Abstract
Currently, thousands of tons of metallic nanoparticles (MNPs) are produced and utilized in nano-enabled devises, personal care, medicinal, food and agricultural products. It is generally accepted that the reaction compounds and the techniques used in industrial production of MNPs are not environmentally friendly. The green synthesis has been proposed as an alternative to reduce the use of hazardous compounds and harsh reaction conditions in the production of MNPs. In this endeavor, investigators have used organic compounds, microbes, plants and plant-derived materials as reducing agents. Research papers are published every year, and each one of them stresses the benefits of the green approach and the advantages over the traditional syntheses. However, after almost two decades since the explosion of the reports about the new approach, the commercial production of green-synthesized nanoparticles does not seem to find a way to scale up commercial production. This review includes descriptions of the traditional and green synthesis and applications of MNPs and highlights the factors limiting the use of plant-based synthesis as a real alternative to the traditional synthesis of MNPs.
Similar content being viewed by others
Introduction
In the first three decades of the twenty-first century, society has been exposed to a large amount of nano-enabled products. The Nanotechnology Consumer Products Inventory currently lists 622 companies in 32 countries, which together produce 1814 nano-enabled consumer products [210]. Despite the progress in the use of tiny materials cataloged as “nano,” there is still a debate in some aspects of the terminology associated with this new technological revolution. There are several expressions associated with nanotechnology, but two of the most frequently used terms are “nanomaterial” and “nanoparticle.” Although sometimes both terms are used as synonymous, the American Society for Testing and Materials (ASTM) [8] has stated that a “nanoparticle (NP) is a sub-classification of ultrafine particle with lengths in two or three dimensions greater than 0.001 µm (1 nm) and smaller than about 0.1 µm (100 nm) and which may or may not exhibit a size-related intensive property.” On the other hand, the European Union has defined nanomaterial as: “Nanomaterial means a natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50 % or more of the particles in the number size distribution, one or more external dimensions are in the size range 1–100 nm” [35].
It is well known that metallic nanoparticels (MNPs) possess unique properties, different from the microsized materials, which allow multiple industrial, agricultural, and household applications. As a result, there has been an accelerated development and massive production of new MNPs to supply the ever-increasing demand for nanotechnology-based goods and devices. The estimated global production of MNPs for 2010 was of 260,000–309,000 metric tons. At the top of the list are: silica, titania, alumina, iron, and zinc oxides [85].
Several publications describe the traditional methods used to produce MNPs for industrial applications. Detailed examples can be found in a publication by The Royal Society [197] and in the review by Charitidis et al. [29]. To briefly summarize, the variety of techniques used for the mass production of MNPs have been grouped into two categories: “top-down” and “bottom-up” techniques. In top-down techniques, the process starts with a block of material that is crushed to the desired shape. Nano-enabled devices like computer chips and high-quality optical mirrors are produced using top-down techniques. In the bottom-up technique, small units (atoms or molecules) are assembled to make a larger structure. This technique is used to produce cosmetics, fuel additives, and molecular devices [197]. Further details will be presented in this review.
Important characteristics of MNPs for industrial and medical applications include type of material, size, shape, composition, and surface charge, among others. The purity and uniformity of the nanomaterial is particularly important in many applications, like in cosmetics, where impurities may have serious effects in users [57]. Similarly, the high-quality performance of electronics devices relies on the high purity of components [55]. In biological sciences, MNPs have gained a preponderant place in multiple applications. Essentially, each application requires particles with unique characteristics. For example, double-stranded DNA separation has shown to be size dependent [77]. Adams et al. [1] reported a size-dependent toxicity of Pd NPs to Escherichia coli and Staphylococcus aureus. In a literature review, Fröhlich [58] analyzed the effects of particle surface charge on its interaction with the cell and subsequent cellular response. The literature on characteristics of drug nanocarriers for cancer treatment was analyzed by Pérez-Herrero and Fernández-Medarde [149]. These authors highlight the importance of the size of carrier particles for their movement within the body, avoiding the action of the defensive body cells and the reticuloendothelial system. The current review includes descriptions of traditional techniques as well as green approaches by using plants/plant-derived materials for the synthesis of MNPs using plants/plant-derived materials. We analyze aspects concerning the most produced and used NPs including Au, Ag, Cu, CeO2, TiO2, ZnO, and Fe NPs. We discuss some of the reasons why the green synthesis approaches have not been used for the mass production of widely applied MNPs.
Chemical synthesis and applications of MNPs
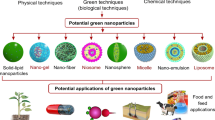
Engineered nanomaterials are designed with a specific purpose or for a specific process. The applications of MNPs are various and include, but are not limited, to the following: catalysis, biomedical, biosensing, environmental remediation/reclamation, pest control, and water treatment. In general, the synthesis of metallic MNPs is achieved through the reduction of the parent ion in different ways. The synthesis of MNPs varies depending on the desired product, and there are numerous methods for their production. Figure 1 shows a summary of methods used for the synthesis of MNPs. The following sections contain a brief description of the methods.
Chemical synthesis
Metal reduction
In a solution containing a metal ion, a reducing agent is added to synthesize the NPs. This method is used for the preparation of copper, gold, and silver NPs. The reduction of the metal ions in solution is achieved with NaBH4, sodium citrate, ascorbic acid, and hydrazine, which is accompanied by a capping/stabilizing agent [25, 41, 207]. The media for the reaction can also act as the reducing and stabilizing agent, which is referred to as the polyol method, where a polyol is the reducing and capping agent [152]. The size and shape of the nanomaterials show control based on a combination of the metal ion concentration, the reducing agent, and the capping/stabilizing agent.
Bottom-up methods
Hydrothermal/solvothermal synthesis
Hydrothermal and solvothermal syntheses are similar techniques; the only difference in the two methods is the solvent system used. In hydrothermal synthesis, water is the medium for the synthesis, whereas any solvent can be used in solvothermal method [32, 86, 91]. The hydrothermal and solvothermal synthesis techniques have generated MNPs with very specific shapes and sizes that enhance the properties of nanomaterials. In addition, hydrothermal and solvothermal processes can be performed under microwave irradiation, which has been shown to affect the properties of the synthesized MNPs.
Sol–gel method
The sol–gel method has been traditionally used for the synthesis of nanomaterials/colloids from solution. In general, the method consists of generating a gel in solution through the hydrolysis of a metal ion; the gel coagulates forming particles [32, 91, 112, 136]. The synthesis can be achieved with different precursors such as the metal ions (from metallic salts) and organo-metal complexes [32]. The MNPs obtained through this process can be tailored to any desired application; in addition, there is good control on the size and morphology of the synthesized NPs.
Sonochemical method
In sonochemical synthesis, ultrasound is used as an energy source [32]. The ultrasound waves form cavitation in the solvent that produces intense energy, causing local heating and high pressures [32]. The local energy results in fast reactions and rapid synthesis of MNPs with controlled sizes and shapes [32, 91, 136]. It is worth mentioning the tutorial developed by Xu et al. [223] where phenomena occurring in sonochemistry were highlighted.
