 and Tables S5 and S6, the dependence of the device performance on the concentration of the BMMIMBF4 treatment was also investigated. The optimized concentration of BMMIMBF4 is 0.03 wt%. The schematic energy-band alignment of the CsPbI2Br PSCs with BMMIMBF4 treatment is illustrated in Fig. 3c, where the VBM of perovskite is located at − 6.0 eV, and the HOMO of BMMIMBF4 is at − 5.54 eV, which leads to a better energy-level alignment with the HOMO (− 5.22 eV) of Spiro-OMeTAD. Such a band alignment between the CsPbI2Br surface and Spiro-OMeTAD facilities hole transport and reduces interfacial recombination [3, 33]. EQE spectra of devices are shown in Fig. 3d. The integrated current density values for control and BMMIMBF4-treated devices are 15.64 and 15.92 mA cm−2, respectively, which coincide with the Jsc values derived from the J-V measurements. Figures 3e and S9 exhibit the statistical photovoltaic parameter distributions of 50 individual devices with or without BMMIMBF4 treatment, which permit the conclusion that BMMIMBF4 treatment can undoubtedly boost the performance of PSCs, mainly stemming from improved Voc and FF. The stabilized PCEs were further measured with devices biased at the initial maximum power point voltage for 120 s. As shown in Fig. 3f, the BMMIMBF4-treated device achieved the stabilized PCE of 17.00% at the maximum power point (1.10 V), which is in good agreement with the PCE obtained from the J-V measurements.
a Schematic image of a CsPbI2Br PSC with the structure FTO/TiO2/CsPbI2Br(IILs)/Spiro-OMeTAD/Au. b J-V curves and c schematic energy-level alignment of the CsPbI2Br PSC with BMMIMBF4 modification. d EQE spectra, e Box charts of PCE, f stable output curves, g Mott-Schottky plots, h Nyquist plots and i open-circuit voltage dependence on light intensity of the CsPbI2Br PSC with BMMIMBF4 modification. j Space-charge-limited current versus voltage for the FTO/TiO2/CsPbI2Br/PCBM/Ag and FTO/TiO2/CsPbI2Br(BMMIMBF4)/PCBM/Ag devices. k J-V curves under dark conditions of the CsPbI2Br PSC without or with BMMIMBF4 modification
A Mott-Schottky analysis was conducted to study the built-in potential (Vbi) in the devices. As illustrated in Fig. 3g, the device with BMMIMBF4 treatment shows a Vbi of 1.29 V, which is larger than that of the control device (1.25 V). The larger Vbi in the treated device is usually related to a higher Voc value. Electrochemical impedance spectroscopy (EIS) was conducted under dark conditions (Fig. 3h) [33, 42]. The fitted parameters are summarized in Table S7. Compared with the control device, the charge transport resistance (Rct) of the device with BMMIMBF4 treatment was decreased significantly from 754.7 to 493.9 Ω, while the recombination resistance (Rrec) was increased significantly from 2.35 to 6.89 kΩ. The smaller Rct and larger Rrec suggest remarkably enhanced carrier transfer and suppressed charge recombination due to low defect density in the modified device, resulting in the enhanced Voc and FF [43, 44].
To further understand the mechanism of trap-assisted carrier recombination during device operation, the relationship between Voc and light intensity was measured (Fig. 3i), which yielded ideality factors (IFs) of the control and treated devices of 1.68 and 1.15 KBT/q, respectively. The largely reduced IF indicates that IILs treatment can effectively reduce the trap-assisted non-radiative recombination, which is favorable for obtaining high Voc and FF values [45,46,47].
To quantitatively evaluate the affects of IILs modification on the trap density of perovskite films, a space-charge limited current (SCLC) characterization was conducted on an electron-transport device (ITO/TiO2/perovskite/PCBM/Ag) [48, 49]. As shown in Fig. 3j, the trap-filled limit voltage (VTFL) of the device with IILs treatment is ~ 0.28 V, corresponding to a relative trap density (nt) of 5.66 × 1015 cm−3, while the control device displays a higher VTFL of 0.45 V with nt of 9.1 × 1015 cm−3. The lower defect density induced by the IILs treatment could be attributed to the effective passivation of the perovskite film by the IILs. Therefore, the SCLC results are consistent with the previous EIS and Voc versus light analysis, in which the perovskite film treated with IILs shows a lower defect density and offers an enhanced Voc and FF in the PSCs. To evaluate the charge transport properties of the devices, dark J-V curves were measured and are shown in Fig. 3k. The device with IIL treatment has a lower leakage current than the control one, indicating a decreased carrier generation rate and reduced background carrier density in the device. Because the carrier generation rate in a solar cell in the dark is related to the trap density in the device [3, 50, 51], the dark J-V result further indicates that the IIL treatment could effectively passivate defects in perovskite.
