Abstract
Colorectal carcinoma (CRC) as a major health problem in industrialized countries is highly preventable and can be successfully treated in the early stages. However, incidence and mortality of CRC has increased over the last two decades. The reason could be that the current recommended options for screening are costly, unpleasant for patients, have low sensitivity and poor accessibility for screening. These reasons provide a strong rationale for the development of a new method. Opto-magnetic imaging spectroscopy (OMIS) as a new imaging method for the characterisation of various materials, including human tissues, is based on light-matter interaction, using a Poincare sphere for light properties and a Bloch sphere for electron properties, and allows the detection of biophysical characteristics within human tissue samples. Compared with histopathology examination, the OMIS method achieved an accuracy of 92.59% using Multilayer Perceptron Neural Network as a classifier, and 89.87% using Naïve-Bayes, respectively. The obtained results, based on the investigation of 316 samples, both tumour and normal mucosa (162 cancer cases), strongly suggest that the new non-invasive OMIS method might be used for tissue characterization ex vivo to discriminate between the healthy and carcinoma state of the colon. However, it opens up the possibility of using the same method in in vivo studies to assist physicians in targeting biopsies of colorectal tissue.









Similar content being viewed by others
Notes
Opto-magnetic imaging spectroscopy.
MLP—multilayer perceptron neural network.
TNM Classification of Malignant Tumours.
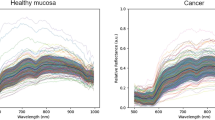
Normalized arbitrary unites.
Fourier Transformed Infrared spectroscopy.
References
Cancer/The problem. Retrieved November 13, 2017 http://www.who.int/mediacentre/factsheets/fs297/en/.
National cancer institute/Cancer statistics. Retrieved November 13, 2017 https://www.cancer.gov/about-cancer/understanding/statistics.
Colorectal cancer statistics. 2017. Retrieved November 13, 2017 http://pressroom.cancer.org/CRCstats2017.
Sali, L., et al. (2013). Screening for colorectal cancer with FOBT, virtual colonoscopy and optical colonoscopy: study protocol for a randomized controlled trial in the Florence district (SAVE study). Trials. https://doi.org/10.1186/1745-6215-14-74.
Franz, M., Scholz, M., Henze, I., Röcki, S., & Gomez, L. (2013). Detection of colon polyps by a novel, polymer pattern-based full blood test. Journal of Translation Medicine. https://doi.org/10.1186/1479-5876-11-278.
Zhan, T., Hielscher, T., Hahn, F., Hauf, C., Betge, J., Ebert, M. P., et al. (2016). Risk factors for local recurrence of large, flat colorectal polyps after endoscopic mucosal resection. Digestion, 93(4), 311–317.
Koruga, D., Tomic, A. (2009). System and method for analysis of light-matter interaction based on spectral convolution. US Patent Pub. No. 2009/0245603.
Coey, J. M. D. (2015). Magnetism and magnetic materials. Cambridge: Cambridge University Press.
Lakshminarayanan, V., Calvo, M. L., & Alieva, T. (2013). Mathematical optics: Classical, quantum, and computational methods. Boca Raton, FL: CRC Press Taylor & Francis Group.
Koruga, D. (2017). Hyperpolarized light: Fundamentals of nanobiomedical photonics. Belgrade: Zepter World Book.
Alonso, M., & Finn, E. J. (1992). Physics. New York: Addison-Wesley Publishing Company.
Malacara, D. (2011). Color vision and colorimetry: Theory and applications (2nd ed.). Washington, DC: SPIE Press.
Rex, D. K. (2000). Colon tumours and colonoscopy. Endoscopy, 32(11), 833–874.
Matija, L., Jeftic, B., Nikolic, G., Dragicevic, A., Mileusnic, I., Muncan, J., et al. (2014). Nanophysical approach to diagnosis of epithelial tissue using opto-magnetic imaging spectroscopy. In A. Seifalian, A. Mel, & D. M. Kalaskar (Eds.), Nanomedicine (pp. 156–186). London: One Central Press.