Top-down methods
Chemical and vapor deposition
In chemical and vapor deposition techniques, the materials are first placed into the gas phase and into the solid phase [32] and are performed under vacuum. If a chemical reaction occurs during the deposition, then the process is called chemical vapor deposition (CVD); if no reaction is observed, then the process is called physical vapor deposition (PVD) [32]. The material is heated to its vaporization point and then condensed onto a target or substrate. In general, CVD and PVD techniques are used to make thin films and nanofilms of materials for specific applications such as optical coatings and photovoltaic applications.
Combustion
Combustion synthesis consists of two sub techniques: solid-state combustion and solution combustion syntheses [146, 147]. In solid-state combustion synthesis, both the starting materials and final products are in the solid phase [146, 147]. The reactants are pressed into a pellet and combusted by an external source. The ignition of the pellet starts an exothermic reaction that is self-propagating and synthesizes the NPs [146, 147]. The solution combustion synthesis consists of rapidly heating a solution containing metal nitrate and a fuel source such as urea/hydrazides [146, 147]. Combustion synthesis techniques have been successfully employed to synthesize metal oxide NPs and composite nanomaterials such as Au on CeO2 and Ag on CeO2 [146, 147]. In addition, Eu3+-activated red phosphors LnMAlO4, where “Ln = La or Y and M = Ca or Sr, having K2Ni4” structure and lamp phosphorus have been prepared using aqueous combustion synthesis and solid-state methods [50, 51].
Applications of engineered and manufactured nanomaterials
For the most part, applications of MNPs are very similar to the application of parent metal ions or the bulk materials. Gold and silver MNPs have numerous applications and are perhaps among the most studied NPs. In the last decade, other MNPs such as CeO2, Cu/CuO, TiO2, ZnO, and Fe/Fe oxide have gained attention due to their application in goods, consumer products, and environmental remediation. Table 1 shows a detailed list of applications of some of the most produced MNPs. As seen in this table, Au, Ag, Cu, and ZnO NPs have biomedical applications [107, 148, 164, 166, 203]. Silver and Au NPs have also been used as sensors and catalysts [36, 37, 48, 88, 106, 108, 123, 125, 161, 166]. Zinc Oxide NPs are used in food packaging [91] and personal care products [85]. Titanium dioxide NPs are used in personal care products [85]; catalysis [124], and solar cells [112, 124]. Iron-based NPs are used in many applications including environmental remediation [205]. More details are shown in Table 1.
Green synthesis and applications of metal-based nanomaterials
According to Cheviron et al. [34], there are three general aspects that need to be considered in green synthesis: solvent medium, non-toxic reducing agents, and environmentally safe nanoparticle stabilizers. Cruz et al. [39] stated that green synthesis by plant extracts can be comparable to some conventional methods; however, they did not give details about nanoparticle yields, stability, and ions reduction mechanisms. The mass production of atomically precise MNPs requires highly reactive substances and/or energy consuming procedures, which are not considered environmentally friendly [144, 216]. To overcome this problem, researchers began to use more environmentally benign methods that are collectively called green synthesis. A simple search using ScienceDirect.com and the keywords “green synthesis of metal nanoparticles” showed 21,488 results, while SciFinder Scholar showed 1326 results (January 11, 2016). Figure 2 shows the annual publications about the green synthesis of MNPs since 2000–2016. As seen in this figure, there has been a constant increase in publications since 2006. The biological synthesis has been accomplished using viruses, bacteria, fungi, and plants [19, 89, 128, 129]. For a complete review on the synthesis of MNPs by microbes, the reader is directed to Narayanan and Sakthivel [137], Hulkoti and Taranath [79], and Virkutyte and Varma [216].
Reports of the reduction of precious metals by inactivated plant materials date back almost 100 years. Molisch [126] and Iwase [81] studied the reduction of Ag by plant chlorophyll and the reduction of Au by extract of fresh leaves, respectively. About 66 years ago, Nagai [133] reported the reduction of Ag(NO)3 by plant cells. A decade ago, Armendariz et al. [10, 11] reported the bioreduction of KAuCl4 by powdered wheat (Triticum aestivum) and oat (Avena sativa) biomasses and the subsequent formation of Au NPs. These researchers reported that the solution pH is a key variable to control the size and to reduce the polydispersity of the biogenic Au NPs. More recently, Montes et al. [127] reported that the biomass solvent extract determined the type of biogenic Au NPs. Water extracts of alfalfa (Medicago sativa) biomass led to the formation of triangular nanoplates, while isopropyl alcohol extract rendered decahedra and icosahedra NPs [127]. Besides biomass, living plants can also reduce KAuCl4 and form metal NPs. Gardea-Torresdey et al. [61, 62] reported, for the first time, the formation of metal NPs by living plants exposed to AuCl4 − solution. Alfalfa plants were grown in an agar medium spiked with AuCl4 − and formed crystalline pure Au NPs, confirming that the NPs grew within the plant [61]. A year later, these researchers reported the formation of silver nanowires inside stems of alfalfa plants exposed to silver chloride [62]. Since then, thousands of papers have reported the formation of MNPs by plant or plant-derived materials. Extensive details can be found in Hong et al. [76] and Virkutyte and Varma [216].
Green synthesis of gold nanoparticles
Gold NPs have been synthesized using different green approaches that include biologically based chemicals, plants/plant-derived materials, algae, bacteria, and microbes. The green-synthesized Au NPs have similar physical characteristics as chemically synthesized nanomaterials (Table 2). Numerous chemicals have been extracted from living systems, which have shown much promise in the reduction of Au ions to Au NPs. These biologically relevant materials include simple organic chemicals such as citric acid and ascorbic acid [207].
Perhaps one of the best known reduction techniques for the synthesis of Au NPs is the Turkevich method, where Au(III) ions are reacted with citric acid/sodium citrate at elevated temperatures, causing the reduction of ions to Au NPs [207, 208]. The reduction technique using citric acid/sodium citrate has shown that the synthesized NPs’ size and shape is depend on the concentration of the citrate. The average particle size of the NPs also depends on the time and the chemicals used [207, 208]. Furthermore, in the synthesis of the Au NPs through the Turkevich method, the oxidized citric acid becomes the stabilizing agent for the produced NPs [207]. Similarly, ascorbic acid and tannic acid have been shown to reduce Au(III) ions to produce Au NPs [2, 175]. Additionally, different polysaccharides and phytochemicals have been used for the synthesis of Au NPs [143]. Studies have shown that gum Arabic, a polysaccharide extracted from Acacia trees, has been successfully used as a reducing agent for the synthesis of Au NPs. Wu and Chen [221] showed that at a temperature of 50 °C, the reduction of the Au ions occurs within the first 15 min of reaction time.
Plant extracts have shown to be effective in the reduction of Au ions to Au NPs [138]. Table 2 shows a summary of the synthesis of Au NPs using plants/plant extracts. As seen in this table, tetrachloroaurate has been used as precursor in all the cases. Although the forms and applications are similar to those observed in chemically synthesized NPs, the size of the green-synthesized NPs, with the exception of those synthesized using honey and banana peel, shows higher variability. This might be one of the reasons that have prevented the massive production of Au NPs using plants/plants extracts.