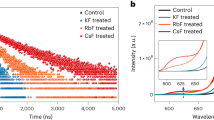
The influence of IILs with different anions on the perovskite devices and films was also studied. As shown in Fig. 4a, IILs with different anions were coated onto the perovskite layer as surface passivators. Figure 4b and Table 2 present the J-V curves and the corresponding photovoltaic parameters of optimized CsPbI2Br PSCs. The device with BMMIMBF4 treatment delivers a champion PCE as high as 16.89%. The EIS curves (Fig. 4c) and their fitted parameters (Table S8) suggest remarkably enhanced carrier transfer and suppressed charge recombination in the BMMIMBF4-treated devices. The XRD patterns and UV–vis spectra of perovskite films with IILs treatment are shown in Fig. 4d and S10, respectively. All the diffraction peaks and absorption edges of the perovskite films have no discernible change, confirming that the crystal structure of the perovskite remains largely unchanged by IILs modification. The PL and TRPL spectra of perovskite films with different IILs treatments were measured and are shown in Fig. 4e, f, respectively. All the PL spectra of these perovskite films show significant quenching and shorter carrier lifetime compared to those of the bare perovskite film, indicating increased charger transfer from the perovskite to the IILs. Among all these perovskite films, the one with BMMIMBF4 treatment shows the strongest quenching and the shortest carrier lifetime (Table S9), indicating the most effective charger transfer process.
a Schematic image of a CsPbI2Br PSC with the structure FTO/TiO2/CsPbI2Br(IILs)/Spiro-OMeTAD/Au. b J-V curves and c Nyquist plots of CsPbI2Br treated using IILs with different anions. d XRD patterns and (200) diffraction peaks, e PL and f TRPL spectra of CsPbI2Br perovskite films treated using IILs with different anions
Upon IILs treatment, as demonstrated by the FTIR, XPS, and NMR results, the IILs could form strong ionic bonds with the uncoordinated Pb and Cs to passivate positively charged halide vacancies [52, 53]. As such, the I− vacancies were diminished, accompanied by reduced defect density. In order to further demonstrate the passivation effect of the IILs, DFT calculations were carried out to adequately clarify the interaction the anions in the IILs with halide vacancies [54,55,56,57]. For reducing the amount of calculation, a 2 × 2 × 3 supercell of CsPbI3 was employed in the study (The detailed models and calculation details are given in the experimental section). Figure 5a shows a calculated structure that illustrates a BF4− anion passivating an I− vacancy at the CsPbI2Br surface. I− vacancy defects often act as non-radiative recombination centers and are responsible for the ionic conductivity of perovskites, causing operational instability. The relative binding affinities of different anions in the IILs to I− vacancies at the surface were estimated. The results exhibited in Fig. 5b reveal that BF4− has the highest binding energy to vacant I− sites in comparison with PF6−, Cl−, Br−, and I−. Pb-I antisites form localized states near the valence band edge of perovskite and act as non-radiative recombination centers. The binding energy between different anions in the IILs and Pb-I antisite was also confirmed by DFT. Figure 5c shows a calculated structure that illustrates a BF4− anion passivating an Pb-I antisite in the CsPbI2Br perovskite. As shown in Fig. 5d, BF4− has the highest binding energy to Pb-I antisite in comparison with other anions, which means that Pb-I antisite could be more effectively passivated by BF4−. Furthermore, the bonding energies of anions and cations in IILs with PbI2 are also calculated to identify the binding preference of the ions in IILs with CsPbI2Br. Figure 5e shows the calculated structures for PbI2 binding with a BF4− anion or BMMIM+ cation. As shown in Fig. 5f, the binding energy is as high as 0.61 eV for the HMIM+ cation, which is the highest among the cations in this study. However, it is still much smaller than that of the anions BF4− (1.08 eV) and PF6− (1.09 eV). The calculation result shows that the PMIM+, BMIM+, HMIM+, BMMIM+, and HMMIM+ cations cannot interact directly with PbI2, while BF4− or PF6− can strongly interact with PbI2. This result explicitly shows that the BF4− or PF6− anions can be incorporated into the CsPbI3 perovskite at the film surface to eliminate I− vacancies to reduce interfacial non-radiative recombination. In addition, compared with BF4− (2.76 Å), the larger size of PF6− (3.00 Å) could induce a certain stress to weaken the interaction energy with the perovskite film [58,59,60]. Therefore, BF4− anion is more suitable for passivating CsPbI2Br perovskite films. From the combined XPS, FTIR, NMR, and DFT calculated results, it can be concluded that anions (BF4−) play a more important role in passivating uncoordinated Pb+, Pb-I antisite defects and eliminating I− vacancy defects than do the cations.
