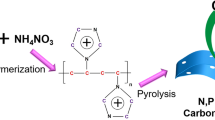
Abstract
A new strategy for the preparation of highly efficient catalyst used in oxygen evolution reaction (OER) in alkaline media was developed. A Co-containing carbonitride polymer network (CoCN) was selected as a structural-directing template and a hypercross-linked polymer containing S and P, which formed on CoCN skeleton in situ, was used as a cover. After calcination at 450°C for 2 h, an interconnected nanostructure was obtained and showed excellent activity and high stability for electrochemical water splitting. Trace amount of Co and other heteroatoms including N, S, P and the formed Co–N and Co–O species are essential for the impressive catalysis performance. The calcination temperature of 450°C is optimal to the catalysis performance. These results suggest that Co in addition to heteroatom-doped (S, P) carbonitride could be used as a supplement and/or an alternative to noble metal oxides for water splitting.
摘要
本文提出了一种制备碱性介质中电化学析氧反应高效催化剂的新方法. 该方法选用一种含钴的碳-氮聚合物网络作为结构模板, 外面包裹一层原位制备的、 含硫和磷的超支化交联聚合物. 450°C煅烧2 h后, 获得可用于电化学析氧反应的、 内部交联、 微观呈现层状结构的催化剂. 该催化剂在1.0 mol L-1的氢氧化钾水溶液中表现出很好的电化学催化活性和高稳定性. 电子衍射图谱(EDS)和X-射线光电子能谱(XPS)研究表明该催化剂含有痕量钴及其他杂原子, 包括氮、 硫、 磷, 且证实了能够大幅提高催化活性的Co-N和Co-O活性物质的存在. 将钴替换为铜和镍之后, 催化剂的催化活性大大降低, 表明当前方法对钴基催化剂的制备最为有效. 煅烧过程中所选用的温度对催化剂的催化活性亦有显著影响, 450°C为最优温度. 这些结果表明, 含钴的、 杂原子(硫、 磷)掺杂的碳氮化物有望成为一类新的电解水催化剂, 以取代贵金属氧化物, 或作为其有益的补充.
Similar content being viewed by others
References
Wang J, Xu F, Jin H, et al. Non-noble metal-based carbon composites in hydrogen evolution reaction: fundamentals to applications. Adv Mater, 2017, 29: 1605838
Zheng Y, Jiao Y, Jaroniec M, et al. Nanostructured metal-free electrochemical catalysts for highly efficient oxygen reduction. Small, 2012, 8: 3550–3566
Dai L, Xue Y, Qu L, et al. Metal-free catalysts for oxygen reduction reaction. Chem Rev, 2015, 115: 4823–4892
Zhu YP, Guo C, Zheng Y, et al. Surface and interface engineering of noble-metal-free electrocatalysts for efficient energy conversion processes. Acc Chem Res, 2017, 50: 915–923
Jiao F, Frei H. Nanostructured cobalt oxide clusters in mesoporous silica as efficient oxygen-evolving catalysts. Angew Chem Int Ed, 2009, 48: 1841–1844
Liang Y, Li Y, Wang H, et al. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat Mater, 2011, 10: 780–786
Koza JA, He Z, Miller AS, et al. Electrodeposition of crystalline Co3O4—a catalyst for the oxygen evolution reaction. Chem Mater, 2012, 24: 3567–3573
Lu X, Zhao C. Highly efficient and robust oxygen evolution catalysts achieved by anchoring nanocrystalline cobalt oxides onto mildly oxidized multiwalled carbon nanotubes. J Mater Chem A, 2013, 1: 12053–12059
Sa YJ, Kwon K, Cheon JY, et al. Ordered mesoporous Co3O4 spinels as stable, bifunctional, noble metal-free oxygen electrocatalysts. J Mater Chem A, 2013, 1: 9992–10001
Singh SK, Dhavale VM, Kurungot S. Low surface energy plane exposed Co3O4 nanocubes supported on nitrogen-doped graphene as an electrocatalyst for efficient water oxidation. ACS Appl Mater Interfaces, 2015, 7: 442–451
Cao X, Zheng X, Tian J, et al. Cobalt sulfide embedded in porous nitrogen-doped carbon as a bifunctional electrocatalyst for oxygen reduction and evolution reactions. Electrochim Acta, 2016, 191: 776–783
Long J, Gong Y, Lin J. Metal-organic framework-derived Co9S8@ CoS@CoO@C nanoparticles as efficient electro- and photo-catalysts for the oxygen evolution reaction. J Mater Chem A, 2017, 5: 10495–10509