Green synthesis of silver nanoparticles
Silver nanoparticles (Ag NPs) have been extensively synthesized and studied because of their unique physical, chemical, and biological properties [17, 212]. Global synthesis of Ag NPs has been estimated to be 55 tons per year [22]. The synthesis process of these NPs involves reduction of the silver ions, nucleation, and growth of the particles [38]. A summary of the methods is shown in Fig. 1. One of the main challenges using traditional methods to synthesize Ag NPs is the high cost of equipment. Moreover, sometimes the addition of surfactants or stabilizers is needed during the synthesis to avoid nanoparticle aggregation [140]. Hart et al. [72] stated that size and monodispersion of the particles define their chemical, optical, and physical properties. Smaller particles are desirable because they have lesser Van der Waals interactions, which avoid aggregation and precipitation.
Lately, researchers have been looking for greener technologies to manufacture NPs [92]. Green technologies to produce Ag NPs are described in Table 3. As indicated in Table 3, Ag(NO)3 has been used as precursor and extracts from different plant parts have been used as reducers. Researchers reported that main compounds involved in Ag(NO)3 reduction can be proteins, carbohydrates, flavonoids, phenols, vitamins, and capsaicinoids [17, 110]. David et al. [43] synthesized Ag NPs from the European black elderberry fruit extract mixed with 1 % of Ag(NO)3. These authors suggest that fruit polyphenols are the reducers, while polyphenols act as capping agents. However, the Ag reduction mechanism was not elucidated in this study. David et al. [43] also tested the anti-inflammatory effects of the green-synthesized Ag NPs but did not compare the effects with those of NPs synthesized by other methods. Cruz et al. [39] reported that the glycoside verbascoside is the major reducing component in the extract of Lippia citrodora and they suggested that this compound acted as reducer and capping agent, promoting the stability of Ag NPs. Kouvaris et al. [92] also synthesized Ag NPs from Arbutus unedo leaf extract and stated that organic compounds are responsible for reduction and stabilization of NPs. Several researchers suggest that green-synthesized Ag NPs can be used for drug delivery due to the compatibility of some pharmaceutical drugs with natural capping agents; however, the feasibility of their use in biomedical areas has not been tested. Most of the studies reported in the literature confirmed the synthesis of Ag NPs by plant extracts as a single step; nonetheless, organic compounds responsible for the Ag ion reduction have not been clearly identified.
Barbinta-Patrascu et al. [17] suggested that alkaloids and flavonoids from Chelidonium majus L. are responsible for the reduction Ag ions and capping agents for the NPs. These authors compared the antimicrobial activity of green-synthesized Ag NPs against E. coli ATCC8738 strain with that synthesized by classical methods. They observed that alkaloids or phenolic compounds present in plant extracts, which have antibacterial activity, increased the inhibition zone diameter of the green-synthesized NPs. Coseri et al. [38] used the polysaccharide pullulan and oxidized pullulan to synthesize and stabilize Ag NPs. They reported that the size of the NPs varied depending upon the concentration of Ag(NO)3 and pullulan used (from 30 to 50 nm for natural pullulan and 8–25 nm for oxidized pullulan). The authors explained the inconsistencies in the results for the NPs’ size due to the sample preparation. Kumar et al. [96, 98, 99] synthesized Ag NPs using Capuli cherry extract exposed to white solar and blue light-emitting diode (LED) light. They showed that silver ions were reduced faster (8 h) when blue LED light was applied, compared to those NPs synthesized in white solar light (96 h). The authors reported different methods for Ag NPs characterization; however, they did not give any information related to the role of some components from the extract on the reduction of Ag. Ag NPs also have been synthesized by other plant parts such as shell, seed, or flower petal extracts [21, 110, 187, 212]. As mentioned before, these studies were mainly focused at the characterization of obtained Ag NPs; however, reduction mechanism, yield of NPs, and feasibility to use them in similar way to chemically synthesized Ag NPs have not been demonstrated.
Green synthesis of Cu-based nanoparticles
Copper and CuO NPS are attracting attention due to their wide applications. The variety of applications of Cu and CuO NPs are described in Table 1. As catalytic or antibacterial materials, Cu and CuO NPs have the advantages of lower cost and increased abundance when compared to Ag, Au, and Pt [24]. However, the use of toxic chemicals during synthesis of Cu and CuO NPs limits their application in the clinical field [27]. In addition, their higher dissolution rates and toxicological properties limit the use of Cu-based NPs. Green synthesis of Cu NPs can reduce or eliminate the use and generation of hazardous substances; therefore, the method has received some attention. Xiong et al. [222] used l-ascorbic acid as a reducing agent and successfully synthesized highly stable Cu NPs. They reported there are three key factors in the reduction of Cu ions to Cu NPs: (1) the solvent medium, (2) the reducing agent, and (3) the capping agent [222]. Plant extracts contain various primary and secondary low molecular weight metabolites, such as amino acid, reducing sugars, and phenolics, which are reported to act as reducing or capping agents during Cu NPs’ synthesis. In recent years, several studies using plant extracts as reducing and capping agents to synthesize Cu and CuO NPs have been published.
Table 4 summarizes studies about the green synthesis of Cu-based NPs. As seen in the table, Cu and CuO NPs have been manufactured using various Cu compounds and extracts from different plant parts. The table also shows that plant extract-synthesized Cu NPs are mainly used as antibacterial, and some were tested in photocatalyst, antioxidant, and conductive nanobiocomposites. It is noteworthy that even though there are wide applications of Cu and Cu NPs in various fields, most of the green-synthesized Cu and CuO NPs are primarily used in antibacterial activity. As seen in Table 4, there is high variability in the precursor concentration (1–50 mM). In addition, the ratio of the amount of precursor to the amount of plant extract used in the synthesis varied from 1:1 to 1:10. Although the majority of the reports have shown that most of the particles formed are spherical and the reported sizes vary from 3 to 250 nm. This, coupled with a lack of data on the performance of the techniques, may be limiting the use of green techniques for the production of high volume of Cu/CuO NPs.
Green synthesis of zinc oxide nanoparticles (ZnO)
Zinc oxide NPs (ZnO NPs) have a wide band gap and large binding energy, which made them widely used in photovoltaic and photocatalytic applications. It is one of the metal oxide NPs that are produced in large quantities with an annual global production estimate of 31,000–34,000 metric tons [102]. The methods used in manufacturing ZnO NPs are shown in Fig. 1. The industrial methods for manufacturing ZnO and other MNPs use harsh chemicals and generate toxic wastes and byproducts. Thus, many scientists are exploring alternative, eco-friendly techniques in synthesizing ZnO NPs [80, 87, 165].
There is an increasing number of reports regarding the green biosynthesis of ZnO NPs using plant extracts [15, 26, 46]. Table 5 presents several studies where the green synthesis of ZnO NPs was investigated. As seen in the table, ZnO NPs were manufactured from different zinc compounds (e.g., zinc acetate, zinc nitrate, zinc sulfate) using extracts from different parts (stem, leaf, flower, and fruit) of various plants (e.g., trees, herbs, ornamentals, and seaweeds). Other researchers reported ZnO NP synthesis using Aloe vera, palm pollen, and dried leaves of zinc hyperaccumulator plants [14, 100, 156]. It is also apparent from the table that the ratio of the amount of precursor Zn compound to the amount of plant extract has the same problem of variability as other NPs. In addition, green-synthesized ZnO NPs had morphologies of hexagonal, spherical, rod, and crystalline, and particle sizes from 9 to 180 nm. The table also shows that the plant extract-synthesized ZnO NPs were tested for various applications and found to exhibit similar properties as chemically synthesized ZnO NPs. Some researchers noted that the green-synthesized ZnO NPs were clean and free of harsh chemicals that make them suitable for biological, pharmaceutical, and medical applications [15, 26, 165], which could give green synthesis an advantage over traditional synthesis.