a Calculated structure illustrating the passivation of an I− vacancy at the CsPbI2Br surface by a BF4− anion. b The relative interaction strengths of different anions with the I− vacancy at the surface of the perovskite. c Calculated structure illustrating the interaction of Pb-I antisite with BF4−. d The binding energy of different anions with Pb-I antisite defect. e Calculated structure illustrating the interaction of PbI2 with BF4− or BMMIM+. f The binding energy of different ions with PbI2
Apart from the efficiency, the device stability was evaluated upon exposure to air and light conditions. The appearance of the perovskite films exposed in air for different times was tracked, as seen in the photographs in Fig. 6a. The BMMIMBF4-treated film remained black even after 12 days exposure in air, while the control film shows obvious fading near the edges, indicating a phase transition from α to δ. As expected, the CsPbI2Br solar cells with BMMIMBF4 treatment show remarkably superior stability compared to the control one, retaining 94.4% of their initial efficiencies after being stored in air for 1440 h under ~ 25% RH. In contrast, the control device lost more than 20% of its PCE over the same period (Fig. 6b). Most importantly, as shown in Fig. 6c, the photostability of the devices with IILs treatment was significantly improved, with ~ 90% of their initial efficiencies remaining after 100 h under AM 1.5G sun illumination at 40 °C in ambient air (RH: ~ 30%), while the PCE of the control device decreased by nearly 50%. Therefore, the IILs surface passivation could significantly improve the ambient and light stability of the CsPbI2Br PSCs, which can be ascribed to the following effects: (1) The long alkyl chain of the IIL enhances the hydrophobicity of the perovskite film as confirmed by the dramatically increased contact angle from 64.9° to 91.2° (Fig. 1d) [25]. (2) Effective defect passivation reduces the active sites for phase transition.
4 Conclusions
A series of IILs with different cations and anions has been applied to unravel the mechanism for passivation of CsPbI2Br inorganic perovskites by the IILs. In contrast to previous studies, this work found that anions of the IILs play a more important role in passivation of lead- and cesium-related defects in inorganic perovskite compared with imidazole cations because they can form strong ionic interactions (Pb-F, Cs-F). These ionic bonds could passivate the surface and grain boundaries of the perovskite to reduce the charge-carrier recombination at the CsPbI2Br/spiro-OMeTAD interface. Due to the large steric hindrance effect, the interaction between the large imidazole cations and lead defects is weakened. Meanwhile, the IILs could improve the energy-level alignment between the perovskite and Spiro-OMeTAD for efficient charge transfer. Further, the IILs modification improves the hydrophobicity of the perovskite film, leading to an improved air stability of PSCs. As a result, the optimized CsPbI2Br PSCs with BMMIMBF4 treatment achieve the highest PCE of 17.02%, which is much higher than the PCE of the control device (15.62%). The unencapsulated device modified with BMMIMBF4 presents outstanding long-term stability when stored in ambient air at 25 °C with a RH of 25%, or under continuous illumination for 100 h. This work provides insightful guidelines toward the design or choice of effective IILs for improving the performance of inorganic PSCs or related photoelectric devices.