Koruga, D., Tomic, A. (2009). Method and algorithm for analysis of light-matter interaction based on spectral convolution. US Pat. App. No. 61/061,852, 2008, PCT/US2009/030347, Publication No: WO/2009/089292.
Koruga, Đ., Miljković, S., Ribar, S., Matija, L., & Kojić, D. (2010). Water hydrogen bonds study by opto-magnetic fingerprint technique. Acta Physica Polonica A, 117(5), 777–781.
Papić-Obradović, M., Kojić, D., & Matija, L. (2010). Opto-magnetic method for Epstein–Barr virus and cytomegalovirus detection in blood plasma samples. Acta Physica Polonica A, 117(5), 782–785.
Dragicevic, A., Krivokapic, Z., Dimitrijevic, I., Markovic, V., Matija, L., & Koruga, D. (2015). Ex vivo preclinical study of colon cancer using opto-magnetic imaging spectroscopy and dual speed spinner magnetometer. European Journal of Cancer, 51(3), S130–S131.
Koruga, Đ., Bandić, J., Janjić, G., Lalović, Č., Munćan, J., & Dobrosavljević-Vukojević, D. (2012). Epidermal layers’ characterisation by opto-magnetic spectroscopy based on digital image of skin. Acta Phisica Polonica A, 121(3), 606–610.
Jeftić, B., Papic-Obradović, M., Munćan, J., Matija, L., & Koruga, Đ. (2017). Optomagnetic imaging spectroscopy application in cervical dysplasia and cancer detection: Comparation of stained 10 and unstained papanicolaou smears. Journal of Medical and Biological Engineering. https://doi.org/10.1007/s40846-017-0255-z.
Papic-Obradovic, M. (2012). Early diagnostics of epithelial tissue cancer (in Serbian). Belgrade: Don Vas.
Papic-Obradovic, M., Jeftic, B., Dragicevic, A., Muncan, J., Matija, L., & Koruga, D. (2015). Optomagnetic Imaging Spectroscopy in characterisation of cervical tissue and cancer detection using unstained sample approach. European Journal of Cancer, 51(Supplement 3), S130.
Stamenković, D., Kojić, D., Matija, L., Miljković, Z., & Babić, B. (2010). Physical properties of contact lenses characterized by scanning probe microscopy and optomagnetic fingerprint. International Journal of Modern Physics B, 24, 825–834.
Iyer, R., Menon, V., Buice, M., Koch, C., & Mihalas, S. (2013). The influence of synaptic weight distribution on neuronal population dynamics. PLoS Computational Biology, 9(10), e1003248.
Ratanamahatana, C., & Gunopulos, D. (2003). Feature selection for the naïve bayesian classifier using decision trees. Applied Artificial Intelligence, 17(5–6), 475–487.
Wang, L.-M., Li, X.-L., Cao, C.-H., & Yuan, S.-M. (2006). Combining decision tree and Naïve Bayes for classification. Knowledge-Based Systems, 19(7), 511–515.
Muralidharan, V., & Sugumaran, V. (2012). A comparative study of Naïve Bayes classifier and Bayes net classifier for fault diagnosis of monoblock centrifugal pump using wavelet analysis. Applied Soft Computing, 12(8), 2023–2029.
Zhang, H. (2004). The Optimality of Naive Bayes. Proceedings of the Seventeenth International Florida Artificial Intelligence Research Society Conference, FLAIRS 2004, AAAI Press.
Domingos, P., & Pazzani, M. (1997). On the optimality of the simple Bayesian classifier under zero-one loss. Machine Learning, 29, 103–130.
Mackanos, M. A., & Contag, C. H. (2010). Fiber-optic probes enable cancer detection with FTIR spectroscopy. Trends in Biotechnology, 28(6), 317–323.
Tuchin, V. V. (2005). Optical clearing of tissues and blood. Washington, DC: SPIE Press.