Liao M, Zeng G, Luo T, et al. Three-dimensional coral-like cobalt selenide as an advanced electrocatalyst for highly efficient oxygen evolution reaction. Electrochim Acta, 2016, 194: 59–66
Ren X, Ge R, Zhang Y, et al. Cobalt-borate nanowire array as a high-performance catalyst for oxygen evolution reaction in nearneutral media. J Mater Chem A, 2017, 5: 7291–7294
Liu M, Li J. Cobalt phosphide hollow polyhedron as efficient bifunctional electrocatalysts for the evolution reaction of hydrogen and oxygen. ACS Appl Mater Interfaces, 2016, 8: 2158–2165
Xie L, Zhang R, Cui L, et al. High-performance electrolytic oxygen evolution in neutral media catalyzed by a cobalt phosphate nanoarray. Angew Chem Int Ed, 2017, 56: 1064–1068
Subbaraman R, Tripkovic D, Chang KC, et al. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr (oxy)oxide catalysts. Nat Mater, 2012, 11: 550–557
Chen P, Xu K, Tong Y, et al. Cobalt nitrides as a class of metallic electrocatalysts for the oxygen evolution reaction. Inorg Chem Front, 2016, 3: 236–242
Chen S, Qiao SZ. Hierarchically porous nitrogen-doped graphene-NiCo2O4 hybrid paper as an advanced electrocatalytic watersplitting material. ACS Nano, 2013, 7: 10190–10196
Shi H, Zhao G. Water oxidation on spinel NiCo2O4 nanoneedles anode: microstructures, specific surface character, and the enhanced electrocatalytic performance. J Phys Chem C, 2014, 118: 25939–25946
Rosen J, Hutchings GS, Jiao F. Ordered mesoporous cobalt oxide as highly efficient oxygen evolution catalyst. J Am Chem Soc, 2013, 135: 4516–4521
Cai P, Huang J, Chen J, et al. Oxygen-containing amorphous cobalt sulfide porous nanocubes as high-activity electrocatalysts for the oxygen evolution reaction in an alkaline/neutral medium. Angew Chem Int Ed, 2017, 56: 4858–4861
Mendoza-Garcia A, Su D, Sun S. Sea urchin-like cobalt-iron phosphide as an active catalyst for oxygen evolution reaction. Nanoscale, 2016, 8: 3244–3247
Miyahara Y, Miyazaki K, Fukutsuka T, et al. Strontium cobalt oxychlorides: enhanced electrocatalysts for oxygen reduction and evolution reactions. Chem Commun, 2017, 53: 2713–2716
Al-Mamun M, Wang Y, Liu P, et al. One-step solid phase synthesis of a highly efficient and robust cobalt pentlandite electrocatalyst for the oxygen evolution reaction. J Mater Chem A, 2016, 4: 18314–18321
Zhuang L, Ge L, Yang Y, et al. Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv Mater, 2017, 29: 1606793
Tang C, Wang B, Wang HF, et al. Defect engineering toward atomic Co-Nx-C in hierarchical graphene for rechargeable flexible solid Zn-air batteries. Adv Mater, 2017, 29: 1703185
Pan T, Liu H, Ren G, et al. Metal-free porous nitrogen-doped carbon nanotubes for enhanced oxygen reduction and evolution reactions. Sci Bull, 2016, 61: 889–896
Bau VM, Bo X, Guo L. Nitrogen-doped cobalt nanoparticles/nitrogen-doped plate-like ordered mesoporous carbons composites as noble-metal free electrocatalysts for oxygen reduction reaction. J Energy Chem, 2017, 26: 63–71
Zheng Y, Jiao Y, Chen J, et al. Nanoporous graphitic-C3N4@carbon metal-free electrocatalysts for highly efficient oxygen reduction. J Am Chem Soc, 2011, 133: 20116–20119
Liang J, Zheng Y, Chen J, et al. Facile oxygen reduction on a threedimensionally ordered macroporous graphitic C3N4/carbon composite electrocatalyst. Angew Chem Int Ed, 2012, 51: 3892–3896