The general procedure for biogenic synthesis of ZnO NPs utilizes plant metabolites in a relatively facile and rapid one-pot experiment [20, 165]. However, the mechanisms for the green synthesis of ZnO NPs are not fully understood. A review of the studies presented in Table 5 revealed that few papers (6 out of 21) tried to elucidate the mechanisms involved in plant extract-mediated synthesis of ZnO NPs. For example, researchers who worked on Azadirachta indica (L.) leaf extract reported that polyols from water-soluble phenolic, flavonoid, and quinone compounds were the major phytochemicals that elicited the formation of ZnO NPs [20, 52]. Kumar et al. [95] hypothesized that free OH/COOH from flavonoids, limonoids, and carotenoid molecules from Citrus paradisi peel extract formed complexes with zinc, which yielded ZnO NPs after oven-drying. In addition, Bala et al. [15] deduced that amino and carboxylic groups in proteins, phenolics, flavonoids, and alkaloids in Hibiscus subdariffa leaf extract functioned as reducing agents for ZnO NPs formation, while amide from proteins and carbonyl and alkyl groups in heterocyclic compounds acted as stabilizer. In another study, Davar et al. [42] used citric acid from lemon fruit extract and sucrose for the green synthesis of ZnO NPs. These researchers indicated that citric acid adsorbed Zn cations that directed the nanostructure formation, while sucrose reduced the crystallization rates, resulting in improved uniformity of ZnO NPs. Similarly, Buazar et al. [26] reported that the long alkyl chain in starch from potato extract served as reducing and capping agents, promoted the monodispersity, and decreased the size of to ZnO NPs.
Several reviews have documented the progress in the emerging field of biogenic synthesis of ZnO NPs [80, 87, 165]. Studies presented in Table 5 are focused on demonstrating the feasibility of plant extract-mediated ZnO NPs synthesis. However, questions arise on whether this method can be adopted for massive production of ZnO NPs to meet industrial needs.
Green synthesis of titanium dioxide NPs (TiO2)
Titanium dioxide (TiO2) NPs are the most widely produced MNPs in the world, with an annual global production estimate of 83,500–88,000 metric tons [102]. The high prevalence of TiO2 NPs in the market is due to their many versatile applications originating from the stability of the chemical structure, physical, optical, and electrical properties. TiO2 exists in three different mineral forms: anatase, rutile, and brookite; usually the former is preferred, due to its increased photocatalytic activity [112]. Several procedures have been developed over the last two decades to synthesize TiO2 NPs. Although these methods effectively control the desired NP properties, they often use materials with potential hazards such as carcinogenicity and toxicity [4].
Despite the high production and wide use of TiO2 NPs, very few attempts have been made for the biogenic production. A few plant species have been investigated for the production of TiO2 NPs (Table 6). Titanium dioxide hydrate has been used as precursor in most of the studies on the green synthesis of TiO2; spherical NPs have been the most produced NPs. In addition, almost all of them have been reported to have antiparasitic properties. However, there are no studies on the comparison of the green-synthesized TiO2 NPs with NPs synthesized with other methods. It is possible that the stability of the bulk TiO2 limits its breakdown by some plant extracts, reducing the availability of Ti ions to form TiO2 NPs.
Green synthesis of cerium dioxide (CeO2)
Cerium oxide NPs (CeO2 NPs) have excellent catalytic properties due to wide band gap energy and large exciton binding energy, showing a wide variety of applications [217]. The CeO2 NP properties (e.g., catalytic activity) could be significantly affected by their solubility, particle size, surface coating/modification, surface charge, crystal structure and morphology [224]. These variations might be controlled by adjusting the synthesis routes and tuning their kinetic parameters [73]. Various techniques have been used for the chemical synthesis of CeO2 NPs [12, 31, 33, 44, 67, 80, 84, 104, 113, 117, 118, 122, 150, 174, 185, 218, 225, 226]. However, conventional techniques used in the synthesis of CeO2 have shown the same drawbacks as the synthesis of other MNPs. Toxic chemical and multiple process are required [12, 80]. At the end, this means threats to the environment and lower cost effectiveness. For example, in solvothermal synthesis, solvents are used under pressures and temperatures above critical points in order to increase the solubility of solids and to accelerate reactions between solids [70]. All these steps require high consumption of energy and might represent safety threats.
Recently, an increasing number of reports have described the green synthesis of CeO2 NPs using plant extracts [12, 116–118, 168, 170, 201] (Table 7). Compounds such as cerium acetate, cerium nitrate, and cerium chloride have been used as precursors, and extracts from different plant parts (leaves and flowers) have been used as reducing agents. Moss of the green-synthesized CeO2 NPs are spherical and crystalline, with particle size varying from 4 to 19 nm. The table also shows that plant extract-synthesized CeO2 NPs have been tested for various applications such as photoluminescence [116] and gamma radiation sensing [117, 118]. They have also shown various properties such as optical [12], photovoltaic and photocatalytic [117, 118], and antimicrobial [12]. The green-synthesized CeO2 NPs can also be used in biomedical applications due to their autocatalytic properties and less toxicity. Sankar et al. prepared CeO2 NPs by using aqueous extract of Centella asiatica and compared their superoxide and hydroxyl radical scavenging activities with bulk cerium. The green-synthesized CeO2 NPs performed better than bulk cerium and had higher cellular uptake and viability in H9c2 rat cardiomyoblasts cell line [168].
Besides CeO2 NPs, cerium oxide nanophosphors with the incorporation of rare earth activators, such as Eu3+ and Ho3+, have been also synthesized via green route using plant extracts [116–118], showing promising application for efficient display and white light emitting.
Green synthesis of other metal-based nanoparticles
Iron-based NPs have been produced using Amaranthus spinosus leaf aqueous extracts. These NPs were spherical with rhombohedral phase structure, smaller size, and large surface with less aggregation than those produced with sodium borohydride (Muthukumar and Matheswaran [130]). Furthermore, these NPs showed better photocatalytic and antioxidant capacity than sodium borohydride-mediated NPs. Similar results were obtained by using tea (Camellia sinensis) polyphenols to synthesize iron NPs, and results reveal that the highest rate of bromothymol blue degradation occurs with green synthesized iron NPs, when compared to Fe-ethylenediaminetetraacetic acid (Fe-EDTA) and Fe-ethylenediaminine-dissuccinic acid (Fe-EDDS) (Hoag et al. [75]). This suggests that, for some applications, better results can be obtained with green synthesized NPs.
Besides iron-based NPs, several other metal-based NPs have been synthesized using plant extracts, including Sm2O3 NPs (Sone et al. [182]), Co3O4 NPs (Diallo et al. [47]), Dy2O3 NPs (Chandrasekhar et al. [28]), CdO NPs (Thovhogi et al. [201]), NiO NPs (Thema et al. [200]), In2O3 NPs (Maensiri et al. [114]), and hydroxyapatite NPs (Klinkaewnarong et al. [90]). Researches have also investigated the green synthesis of rare earth-doped lanthanide oxides NPs, such as Gd2O3 (Vidya et al. [213]) and Y2O3 (Prasannakumar et al. [155]; Kumar et al. [96]; Kumar et al. [98]). Magnetic nanocrystalline spinel ferrite, CoFe2O4 NPs, MgFe2O4 NPs, MnFe2O4 NPs, and NiFe2O4 NPs were also synthesized by using plant-derived oil (Gherca et al. [65]; Phumying et al. [154]; Gherca et al. [63]) (Table 8).