References
A. Ho-Baillie, M. Zhang, C.F.J. Lau, F.J. Ma, S. Huang, Untapped potentials of inorganic metal halide perovskite solar cells. Joule 3(4), 938–955 (2019). https://doi.org/10.1016/j.joule.2019.02.002
Q. Tai, K.C. Tang, F. Yan, Recent progress of inorganic perovskite solar cells. Energy Environ. Sci. 12(8), 2375–2405 (2019). https://doi.org/10.1039/c9ee01479a
C. Li, Z. Song, D. Zhao, C. Xiao, B. Subedi et al., Reducing saturation-current density to realize high-efficiency low-bandgap mixed tin–lead halide perovskite solar cells. Adv. Energy Mater. 9(3), 1803135 (2018). https://doi.org/10.1002/aenm.201803135
Y. Wang, Y. Chen, T. Zhang, X. Wang, Y. Zhao, Chemically stable black phase CsPbI3 inorganic perovskites for high-efficiency photovoltaics. Adv. Mater. 32(45), 2001025 (2020). https://doi.org/10.1002/adma.202001025
Y. Zheng, X. Yang, R. Su, P. Wu, Q. Gong et al., High-performance CsPbIxBr 3–x all-inorganic perovskite solar cells with efficiency over 18% via spontaneous interfacial manipulation. Adv. Funct. Mater. 30(46), 2000457 (2020). https://doi.org/10.1002/adfm.202000457
Z. Ni, C. Bao, Y. Liu, Q. Jiang, W.Q. Wu et al., Resolving spatial and energetic distributions of trap states in metal halide perovskite solar cells. Science 367(6484), 1352–1358 (2020). https://doi.org/10.1126/science.aba0893
J.M. Ball, A. Petrozza, Defects in perovskite-halides and their effects in solar cells. Nat. Energy 1, 16149 (2016). https://doi.org/10.1038/nenergy.2016.149
D.H. Kim, J.B. Whitaker, Z. Li, M.F.A.M. Hest, K. Zhu, Outlook and challenges of perovskite solar cells toward terawatt-scale photovoltaic module technology. Joule 2(8), 1437–1451 (2018). https://doi.org/10.1016/j.joule.2018.05.011
E. Aydin, M.D. Bastiani, S.D. Wolf, Defect and contact passivation for perovskite solar cells. Adv. Mater. 31(25), 1900428 (2019). https://doi.org/10.1002/adma.201900428
F. Qian, S. Yuan, Y. Cai, Y. Han, H. Zhao et al., Novel surface passivation for stable FA0.85MA0.15PbI3 perovskite solar cells with 21.6% efficiency. Sol. RRL 3(7), 1900072 (2019). https://doi.org/10.1002/solr.201900072
Q. Jiang, Y. Zhao, X. Zhang, X. Yang, Y. Chen et al., Surface passivation of perovskite film for efficient solar cells. Nat. Photon. 13, 460–466 (2019). https://doi.org/10.1038/s41566-019-0398-2
Q. Jiang, Z. Ni, G. Xu, Y. Lin, P.N. Rudd et al., Interfacial molecular doping of metal halide perovskites for highly efficient solar cells. Adv. Mater. 32(31), 2001581 (2020). https://doi.org/10.1002/adma.202001581
L. Meng, C. Sun, R. Wang, W. Huang, Z. Zhao et al., Tailored phase conversion under conjugated polymer enables thermally stable perovskite solar cells with efficiency exceeding 21%. J. Am. Chem. Soc. 140(49), 17255–17262 (2018). https://doi.org/10.1021/jacs.8b10520
L. Zuo, H. Guo, D.W. Quilettes, S. Jariwala, N.D. Marco et al., Polymer-modified halide perovskite films for efficient and stable planar heterojunction solar cells. Sci. Adv. 3(8), 1700106 (2017). https://doi.org/10.1126/sciadv.1700106
S. Wang, Z. Li, Y. Zhang, X. Liu, J. Han et al., Water-soluble triazolium ionic-liquid-induced surface self-assembly to enhance the stability and efficiency of perovskite solar cells. Adv. Funct. Mater. 29(15), 1900417 (2019). https://doi.org/10.1002/adfm.201900417
H. Choi, X. Liu, H.I. Kim, D. Kim, T. Park et al., A facile surface passivation enables thermally stable and efficient planar perovskite solar cells using a novel IDTT-based small molecule additive. Adv. Energy Mater. 11(16), 2003829 (2021). https://doi.org/10.1002/aenm.202003829