Ma TY, Dai S, Jaroniec M, et al. Graphitic carbon nitride nanosheet-carbon nanotube three-dimensional porous composites as high-performance oxygen evolution electrocatalysts. Angew Chem Int Ed, 2014, 53: 7281–7285
Qiu Y, Xin L, Jia F, et al. Three-dimensional phosphorus-doped graphitic-C3N4 self-assembly with NH2-functionalized carbon composite materials for enhanced oxygen reduction reaction. Langmuir, 2016, 32: 12569–12578
Gong K, Du F, Xia Z, et al. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science, 2009, 323: 760–764
Li L, Yang H, Miao J, et al. Unraveling oxygen evolution reaction on carbon-based electrocatalysts: effect of oxygen doping on adsorption of oxygenated intermediates. ACS Energy Lett, 2017, 2: 294–300
Schwab MG, Fassbender B, Spiess HW, et al. Catalyst-free preparation of melamine-based microporous polymer networks through schiff base chemistry. J Am Chem Soc, 2009, 131: 7216–7217
Li G, Zhang B, Yan J, et al. The cost-effective synthesis of furanand thienyl-based microporous polyaminals for adsorption of gases and organic vapors. Chem Commun, 2016, 52: 1143–1146
Yang S, Peng L, Huang P, et al. Nitrogen, phosphorus, and sulfur Co-doped hollow carbon shell as superior metal-free catalyst for selective oxidation of aromatic alkanes. Angew Chem Int Ed, 2016, 55: 4016–4020
Wei J, Hu Y, Liang Y, et al. Nitrogen-doped nanoporous carbon/graphene nano-sandwiches: synthesis and application for efficient oxygen reduction. Adv Funct Mater, 2015, 25: 5768–5777
Zhong H, Wang J, Zhang Y, et al. ZIF-8 derived graphene-based nitrogen-doped porous carbon sheets as highly efficient and durable oxygen reduction electrocatalysts. Angew Chem Int Ed, 2014, 53: 14235–14239
Razmjooei F, Singh KP, Song MY, et al. Enhanced electrocatalytic activity due to additional phosphorous doping in nitrogen and sulfur-doped graphene: a comprehensive study. Carbon, 2014, 78: 257–267
Wang C, Sun L, Zhou Y, et al. P/N co-doped microporous carbons from H3PO4-doped polyaniline by in situ activation for supercapacitors. Carbon, 2013, 59: 537–546
Bayatsarmadi B, Zheng Y, Tang Y, et al. Significant enhancement of water splitting activity of N-carbon electrocatalyst by trace level Co doping. Small, 2016, 12: 3703–3711
Tüysüz H, Hwang YJ, Khan SB, et al. Mesoporous Co3O4 as an electrocatalyst for water oxidation. Nano Res, 2013, 6: 47–54
Wu J, Xue Y, Yan X, et al. Co3O4 nanocrystals on single-walled carbon nanotubes as a highly efficient oxygen-evolving catalyst. Nano Res, 2012, 5: 521–530
Hou Y, Li J, Wen Z, et al. Co3O4 nanoparticles embedded in nitrogen- doped porous carbon dodecahedrons with enhanced electrochemical properties for lithium storage and water splitting. Nano Energy, 2015, 12: 1–8
Guo Y, Tong Y, Chen P, et al. Engineering the electronic state of a perovskite electrocatalyst for synergistically enhanced oxygen evolution reaction. Adv Mater, 2015, 27: 5989–5994
Hou Y, Wen Z, Cui S, et al. An advanced nitrogen-doped graphene/cobalt-embedded porous carbon polyhedron hybrid for efficient catalysis of oxygen reduction and water splitting. Adv Funct Mater, 2015, 25: 872–882
Qu K, Zheng Y, Dai S, et al. Graphene oxide-polydopamine derived N, S-codoped carbon nanosheets as superior bifunctional electrocatalysts for oxygen reduction and evolution. Nano Energy, 2016, 19: 373–381