Factors limiting the development of green synthesis of MNPs
Studies support the feasibility of a facile synthesis of NPs using plant extracts. However, an analysis of the pertinent literature revealed challenging issues and shortcomings limiting the advancement of the green synthesis. Major issues, as depicted in Fig. 3, include: technical, engineering, and economical limitations associated with the source/type and concentration of plant extracts, stoichiometric ratios of the reagents, optimal experimental conditions (temperature, pH, time), yield, and product characterization/application. In addition, process-engineering limitations, operational scalability, and a lack of life cycle assessment are also considered major issues.
Technical factors
Variability in bioactive compounds
Variability in the source/type (i.e., bioactive compounds) of plant extracts is probably the most limiting factor to standardize the green synthesis of metal-based NPs. Most of the reports only show the description of the preparation, collection, washing, and extraction of the plant extract with very little attempt to document which active ingredient triggers the NP formation. For example, Nagarajan and Kuppusamy [134] made comparative studies on the ability of seaweed (Caulerpa peltata, Hypnea valencia, and Sargassum myriocystum) extracts to synthesize ZnO NPs. He found that only S. myriocystum extract could trigger the formation of ZnO NPs. The lack of extract characterization seems to be a common element to the most reports. Only 1 out of 21 articles (Table 5) included the characterization of the plant and the extract used in the synthesis [15], while other researchers made the assumption that extracts were rich in plant metabolites. In case of CeO2 NPs, Thovhogi et al. [201] found several bioactive compounds (hibiscetin, gossypetine, quercetin, delphinidin, pectin, and cyanidin 3-sambubioside) in Hibiscus sabdariffa flower extract, but failed to identify which substance was responsible for the formation of CeO2 NPs. The antioxidant power of leaf extracts of 26 different plant species was found to have direct effect on their capacity to reduce Fe(III) to form ZVIs NPs; however, authors just characterized the extracts by determining the “ferric reducing antioxidant power” [111]. Most articles on the biogenic synthesis of other NPs have a similar problem, which leave a huge knowledge gap regarding which metabolites in plant extracts are the reducing or capping agents to convert metallic substrates to NPs. Unfortunately, this very broad generalization needs further examination. Obviously, the best source/type of plant extract for the synthesis of each NP has to be identified. Studies cataloging the contribution of the different phytochemicals may be necessary.
A corollary to the lack of knowledge on the bioactive components of plant extracts is the poor understanding on the underlying reaction mechanisms in green synthesis. Despite attempts made by several researchers to unravel the mechanism, it is still a major knowledge gap in green synthesis. Shankar et al. [176, 177] employed IR techniques in deducing the mechanism involved in the green synthesis of Au NPs and concluded that the aldehyde and ketones from sugars in lemon grass extract are involved in the reaction intermediates. IR analyses also suggested that alcohol, amine, and acid groups are responsible for the reduction of the Au(III) ions to form the Au NPs [207, 208]. In another study, Terenteva et al. [196] suggested that flavonoid compounds with lower redox potential facilitate the electron transfer such that flavonoids containing higher hydroxyl groups in their structures are able to produce more Ag NPs. Velmurugan et al. [212] also observed the interaction between Ag ions and hydroxyl groups in phenolic and other compounds from peanut shell extract. It is clear that the existing studies lack description of reaction mechanisms to control the morphology and particle size, which are characteristics inherent to the current physical and chemical synthesis processes. It is understandable that with the difficulty in identifying the active components, it is impossible to comprehend the underlying mechanisms for the green synthesis of NPs. The number of potential reducing agents complicates the investigation of the mechanism. This suggests performing studies using pure solutions of active components found in plant extracts to gain more insights into the efficacy of such compounds in green synthesis.
Variability in experimental conditions
The literature is also lacking analysis of experimental conditions for plant extract-assisted synthesis of NPs. Since plant extracts have very different properties, it is possible that operational conditions would greatly vary for every procedure. Villanueva-Ibáñez et al. [214] compared the effect of pH on the biogenic synthesis of Ag NPs. They obtained NPs of about 10 nm at neutral pH, while those synthesized at lower pH (4.5) had a size of about 25 nm. Authors stated that alkaline condition of silver nitrate solution prevents Ag reduction, while acidic condition promotes Ag solubility and NPs agglomeration. A study by Terenteva et al. [196] showed an optimum pH range of 8–10 to get the highest yield of Ag NPs. In a separate study, Bonatto and Silva [21] described the influence of temperature on the hydrodynamic diameters of Ag NPs. Suspension incubated at 0 °C revealed three size populations, while those at 50 and 75 °C only had one. More negative zeta potential values for Ag NPs were recorded at 25 and 75 °C. Similarly, plant extract reduction of Au ions to Au NPs is temperature dependent [207, 208].
In a related study, Rafaie et al. [157] reported the synthesis of ZnO NPs using Citrus aurantifolia extracts at pH values of 5, 7, and 9. Authors reported that the pH had direct influence on the growth and aspect ratios of ZnO nanorods. These researchers observed that the nanorods’ growth was inhibited in acidic conditions, compared to those in neutral and alkaline solutions. The NPs synthesized at pH 5 were smaller and shorter than those formed at pH 7 and 9. Likewise, Nagarajan and Kuppusamy [134] found that formation of ZnO NPs using S. myriocystum extract yielded better results at pH 8, compared to other pH values (i.e., 5, 6, 7, 9, and 10) tested. The same researchers also observed that the reaction temperature affects the nanoparticle formation. They found that 80 °C was the optimum condition for the synthesis of ZnO NPs [134]. In contrast, other procedures describe reaction temperatures as low as 50 °C to as high as 300, 400 and 450 °C [7, 13, 15, 157]. The reaction time is another factor that could vary from 1 to 8 h [121, 134]. A review of publications shown in Tables 2, 3, 4, 5, 6, 7 and 8 reveals that the majority of these studies did not explain on the optimization of conditions employed in the synthesis. A more thorough investigation on the experimental conditions is needed to determine how they affect the mechanisms involved in the synthesis of each NP.
Engineering factors
Commercial NPs are produced under rigorous standards that guarantee the quality and performance of the products. This includes uniformity in size and surface composition to assure similar behavior. In fact, there are several parameters controlling the plant-based synthesis of MNPs that need further studies (Table 9). So far, most of the green-synthesized MNPs have not been characterized for properties like zeta potential, thermal and electrical conductivity, and layer of atoms in the external part. In addition, only a few reports (Tables 2, 3, 4, 5, 6, 7, 8) show monodispersion of the green-synthesized MNPs. Works on the green synthesis of Au NPs suggest that uniform material is not produced due to the diversity in bioactive compounds present in different plant materials [60, 162, 178]. Shankar et al. [176, 177] reported that sugars present in lemon grass extracts induced the production of triangular Au nanoplatelets, whereas Fayaz et al. [54] showed that aqueous extracts of Madhuca longifolia leafs produced Au NPs and Au platelets varying from 7 to 3000 nm. On the other hand, Smitha et al. [181] showed that an aqueous extract from Cinnamomum zeylanicum was able to reduce Au ions from HAuCl4 solution to form Au NPs. The morphology of the smaller NPs was spherical, whereas that of the larger particles had mixed geometries consisting of triangular platelets and spherical particles. The literature on the biogenic synthesis of Au NPs (Table 2) reveals similar problems in Au reduction, which clearly hinders the possibility of mass production of Au NPs via green synthesis. These problems have been present since the very beginning of the green synthesis of MNPs and have been continually reported.
