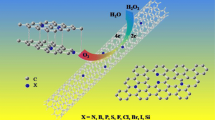
Abstract
Oxygen reduction reaction (ORR) is key to fuel cells and metal-air batteries which are considered as the alternative clean energy. Various carbon materials have been widely researched as ORR electrocatalysts. It has been accepted that heteroatom doping and exposure of the edge sites can effectively improve the activity of carbon materials. In this work, we used a simple method to prepare a novel N, P-dual doped carbon-based catalyst with many holes on the surface. In addition, trace level Co doping in the carbon material forming Co–N–C active species can further enhance the ORR performance. On one hand, the doping can adjust the electronic structure of carbon atoms, which would induce more active sites for ORR. And on the other hand, the holes formed on the surface of carbon nanosheets would expose more edge sites and can improve the intrinsic activity of carbon. Due to the heteroatom doping and the exposed edge sites, the prepared carbon materials showed highly excellent ORR performance, close to that of commercial Pt/C.
摘要
本文使用有机分子配位聚合作用一步聚合、 碳化、 酸洗得到了一种N,P双掺杂碳材料. 其具有痕量掺杂的金属钴、 且具有更多活性边缘. X射线光电子能谱显示杂原子成功进入碳材料当中, 并且发现酸洗后钴的信号非常低, 证明酸洗后, 材料表面形成非常多的孔, 暴露出更多的边缘催化位点. 制备的碳材料具有大量催化活性位点, 因此表现出极其优异的电催化氧还原性能. 另外, 与Pt/C相比, 制备的多孔碳材料还具有较好的抗毒性与稳定性, 进一步显示了其在新能源电池领域的应用前景.
Similar content being viewed by others
References
Dai L, Xue Y, Qu L, et al. Metal-free catalysts for oxygen reduction reaction. Chem Rev, 2015, 115: 4823–4892
Gong K, Du F, Xia Z, et al. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science, 2009, 323: 760–764
Jasinski R. A new fuel cell cathode catalyst. Nature, 1964, 201: 1212–1213
Wang S, Jiang SP. Prospects of fuel cell technologies. Nat Sci Rev, 2017, 4: 163–166
Shao M, Chang Q, Dodelet JP, et al. Recent advances in electrocatalysts for oxygen reduction reaction. Chem Rev, 2016, 116: 3594–3657
Wang S, Zhang L, Xia Z, et al. BCN graphene as efficient metalfree electrocatalyst for the oxygen reduction reaction. Angew Chem Int Ed, 2012, 51: 4209–4212
Zhao C, Yu C, Liu S, et al. 3D porous N-doped graphene frameworks made of interconnected nanocages for ultrahigh-rate and long-life Li-O2 batteries. Adv Funct Mater, 2015, 25: 6913–6920
Zhou T, Du Y, Yin S, et al. Nitrogen-doped cobalt phosphate@ nanocarbon hybrids for efficient electrocatalytic oxygen reduction. Energy Environ Sci, 2016, 9: 2563–2570
Yang L, Jiang S, Zhao Y, et al. Boron-doped carbon nanotubes as metal-free electrocatalysts for the oxygen reduction reaction. Angew Chem Int Ed, 2011, 50: 7132–7135
Xia BY, Yan Y, Li N, et al. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat Energy, 2016, 1: 15006
Wang S, Yu D, Dai L. Polyelectrolyte functionalized carbon nanotubes as efficient metal-free electrocatalysts for oxygen reduction. J Am Chem Soc, 2011, 133: 5182–5185
Guo D, Shibuya R, Akiba C, et al. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science, 2016, 351: 361–365
Liang HW, Wei W, Wu ZS, et al. Mesoporous metal–nitrogendoped carbon electrocatalysts for highly efficient oxygen reduction reaction. J Am Chem Soc, 2013, 135: 16002–16005
Zhang G, Jin X, Li H, et al. N-doped crumpled graphene: bottomup synthesis and its superior oxygen reduction performance. Sci China Mater, 2016, 59: 337–347
Wu S, Zhu Y, Huo Y, et al. Bimetallic organic frameworks derived CuNi/carbon nanocomposites as efficient electrocatalysts for oxygen reduction reaction. Sci China Mater, 2017, 60: 654–663
Wang L, Jia W, Liu X, et al. Sulphur-doped ordered mesoporous carbon with enhanced electrocatalytic activity for the oxygen reduction reaction. J Energy Chem, 2016, 25: 566–570
Seredych M, László K, Rodríguez-Castellón E, et al. S-doped carbon aerogels/GO composites as oxygen reduction catalysts. J Energy Chem, 2016, 25: 236–245
Preuss K, Kannuchamy VK, Marinovic A, et al. Bio-inspired carbon electro-catalysts for the oxygen reduction reaction. J Energy Chem, 2016, 25: 228–235
Zhang J, Dai L. Heteroatom-doped graphitic carbon catalysts for efficient electrocatalysis of oxygen reduction reaction. ACS Catal, 2015, 5: 7244–7253
Zhang L, Xia Z. Mechanisms of oxygen reduction reaction on nitrogen-doped graphene for fuel cells. J Phys Chem C, 2011, 115: 11170–11176
Xiao Z, Wang Y, Huang YC, et al. Filling the oxygen vacancies in Co3O4 with phosphorus: an ultra-efficient electrocatalyst for overall water splitting. Energy Environ Sci, 2017, 44
Dou S, Dong CL, Hu Z, et al. Atomic-scale CoOx species in metalorganic frameworks for oxygen evolution reaction. Adv Funct Mater, 2017, 27: 1702546