Wiesner, W., Mortelé, K. J., Ji, H., & Ros, P. R. (2002). Normal colonic wall thickness at CT and its relation to colonic distension. Journal of Computer Assisted Tomography, 26(1), 102–106.
Atanackovic, M., Bacetić, D., Begić-Janeva, G., Boričić, I., Brašanac, D., Cvetković-Dožić, D., et al. (2003). Patologija. Beograd: Medicinski fakultet Univerziteta u Beogradu, Katedra za patološku anatoniju.
Edge, S. B., Byrd, D. R., Compton, C. C., Fritz, A. G., Greene, F. L., & Trotti, A. (2010). AJCC cancer staging manual (7th ed.). New York: Springer.
Hecht-Nielsen, R. (1989). Neurocomputing. Boston, MA: Addison-Wesley Longman Publishing Co.
Recursive Feature Elimination. Retrieved November 21, 2017 http://topepo.github.io/caret/recursive-feature-elimination.html.
Xie, Z. X., Hu, Q. H., & Yu, D. R. (2006). Improved feature selection algorithm based on SVM and correlation. In J. Wang, Z. Yi, J. M. Zurada, B. L. Lu, & H. Yin (Eds.), Advances in neural networks-ISNN 2006. Berlin: Springer.
Fawcett, T., & Provost, F. (1996). Combining data mining and machine learning for effective user profiling. In Simoudis, E., Han, J., & Fayyad, U. (Eds.), Proceedings of the Second International Conference on Knowledge Discovery and Data Mining (pp. 8–13). Menlo Park, CA: AAAI Press. Provost et al. 1998.
Fawcett, T., & Provost, F. (1997). Adaptive fraud detection. Data Mining and Knowledge Discovery, 1(3), 291–316.
Winawer, S. J., Zauber, A. G., Ho, M. H., et al. (1993). The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopy polypectomy. New England Journal of Medicine, 329(27), 1977–1981.
American Cancer Society. Cancer Facts & Figures, 2010. American Cancer Society; Retrieved April 26, 2016 http://www.cancer.org/Research/CancerFactsFigures/index.
Krivokapić, Z. (2012). Karcinom rektuma. Zavod za Udzbenike: Beograd.
Mamazza, J., & Gordon, P. H. (1982). The changing distribution of large intestinal cancer. Diseases of the Colon and Rectum, 25, 558–562.
Endocrine Tumour-Risk Factors-Cncer.net. Retrieved May 27, 2016 http://www.cancer.net/node/19207.
Rex, D. K., Johnson, D. A., Lieberman, D. A., Burt, R. W., & Sonnenberg, A. (2000). Colorectal cancer prevention 2000: Screening recommendations of the American College of Gastroenterology. American College of Gastroenterology. American Journal of Gastroenterology, 95(4), 868–877.
Pitris, C., Jesser, C., Boppart, S. A., Stamper, D., Brezinski, M. E., & Fujimoto, J. G. (2000). Feasibility of optical coherence tomography for high-resolution imaging of human gastrointestinal tract malignancies. Journal of Gastroenterology, 35(2), 87–92.
Kong, K., Kendall, C., Stone, N., & Notingher, I. (2015). Raman spectroscopy for medical diagnostics: From in vitro biofluid assays to in vivo cancer detection. Advanced Drug Delivery Reviews, 89, 121–134.
Lasch, P., Haensch, W., Naumann, D., & Diem, M. (2004). Imaging of colorectal adenocarcinoma using FT-IR microspectroscopy and cluster analysis. Biochimica et Biophysica Acta (BBA), 1688(2), 176–186.
Li, Q. B., Xu, Z., Zhang, N. W., Zhang, L., Wang, F., Yang, L. M., et al. (2005). In vivo and in situ detection of colorectal cancer using Fourier transform infrared spectroscopy. World Journal of Gastroenterology, 11(3), 327–330.