The stoichiometric ratio of the extract and metal precursor is a factor that affects the size, shape, yield, and other characteristics of green-synthesized MNPs. The reduction of Au ions using plant extracts has been shown to produce smaller particles with higher plant extract concentrations [207, 208]. Biogenic synthesis of Ag NPs (Table 3) revealed that silver nitrate/extract ratio is the main parameter controlling the synthesis and stability of Ag NPs. Villanueva-Ibáñez et al. [214] observed that Ag NPs synthesized using 1–5 mL of corn husk extract had smaller particle size than those formed at higher extract volume (8 mL), while [196] reported an optimum silver nitrate concentration ranging between 0.8 and 2 mM to get the highest yield of Ag NPs.
The concentration of plant extract is also a critical factor influencing the shapes and crystallinity MNPs. Davar et al. [42] reported that size distribution of ZnO NPs increased with the volume of lemon extract used in the synthesis. These researchers found a narrower size distribution at 30 mL lemon juice, which increased to submicrometer size at 50 and 70 mL extract. Similarly, Nagarajan and Kuppusamy [134] detected formation of ZnO NPs only at 5 mL of S. myriocystum extract, while [121] observed varying degrees of crystallinity at different concentrations of Sapindus rarak extracts. Likewise, other reports illustrated the influence of the precursor zinc concentration on the synthesis of ZnO NPs. Nagarajan and Kuppusamy [134] detected the formation ZnO NPs at 1 mM zinc nitrate but not at other concentrations (0.25–0.75 mM), whereas [167] observed morphological variations at different concentrations of zinc acetate (0.05–0.20 M). In addition, an analysis of the stability of plant-based synthesized NPs has not been included in the particle characterization. Very few reports have mentioned that plant-based synthesized NPs were surface capped, but none of them report characterization of the capping. For example, Jang et al. [82] reported that the extract of Lonicera hypoglauca flowers functions as silver nitrate reducer and capping agent of the Ag NPs. They used UV–Vis spectroscopy, FTIR, SEM-ED, TEM, and SAED to characterize the NPs but did not characterize the surface coat. In fact, plant-based synthesized MNPs can be capped with several organic molecules [97]. Therefore, much more data are needed to assess and determine the feasibility of the whole process and to obtain a comprehensive analysis on the direction where the green synthesis is headed.
Economical and environmental factors
Some other factors are overlooked in current studies. Claims that biogenic synthesis of NPs is more environmental friendly and compatible with biological systems, compared with the chemical approach, are not widely supported. There are no clear evidences that green approaches are environmental friendly. Case studies appraising how much energy is saved, how many toxic reagents are avoided in lieu of non-toxic alternatives, and the ecological and economic impacts of using plant materials, among others, might be helpful in understanding the environmental benefits of the green synthesis. The effects of plant material conditions (e.g., fresh or dried), extraction conditions (temperature, contact time, volume/mass ratio), and extract storage, in the efficiency of the process have to be studied. There is also a lack of research demonstrating the feasibility of adopting the process to an industrial and more massive scale. Scaling up the green production will increase tremendously the required volume of plant source and extracts, which may be a limiting factor to achieve the long-term demands. Unfortunately, these factors are not well understood. Other factors to evaluate are the yield and stability of the green-synthesized NPs. Yield is a sound measure in assessing the efficiency, practicality, and profitability of the green synthesis. The amount of starting reagents, both the metal compounds and the extract, is reported in the studies (Tables 2, 3, 4, 5, 6, 7, 8), but the yield efficiency of the process is staggeringly missing in the reports. To authors’ knowledge, an economic analysis of plant-based production of MNPs is missing. In addition, nobody has characterized the metrics to evaluate the green synthesis of MNPs. For example, the effective mass yield, mass intensity, and the stoichiometric factor, among others, are parameters to be calculated for a better assessment of the green synthesis of MNPs [204] (Fig. 3).
Conclusions and future work
From the beginning, the methods for the synthesis of metal-based NMs using green processes have been touted in the last three decades as being “environmentally friendly and cost-effective” alternatives to traditional approaches. There has been an explosion of research publications highlighting the benefits of the green synthesis. In practice, all industrially produced MNPs have also been synthesized using plants/plant extracts or microorganisms. Several species of plants and microbes have shown to be promising sources for the green synthesis of MNPs. State-of-the-art analytical techniques such as transmission electron microscopy, X-ray absorption spectroscopy, and X-ray diffraction, among others, have been used to characterize the different NMs generated by green syntheses. Nanoparticles and nanoplates of different forms and sizes have been reported. More than 50 plant species and several microbial species have been used. Extracts of leaves, flowers, and fruits, as well as several plant-derived materials, have shown to reduce metals, forming nanostructures. The green-synthesized MNPs have shown similar properties as their chemically synthesized counterparts. However, almost all of the reports have shown a lack of regularity in size and forms of the synthesized MNPs and, practically, none of them have quantified the production. Other aspects related to the green synthesis are the variety of plant compounds with potential to reduce the metals. Phenolic, amino, carboxyl and hydroxyl groups, proteins, and aminoacids, among others, have shown reducing capacity, resulting in the production of NPs. Moreover, variations in the production conditions result in differences in the synthesized materials. In most of the reports, there is no clear mention of the reducing factor, and none of them have presented stoichiometric calculations. A clear description of the resulting nanoparticles in each extracts condition may allow to fine tune of nano properties. The problems mentioned above raise questions like: How much reducing agent can be obtained from the target plant? How much biomass will be needed for a sustainable production of the respective NP? The literature shows that in 2010 there was a global production of 260,000–309,000 metric tons of MNPs [85]; how much time will be needed for the production, for example, of one ton of green-synthesized NPs? These queries suggest that the future work has to be focused to answer the following research questions:
-
1.
Quantification of the NP production (ratio of starting material/reducing agent for a specific volume of MNP).
-
2.
Establishment of the production time for a required volume of determined MNPs.
-
3.
Standardization of conditions for the production of monodisperse specific particles.
-
4.
Stability of the green-synthesized MNPs.
-
5.
Identification with no doubt of best plant/plant-derived materials for the synthesis of specific MNPs.
-
6.
Clarification of the biochemical and molecular mechanisms involved in the formation of specific NPs.
-
7.
Definition of costs of production of specific amounts of MNPs.
-
8.
Development of chemistry metrics for the green synthesis of MNPs.
Until these questions are answered, the green synthesis of nanomaterials is a scientific curiosity with no possibilities of scaling up to industrial levels.