T. Niu, J. Lu, R. Munir, J. Li, D. Barrit et al., Stable high-performance perovskite solar cells via grain boundary passivation. Adv. Mater. 30(16), 1706576 (2018). https://doi.org/10.1002/adma.201706576
M. Qin, J. Cao, T. Zhang, J. Mai, T.K. Lau et al., Fused-ring electron acceptor ITIC-Th: a novel stabilizer for halide perovskite precursor solution. Adv. Energy Mater. 8(18), 1703399 (2018). https://doi.org/10.1002/aenm.201703399
T. Niu, L. Chao, W. Gao, C. Ran, L. Song et al., Ionic liquids-enabled efficient and stable perovskite photovoltaics: progress and challenges. ACS Energy Lett. 6(4), 1453–1479 (2021). https://doi.org/10.1021/acsenergylett.0c02696
S. Wang, B. Yang, J. Han, Z. He, T. Li et al., Polymeric room-temperature molten salt as a multifunctional additive toward highly efficient and stable inverted planar perovskite solar cells. Energy Environ. Sci. 13(12), 5068–5079 (2020). https://doi.org/10.1039/d0ee02043e
D. Yang, R. Yang, X. Ren, X. Zhu, Z. Yang et al., Hysteresis-suppressed high-efficiency flexible perovskite solar cells using solid-state ionic-liquids for effective electron transport. Adv. Mater. 28(26), 5206–5213 (2016). https://doi.org/10.1002/adma.201600446
W. Cai, Z. Zang, L. Ding, Ionic liquids in perovskite solar cells. J. Semicond. 42, 080201 (2021). https://doi.org/10.1088/1674-4926/42/8/080201
P. Caprioglio, D.S. Cruz, S. Caicedo-Dávila, F. Zu, A.A. Sutanto et al., Bi-functional interfaces by poly(ionic liquid) treatment in efficient pin and nip perovskite solar cells. Energy Environ. Sci. 14(8), 4508–4522 (2021). https://doi.org/10.1039/D1EE00869B
B. Yu, C. Zuo, J. Shi, Q. Meng, L. Ding, Defect engineering on all-inorganic perovskite solar cells for high efficiency. J. Semicond. 42, 050203 (2021). https://doi.org/10.1088/1674-4926/42/5/050203
S. Bai, P. Da, C. Li, Z. Wang, Z. Yuan et al., Planar perovskite solar cells with long-term stability using ionic liquid additives. Nature 571, 245–250 (2019). https://doi.org/10.1038/s41586-019-1357-2
D. Yang, X. Zhou, R. Yang, Z. Yang, W. Yu et al., Surface optimization to eliminate hysteresis for record efficiency planar perovskite solar cells. Energy Environ. Sci. 9(10), 3071–3078 (2016). https://doi.org/10.1039/c6ee02139e
W. Zhang, Z. Ren, Y. Guo, X. He, X. Li, Improved the long-term air stability of ZnO-based perovskite solar cells prepared under ambient conditions via surface modification of the electron transport layer using an ionic liquid. Electrochim. Acta 268, 539–545 (2018). https://doi.org/10.1016/j.electacta.2018.02.103
X. Zhou, M. Hu, C. Liu, L. Zhang, X. Zhong et al., Synergistic effects of multiple functional ionic liquid-treated PEDOT:PSS and less-ion-defects S-acetylthiocholine chloride-passivated perovskite surface enabling stable and hysteresis-free inverted perovskite solar cells with conversion efficiency over 20%. Nano Energy 63, 103866 (2019). https://doi.org/10.1016/j.nanoen.2019.103866
N.K. Noel, S.N. Habisreutinger, B. Wenger, Y.H. Lin, F. Zhang et al., Elucidating the role of a tetrafluoroborate-based ionic liquid at the n-type oxide/perovskite interface. Adv. Energy Mater. 10(4), 1903231 (2019). https://doi.org/10.1002/aenm.201903231
W. Zhang, X. Liu, B. He, Z. Gong, J. Zhu et al., Interface engineering of imidazolium ionic liquids toward efficient and stable CsPbBr 3 perovskite solar cells. ACS Appl. Mater. Interfaces 12(4), 4540–4548 (2020). https://doi.org/10.1021/acsami.9b20831
X. Zhu, M. Du, J. Feng, H. Wang, Z. Xu et al., High-efficiency perovskite solar cells with imidazolium-based ionic liquid for surface passivation and charge transport. Angew. Chem. Int. Ed. 60(8), 4238–4244 (2021). https://doi.org/10.1002/anie.202010987