Mavarani, L., Petersen, D., El-Mashtoly, S. F., Mosig, A., Tannapfel, A., Kötting, C., et al. (2013). Spectral histopathology of colon cancer tissue sections by Raman imaging with 532 nm excitation provides label free annotation of lymphocytes, erythrocytes and proliferating nuclei of cancer cells. Analyst, 138(14), 4035–4039.
Shim, M. G., Song, L. M. W. K., Marcon, N. E., & Wilson, B. C. (2000). In vivo near-infrared raman spectroscopy: Demonstration of feasibility during clinical gastrointestinal endoscopy. Photochemistry and Photobiology, 72(1), 146–150.
Molckovsky, A., Song, L. M. W. K., Shim, M. G., Marcon, N. E., & Wilson, B. C. (2003). Diagnostic potential of near-infrared Raman spectroscopy in the colon: Differentiating adenomatous from hyperplastic polyps. Gastrointestinal Endoscopy, 57(3), 396–402.
Widjaja, E., Zheng, W., & Huang, Z. (2008). Classification of colonic tissues using near-infrared Raman spectroscopy and support vector machines. International Journal of Oncology, 32(3), 653–662.
Short, M. A., Tai, I. T., Owen, D., & Zeng, H. (2013). Using high frequency Raman spectra for colonic neoplasia detection. Optics Express, 21(4), 5025–5034.
Argov, S., Ramesh, J., Salman, A., et al. (2002). Diagnostic potential of Fourier-transform infrared microspectroscopy and advanced computational methods in colon cancer patients. Journal of Biomeical Optics, 7(2), 248–254.
Swartling, J., Dam, J. S., & Andersson-Engels, S. (2003). Comparison of spatially and temporally resolved diffuse-reflectance measurement systems for determination of biomedical properties. Applied Optics, 42(22), 4612–4620.
Mourant, J. R., Hielscher, A. H., Eick, A. A., Johnson, T. M., & Freyer, J. P. (1998). Evidence of intrinsic differences in the light scattering properties of tumorigenic and nontumorigenic cells. Cancer Cytopathology, 84(6), 366–374.
Hidovic-Rowe, D., & Claridge, E. (2005). Modelling and validation of spectral reflectance for the colon. Physics in Medicine & Biology, 50, 1071–1093.
Old, O. J., Fullwood, L. M., Scott, R., Lloyd, G. R., Almond, L. M., Shepherd, N. A., et al. (2014). Vibrational spectroscopy for cancer diagnostics. Analytical Methods, 6(12), 3901–3917.
Kallenbach-Thieltges, A., Großerüschkamp, F., Mosig, A., Diem, M., Tannapfel, A., & Gerwert, K. (2013). Immunohistochemistry, histopathology and infrared spectral histopathology of colon cancer tissue sections. Journal of Biophotonics, 6(1), 88–100.
Krafft, C., Ramoji, A. A., Bielecki, C., Vogler, N., Meyer, T., Akimov, D., et al. (2009). A comparative Raman and CARS imaging study of colon tissue. Journal of Biophotonics, 2(5), 303–312.
Wei, H., Xing, D., Wu, G., Gu, H., Jin, Y., & Li, X.-Y. (2005). Differences in optical properties between healthy and pathological human colon tissues using a Ti:sapphire laser: An in vitro study using the Monte Carlo inversion technique. Journal of Biomedical Optics. https://doi.org/10.1117/1.1990125.
Waterhouse, B. R., & Farmery, A. D. (2012). The organization and composition of body fluids. Anaesthesia and intensive care medicine, 13(12), 603–608.
Acknowledgments
The research is supported by the Ministry of Education, Science and Technological Development, project III41006.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Dragicevic, A., Matija, L., Krivokapic, Z. et al. Classification of Healthy and Cancer States of Colon Epithelial Tissues Using Opto-magnetic Imaging Spectroscopy. J. Med. Biol. Eng. 39, 367–380 (2019). https://doi.org/10.1007/s40846-018-0414-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-018-0414-x