References
Adams CP, Walker KA, Obare SO, Docherty KM (2014) Size-dependent antimicrobial effects of novel palladium nanoparticles. PLoS ONE 9:e85981. doi:10.1371/journal.pone.0085981
Ahmad T (2014) Reviewing the tannic acid mediated synthesis of metal nanoparticles. J Nanotechnol. doi:10.1155/2014/954206
Ahmmad B, Leonard K, Islam MS, Kurawaki J, Muruganandham M, Ohkubo T, Kuroda Y (2013) Green synthesis of mesoporous hematite (alpha-Fe2O3) nanoparticles and their photocatalytic activity. Adv Powder Technol 24(1):160–167. doi:10.1016/j.apt.2012.04.005
Ai J, Biazar E, Jafarpour M, Montazeri M, Majdi A, Aminifard S, Rad HG (2011) Nanotoxicology and nanoparticle safety in biomedical designs. Int J Nanomed 6:1117–1127
Alagiri M, Hamid SBA (2014) Green synthesis of alpha-Fe2O3 nanoparticles for photocatalytic application. J Mater Sci Mater Electon 25(8):3572–3577. doi:10.1007/s10854-014-2058-0
Ali DM, Thajuddina N, Jeganathanb K, Gunasekaranc M (2011) Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloid Surf B Biointerfaces 85:360–365
Ambika S, Sundrarajan M (2015) Green biosynthesis of ZnO nanoparticles using Vitex negundo L. extract: spectroscopic investigation of interaction between ZnO nanoparticles and human serum albumin. J Photochem Photobiol B 149:143–148
American Society for Testing and Materials (2012) Standard terminology relating to nanotechnology. E 2456-06. West Conshohocken, PA
Anilkumar MR, Nagaswarupa HP, Nagabhushana H, Sharma SC, Vidya YS, Anantharaju KS, Prashantha SC, Shivakuamra C, Gurushantha K (2015) Bio-inspired route for the synthesis of spherical shaped MgO:Fe3+ nanoparticles: structural, photoluminescence and photocatalytic investigation. Spectrochim Acta A 149:703–713. doi:10.1016/j.saa.2015.05.003
Armendariz V, Jose-Yacaman M, Duarte-Moller A, Peralta-Videa JR, Troiani H, Herrera I, Gardea-Torresdey JL (2004) HRTEM characterization of gold nanoparticles produced by wheat biomass. Rev Mex Fis 50(1):7–11
Armendariz V, Herrera I, Peralta-Videa JR, Jose-Yacaman M, Troiani H, Santiago P, Gardea-Torresdey JL (2004) Size controlled gold nanoparticle formation by Avena sativa biomass: use of plants in nanobiotechnology. J Nanopart Res 6:377–382
Arumugam A, Karthikeyan C, Hameed ASH, Gopinath K, Gowri S, Karthika V (2015) Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Mater Sci Eng C Mater 49:408–415. doi:10.1016/j.msec.2015.01.042
Azizi S, Ahmad MB, Namvar F, Mohamad R (2014) Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater Lett 116:275–277
Azizi S, Namvar F, Mohamad R, Tahir PM, Mahdavi M (2015) Facile biosynthesis and characterization of palm pollen stabilized ZnO nanoparticles. Mater Lett 148:106–109
Bala N, Saha S, Chakraborty M, Maiti M, Das S, Basu R, Nandy P (2015) Green synthesis of zinc oxide nanoparticle using Hibiscus subdariffa leaf extract: effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv 5:4993–5003
Bankar A, Joshi B, Ra Kumar A, Zinjarde S (2010) Banana peel extract mediated synthesis of gold nanoparticles. Colloid Surf B Biointerfaces 80:45–50
Barbinta-Patrascu ME, Badea N, Ungureanu C, Constantin M, Pirvu C, Rau I (2015) Silver-based biohybrids “green” synthesized from Chelidonium majus L. Opt Mater. doi:10.1016/j.optmat.2015.10.021
Basavegowda N, Mishra K, Lee YR (2014) Sonochemically synthesized ferromagnetic Fe3O4 nanoparticles as a recyclable catalyst for the preparation of pyrrolo[3,4-c]quinoline-1,3-dione derivatives. RSC Adv 4(106):61660–61666. doi:10.1039/c4ra11623b
Beveridge TJ, Murray RGE (1980) Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol 141:876–887
Bhuyan T, Mishra K, Khanuja M, Prasad R (2015) Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Proc 32:55–61
Bonatto CC, Silva LP (2014) Higher temperatures speed up the growth and control the size and optoelectrical properties of silver nanoparticles greenly synthesized by cashew nutshells. Ind Crops Prod 58:46–54
Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A (2013) Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol 87:1181–1200
Bradley B (2015) The great big question about really tiny materials. Fortune 171(4):192–196, 198, 200-202
Brumbaugh AD, Cohen KA, Angelo SKS (2014) Ultrasmall copper nanoparticles synthesized with a plant tea reducing agent. ACS Sustain Chem Eng 2:1933–1939
Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R (1994) Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J Chem Soc Chem Commun 7:801–802 doi:10.1039/C39940000801
Buazar F, Bavi M, Kroushawi F, Halvani M, Khaledi-Nasab A, Hossieni SA (2015) Potato extract as reducing agent and stabilizer in a facile green one-step synthesis of ZnO nanoparticles. J Exp Nanosci. doi:10.1080/17458080.2015.1039610
Caroling G, Vinodhini E, Ranjitham AM, Shanthi P (2015) Biosynthesis of copper nanoparticles using aqueous Phyllanthus embilica (Gooseberry) extract- characterisation and study of antimicrobial effects. Int J Nano Chem 1:53–63
Chandrasekhar M, Nagabhushana H, Sudheerkumar KH, Dhananjaya N, Sharma SC, Kavyashree D, Shivakumara C, Nagabhushana BM (2014) Comparison of structural and luminescence properties of Dy2O3 nanopowders synthesized by co-precipitation and green combustion routes. Mater Res Bull 55:237–245. doi:10.1016/j.materresbull.2014.04.013
Charitidis CA, Georgiou P, Koklioti MA, Trompeta A-F, Markakis V (2014) Manufacturing nanomaterials: from research to industry. Manuf Rev 1:11. doi:10.1051/mfreview/2014009
Cheirmadurai K, Biswas S, Murali R, Thanikaivelan P (2014) Green synthesis of copper nanoparticles and conducting nanobiocomposites using plant and animal sources. RSC Adv 4:19507–19511
Chen H-L, Zhu H-Y, Wang H, Dong L, Zhu J-J (2006) Sonochemical fabrication and characterization of ceria (CeO2) nanowires. J Nanosci Nanotechnol 6(1):157–161
Chen X, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107:2891–2959
Chen S-Y, Lu Y-H, Huang T-W, Yan D-C, Dong C-L (2010) Oxygen vacancy dependent magnetism of CeO2 nanoparticles prepared by thermal decomposition method. J Phys Chem C 114(46):19576–19581
Cheviron P, Gouanvé F, Espuche E (2014) Green synthesis of colloid silver nanoparticles and resulting biodegradable starch/silver nanocomposites. Carbohydr Polym 108:291–298
Commission Joint Research Centre Reference Report (2011) Official Journal of the European Union. http://ec.europa.eu/environment/index_en.htm