Zhang J, Qu L, Shi G, et al. N,P-codoped carbon networks as efficient metal-free bifunctional catalysts for oxygen reduction and hydrogen evolution reactions. Angew Chem Int Ed, 2016, 55: 2230–2234
Chang Y, Hong F, He C, et al. Nitrogen and sulfur dual-doped non-noble catalyst using fluidic acrylonitrile telomer as precursor for efficient oxygen reduction. Adv Mater, 2013, 25: 4794–4799
Wang X, Wang J, Wang D, et al. One-pot synthesis of nitrogen and sulfur co-doped graphene as efficient metal-free electrocatalysts for the oxygen reduction reaction. Chem Commun, 2014, 50: 4839–4842
Shen A, Zou Y, Wang Q, et al. Oxygen reduction reaction in a droplet on graphite: direct evidence that the edge is more active than the basal plane. Angew Chem Int Ed, 2014, 53: 10804–10808
Tang C, Wang HF, Chen X, et al. Topological defects in metal-free nanocarbon for oxygen electrocatalysis. Adv Mater, 2016, 28: 6845–6851
Yan D, Li Y, Huo J, et al. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions. Adv Mater, 2017, 414: 1606459
Tang C, Wang B, Wang HF, et al. Defect engineering toward atomic Co-Nx-C in hierarchical graphene for rechargeable flexible solid Zn-air batteries. Adv Mater, 2017, 29: 1703185
Liu Z, Zhao Z, Wang Y, et al. In situ exfoliated, edge-rich, oxygenfunctionalized graphene from carbon fibers for oxygen electrocatalysis. Adv Mater, 2017, 29: 1606207
Wu G, More KL, Johnston CM, et al. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science, 2011, 332: 443–447
Chen Y, Gokhale R, Serov A, et al. Novel highly active and selective Fe-N-C oxygen reduction electrocatalysts derived from in-situ polymerization pyrolysis. Nano Energy, 2017, 38: 201–209
Hu K, Xiao Z, Cheng Y, et al. Iron phosphide/N, P-doped carbon nanosheets as highly efficient electrocatalysts for oxygen reduction reaction over the whole pH range. Electrochim Acta, 2017, 254: 280–286
Jiang Y, Yang L, Sun T, et al. Significant contribution of intrinsic carbon defects to oxygen reduction activity. ACS Catal, 2015, 5: 6707–6712
Tao L, Wang Q, Dou S, et al. Edge-rich and dopant-free graphene as a highly efficient metal-free electrocatalyst for the oxygen reduction reaction. Chem Commun, 2016, 52: 2764–2767
Yan D, Dou S, Tao L, et al. Electropolymerized supermolecule derived N, P co-doped carbon nanofiber networks as a highly efficient metal-free electrocatalyst for the hydrogen evolution reaction. J Mater Chem A, 2016, 4: 13726–13730
Sun M, Zhang G, Liu H, et al. α- and γ-Fe2O3 nanoparticle/nitrogen doped carbon nanotube catalysts for high-performance oxygen reduction reaction. Sci China Mater, 2015, 58: 683–692
Zhang J, Zhao Z, Xia Z, et al. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat Nanotechnol, 2015, 10: 444–452
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21701043, 21573066, and 51402100), the Provincial Natural Science Foundation of Hunan (2016JJ1006 and 2016TP1009), the Key Laboratory of Optoelectronic Devices and Systems of Ministry of Education and Guangdong Province and Shenzhen Science and Technology Program (JCYJ20170306141659388).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dafeng Yan received his BSc degree in 2014 from the Department of Chemistry, Hunan University, China. He is currently pursuing his PhD degree under the supervision of Prof. Shuangyin Wang. His current interests include the synthesis and characterization of nanomaterials with various defects and the relationship between the defects and the electrocatalytic performance of renewable energy-related reactions.
Shuangyin Wang received his BSc degree in 2006 from Zhejiang University and PhD in 2010 from Nanyang Technological University, Singapore. He was a postdoctoral fellow working with Prof. Liming Dai (2010–2011) and Prof. A. Manthiram (2011–2012). He is currently a Professor of Hunan University. His research interests are in novel carbon catalysts, defects in various crystals and their application on electrocatalysis and batteries.
Electronic supplementary material
40843_2017_9174_MOESM1_ESM.pdf
N, P-Dual Doped Carbon with Trace Co and Rich Edge Sites As Highly Efficient Electrocatalyst for Oxygen Reduction Reaction
Rights and permissions
About this article
Cite this article
Yan, D., Guo, L., Xie, C. et al. N, P-dual doped carbon with trace Co and rich edge sites as highly efficient electrocatalyst for oxygen reduction reaction. Sci. China Mater. 61, 679–685 (2018). https://doi.org/10.1007/s40843-017-9174-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-017-9174-1