C. Duan, J. Cui, M. Zhang, Y. Han, S. Yang et al., Precursor engineering for ambient-compatible antisolvent-free fabrication of high-efficiency CsPbI2Br perovskite solar cells. Adv. Energy Mater. 10(22), 2000691 (2020). https://doi.org/10.1002/aenm.202000691
H. Zhao, Y. Han, Z. Xu, C. Duan, S. Yang et al., A novel anion doping for stable CsPbI2Br perovskite solar cells with an efficiency of 15.56% and an open circuit voltage of 1.30 V. Adv. Energy Mater. 9(40), 1902279 (2019). https://doi.org/10.1002/aenm.201902279
Y.H. Lin, N. Sakai, P. Da, J. Wu, H.C. Sansom et al., A piperidinium salt stabilizes efficient metal-halide perovskite solar cells. Science 369(6499), 96–102 (2020). https://doi.org/10.1126/science.aba1628
S. Fu, X. Li, L. Wan, W. Zhang, W. Song et al., Effective surface treatment for high-performance inverted CsPbI2Br perovskite solar cells with efficiency of 15.92%. Nano-Micro Lett. 12, 170 (2020). https://doi.org/10.1007/s40820-020-00509-y
W.Q. Wu, Q. Wang, Y. Fang, Y. Shao, S. Tang et al., Molecular doping enabled scalable blading of efficient hole-transport-layer-free perovskite solar cells. Nat. Commun. 9, 1625 (2018). https://doi.org/10.1038/s41467-018-04028-8
N.K. Noel, S.N. Habisreutinger, A. Pellaroque, F. Pulvirenti et al., Interfacial charge-transfer doping of metal halide perovskites for high performance photovoltaics. Energy Environ. Sci. 12(10), 3063–3073 (2019). https://doi.org/10.1039/c9ee01773a
X. Guo, J. Su, Z. Lin, X. Wang, Q. Wang et al., Synergetic surface charge transfer doping and passivation toward high efficient and stable perovskite solar cells. iScience 24(4), 102276 (2021). https://doi.org/10.1016/j.isci.2021.102276
J. Lu, X. Lin, X. Jiao, T. Gengenbach, A.D. Scully et al., Interfacial benzenethiol modification facilitates charge transfer and improves stability of cm-sized metal halide perovskite solar cells with up to 20% efficiency. Energy Environ. Sci. 11(7), 1880–1889 (2018). https://doi.org/10.1039/c8ee00754c
D.G. Lee, D.H. Kim, J.M. Lee, B.J. Kim, J.Y. Kim et al., High efficiency perovskite solar cells exceeding 22% via a photo-assisted two-step sequential deposition. Adv. Funct. Mater. 31(9), 2006718 (2020). https://doi.org/10.1002/adfm.202006718
F. Cai, J. Cai, L. Yang, W. Li, R.S. Gurney et al., Molecular engineering of conjugated polymers for efficient hole transport and defect passivation in perovskite solar cells. Nano Energy 45, 28–36 (2018). https://doi.org/10.1016/j.nanoen.2017.12.028
S. Yuan, Y. Cai, S. Yang, H. Zhao, F. Qian et al., Simultaneous cesium and acetate coalloying improves efficiency and stability of FA0.85MA0.15PbI3 perovskite solar cell with an efficiency of 21.95%. Sol. RRL 3(9), 1900220 (2019). https://doi.org/10.1002/solr.201900220
C. Xu, Z. Zhang, S. Zhang, H. Si, S. Ma et al., Manipulation of perovskite crystallization kinetics via Lewis base additives. Adv. Funct. Mater. 31(13), 2009425 (2021). https://doi.org/10.1002/adfm.202009425
H. Si, Z. Zhang, Q. Liao, G. Zhang, Y. Ou et al., A-site management for highly crystalline perovskites. Adv. Mater. 32(4), 1904702 (2020). https://doi.org/10.1002/adma.201904702
M. Du, X. Zhu, L. Wang, H. Wang, J. Feng et al., High-pressure nitrogen-extraction and effective passivation to attain highest large-area perovskite solar module efficiency. Adv. Mater. 32(47), 2004979 (2020). https://doi.org/10.1002/adma.202004979
J. Tian, Q. Xue, X. Tang, Y. Chen, N. Li et al., Dual interfacial design for efficient CsPbI2Br perovskite solar cells with improved photostability. Adv. Mater. 31(23), 1901152 (2019). https://doi.org/10.1002/adma.201901152