Kong D, Wang H, Lu Z, et al. CoSe2 nanoparticles grown on carbon fiber paper: an efficient and stable electrocatalyst for hydrogen evolution reaction. J Am Chem Soc, 2014, 136: 4897–4900
Jiang J, Li Y, Liu J, et al. Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv Mater, 2012, 24: 5166–5180
Tian J, Liu Q, Asiri AM, et al. Self-supported nanoporous cobalt phosphide nanowire arrays: an efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J Am Chem Soc, 2014, 136: 7587–7590
Gao H, Pan J, Han D, et al. Facile synthesis of microcellular foam catalysts with adjustable hierarchical porous structure, acid-base strength and wettability for biomass energy conversion. J Mater Chem A, 2015, 3: 13507–13518
Liu ZW, Peng F, Wang HJ, et al. Phosphorus-doped graphite layers with high electrocatalytic activity for the O2 reduction in an alkaline medium. Angew Chem Int Ed, 2011, 50: 3257–3261
Zhang C, Mahmood N, Yin H, et al. Synthesis of phosphorus-doped graphene and its multifunctional applications for oxygen reduction reaction and lithium ion batteries. Adv Mater, 2013, 25: 4932–4937
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21603243, 21402215 and 61474124), the Natural Science Foundation of Gansu Province (1606RJZA112) and the Natural science research project of Education Department of Shaanxi Province (17JK0093).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jiamei Cao obtained her BSc degree from the Department of Northwest Normal University in 2014, and her MSc degree from Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, under the supervision of Prof. Hongguang Li, in July 2017. Her research interest is focused on microporous polymeric materials and functional fullerenes for applications in clean energy.
Yongqiang Feng obtained his BSc degree from Sichuan University in 2010 and PhD degree from the Institute of Chemistry, Chinese Academy of Sciences in 2015. After that, he joined Prof. Hongguang Li’s group in Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences as an assistant professor. Now, he is an associate professor in Shannxi University of Science & Technology. His current interest is focused on the design of carbon-based nanomaterials for applications in optoelectronic devices.
Baoyong Liu obtained his BSc degree from Shandong University in 2003. And he received his Master’s degree in chemical engineering from China University of Petroleum (East China) in 2008. He is currently a PhD candidate at Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences under the supervision of Prof. Hongguang Li. His research interest includes carbon nanomaterials and tribology.
Hongguang Li obtained his BSc degree and PhD degree from Shandong University in 2003 and 2008, respectively. After three-year postdoc research, he joined the Laboratory of Clean Energy Chemistry and Materials, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences as a professor. His current interest focuses on the design of novel π-conjugated molecules and engineering carbon nanomaterials for the applications in optoelectronic devices.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Cao, J., Feng, Y., Liu, B. et al. Carbon skeleton doped with Co, N, S and P as efficient electrocatalyst for oxygen evolution reaction. Sci. China Mater. 61, 686–696 (2018). https://doi.org/10.1007/s40843-017-9149-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-017-9149-y