D. Yang, R. Yang, K. Wang, C. Wu, X. Zhu et al., High efficiency planar-type perovskite solar cells with negligible hysteresis using EDTA-complexed SnO2. Nat. Commun. 9, 3239 (2018). https://doi.org/10.1038/s41467-018-05760-x
Y. Han, H. Zhao, C. Duan, S. Yang, Z. Yang et al., Controlled n-doping in air-stable CsPbI2Br perovskite solar cells with a record efficiency of 16.79%. Adv. Funct. Mater. 30(12), 1909972 (2020). https://doi.org/10.1002/adfm.201909972
S. Yang, W. Liu, Y. Han, Z. Liu, W. Zhao et al., 2D Cs2PbI2Cl2 nanosheets for holistic passivation of inorganic CsPbI2Br perovskite solar cells for improved efficiency and stability. Adv. Energy Mater. 10(46), 2002882 (2020). https://doi.org/10.1002/aenm.202002882
J. Cao, G. Tang, P. You, T. Wang, F. Zheng et al., Enhanced performance of planar perovskite solar cells induced by van der Waals epitaxial growth of mixed perovskite films on WS2 flakes. Adv. Funct. Mater. 30(38), 2002358 (2020). https://doi.org/10.1002/adfm.202002358
S.M. Sze, (2008) Semiconductor Devices: Physics and Technology. John wiley & sons
S. Xiong, Y. Dai, J. Yang, W. Xiao, D. Li et al., Surface charge-transfer doping for highly efficient perovskite solar cells. Nano Energy 79, 105505 (2021). https://doi.org/10.1016/j.nanoen.2020.105505
X. Zheng, B. Chen, J. Dai, Y. Fang, Y. Bai et al., Defect passivation in hybrid perovskite solar cells using quaternary ammonium halide anions and cations. Nat. Energy 2, 17102 (2017). https://doi.org/10.1038/nenergy.2017.102
G. Kresse, J. Hafner, Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993). https://doi.org/10.1103/physrevb.47.558
G. Kresse, J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996). https://doi.org/10.1103/PhysRevB.54.11169
J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple. Phys. Rev. Lett. 77(18), 3865–3868 (1996). https://doi.org/10.1103/PhysRevLett.77.3865
A. Tkatchenko, M. Scheffler, Accurate molecular van der Waals interactions from ground-state electron density and free-atom reference data. Phys. Rev. Lett. 102(7), 073005 (2009). https://doi.org/10.1103/PhysRevLett.102.073005
J. Chen, Y. Rong, A. Mei, Y. Xiong, T. Liu et al., Hole-conductor-free fully printable mesoscopic solar cell with mixed-anion perovskite CH3NH3PbI(3–x)(BF4)x. Adv. Energy Mater. 6(5), 1502009 (2009). https://doi.org/10.1002/aenm.201502009
J. Chen, S.G. Kim, N.G. Park, FA0.88Cs0.12PbI3−x(PF6)x interlayer formed by ion exchange reaction between perovskite and hole transporting layer for improving photovoltaic performance and stability. Adv. Mater. 30(40), 1801948 (2018). https://doi.org/10.1002/adma.201801948
S. Nagane, U. Bansode, O. Game, S. Chhatre, S. Ogale, CH3NH3PbI(3–x)(BF4)x: molecular ion substituted hybrid perovskite. Chem. Commun. 50(68), 9741–9744 (2014). https://doi.org/10.1039/c4cc04537h
Acknowledgements
J. Xu and J. Cui contributed equally to this work. The authors acknowledge support from the National Natural Science Foundation of China (62074095), the National Key Research and Development Program of China (2016YFA0202403), the Fundamental Research Funds for the Central Universities (GK202002001), the 111 Project (Grant No. B21005), and the DNL Cooperation Fund CAS (DNL180311). J. Cui would like to thank the support of H2 cluster in Xi’an Jiaotong University.
Funding
Open access funding provided by Shanghai Jiao Tong University.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, J., Cui, J., Yang, S. et al. Unraveling Passivation Mechanism of Imidazolium-Based Ionic Liquids on Inorganic Perovskite to Achieve Near-Record-Efficiency CsPbI2Br Solar Cells. Nano-Micro Lett. 14, 7 (2022). https://doi.org/10.1007/s40820-021-00763-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40820-021-00763-8