Cong H, Becker CF, Elliott SJ, Grinstaff MW, Porco JA Jr (2010) Silver nanoparticle-catalyzed Diels–Alder cycloadditions of 2′-hydroxychalcones. J Am Chem Soc 132:7514–7518
Corma A, Garcia H (2008) Supported gold nanoparticles as catalysts for organic reactions. Chem Soc Rev 37:2096–2126
Coseri S, Spatareanu A, Sacarescu L, Rimbu C, Suteu D, Spirk S, Harabagiu V (2015) Green synthesis of the silver nanoparticles mediated by pullulan and 6-carboxypullulan. Carbohydr Polym 116:9–17
Cruz D, Falé PL, Mourato A, Vaz PD, Luisa Serralheiro M, Lino ARL (2010) Preparation and physicochemical characterization of Ag nanoparticles biosynthesized by Lippia citriodora (Lemon Verbena). Colloid Surf B 81:67–73
Dahle JT, Arai Y (2015) Environmental geochemistry of cerium: applications and toxicology of cerium oxide nanoparticles. Int J Environ Res Public Health 12(2):1253–1278
Dang TMD, Le TTT, Fribourg-Blanc E, Dang MC (2011) Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Adv Nat Sci Nanosci Nanotechnol 2:015009
Davar F, Majedi A, Mirzaei A (2015) Green synthesis of ZnO nanoparticles and its application in the degradation of some dyes. J Am Ceram Soc 98:1739–1746
David L, Moldovan B, Vulcu A, Olenic L, Perde-Schrepler M, Fischer-Fodor E, Florea A, Crisan M, Chiorean I, Clichici S, Filip GA (2014) Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloid Surf B Biointerface 122:767–777
Devaraju MK, Yin S, Sato T (2009) Morphology control of cerium oxide particles synthesized via a supercritical solvothermal method. ACS Appl Mater Interface 1(11):2694–2698
Devi HS, Singh TD (2014) Synthesis of copper oxide nanoparticles by a novel method and its application in the degradation of methyl orange. Adv Electron Electr Eng 4:83–88
Diallo A, Beye AC, Doyle TB, Park E, Maaza M (2015) Green synthesis of Co3O4 nanoparticles via Aspalathus linearis: physical properties. Green Chem Lett Rev 8(3–4):30–36. doi:10.1080/17518253.2015.1082646
Diallo A, Ngom BD, Park E, Maaza M (2015) Green synthesis of ZnO nanoparticles by Aspalathus linearis: structural and optical properties. J Alloys Compd 646:425–430
Dong X-Y, Gao Z, Yang K-F, Zhanga W-Q, Xu L-W (2015) Nanosilver as a new generation of silver catalysts in organic transformations for efficient synthesis of fine chemicals. Catal Sci Technol 5:2554–2574
Duan Z, Ma G, Zhang W (2012) Preparation of copper nanoparticles and catalytic properties for the reduction of aromatic nitro compounds. Bull Korean Chem Soc 33(12):4003
Ekambaram S, Patil KC, Maaza M (2005) Synthesis of lamp phosphors: facile combustion approach. J Alloys Compd 393:81–92
Ekambaram S, Maaza S (2005) Combustion synthesis and luminescent properties of Eu3+-activated cheap red phosphors. J Alloys Compd 395:132–134
Elumalai K, Velmurugan S (2015) Green synthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from the leaf of Azadirachta indica (L.). Appl Surf Sci 345:329–336
Elumalai K, Velmurugan S, Ravi S, Kathiravan V, Ashokkumar S (2015) Green synthesis of zinc oxide nanoparticles using Moringa oleifera leaf extract and evaluation of its antimicrobial activity. Spectrochim Acta A 143:158–164
Fayaz AM, Girilal M, Venkatesan R, Kalaichelvan PT (2011) Biosynthesis of anisotropic gold nanoparticles using Maduca longifolia extract and their potential in infrared absorption. Colloid Surf B Biointerfaces 88:287–291
Ferain I, Colinge CA, Colinge J-P (2011) Multigate transistors as the future of classical metal-oxide-semiconductor field-effect transistors. Nature 479:310–316. doi:10.1038/nature10676
Filippo E, Manno D, Buccolieri A, Serra A (2013) Green synthesis of sucralose-capped silver nanoparticles for fast colorimetric triethylamine detection. Sens Actuators B Chem 178:1–9
Food and Drug Administration (2014) Guidance for industry safety of nanomaterials in cosmetic products. Center for Food Safety and Applied Nutrition, U.S. Department of Health and Human Services, Rockville, MD. http://www.fda.gov/downloads/Cosmetics/GuidanceRegulation/GuidanceDocuments/UCM300932.pdf
Fröhlich E (2012) The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomed 7:5577–5591. doi:10.2147/IJN.S36111
Fu L, Fu Z (2015) Plectranthus amboinicus leaf extract-assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceram Int 41:2492–2496
Gangula A, Podila R, Ramakrishna M, Karanam L, Janardhana C, Rao AM (2011) Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived from Breynia rhamnoides. Langmuir 27:15268–15274
Gardea-Torresdey JL, Parsons JG, Gomez E, Peralta-Videa J, Troiani HE, Santiago P, Jose-Yacaman M (2002) Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett 2:397–401. doi:10.1021/nl015673+
Gardea-Torresdey JL, Gomez E, Peralta-Videa JR, Parsons JG, Troiani H, Jose-Yacaman M (2003) Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir 19:1357–1361. doi:10.1021/la020835i
Gherca D, Pui A, Cornei N, Cojocariu A, Nica V, Caltun O (2012) Synthesis, characterization and magnetic properties of mFe2O4 (m = co, mg, mn, ni) nanoparticles using ricin oil as capping agent. J Magn Magn Mater 324(22):3906–3911. doi:10.1016/j.jmmm.2012.06.027
Gherca D, Cornei N, Mentré O, Kabbour H, Daviero-Minaud S, Pui A (2013) In situ surface treatment of nanocrystalline mFe2O4 (m = co, mg, mn, ni) spinel ferrites using linseed oil. Appl Surf Sci 287:490–498. doi:10.1016/j.apsusc.2013.10.018
Gherca D, Pui A, Nica V, Caltun O, Cornei N (2014) Eco-environmental synthesis and characterization of nanophase powders of Co, Mg, Mn and Ni ferrites. Ceram Int 40(7):9599–9607. doi:10.1016/j.ceramint.2014.02.036
Ghodake G, Lim SR, Lee DS (2013) Casein hydrolytic peptides mediated green synthesis of antibacterial silver nanoparticles. Colloid Surf B Biointerface 108:147–151
Gnanam S, Rajendran V (2011) Synthesis of CeO2 or α-Mn2O3 nanoparticles via sol–gel process and their optical properties. J Sol-Gel Sci Technol 58(1):62–69
Gopalakrishnan K, Ramesh C, Ragunathan V, Thamilselvan M (2012) Antibacterial activity of Cu2O nanoparticles on E. coli synthesized from Tridax procumbens leaf extract and surface coating with polyaniline. Dig J Nanomater Biostruct 7:833–839
Gopinath M, Subbaiya R, Selvam MM, Suresh D (2014) Synthesis of copper nanoparticles from Nerium oleander leaf aqueous extract and its antibacterial activity. Int J Curr Microbiol Appl Sci 3:814–818
Gu H, Soucek MD (2007) Preparation and characterization of monodisperse cerium oxide nanoparticles in hydrocarbon solvents. Chem Mater 19(5):1103–1110
Guidelli EJ, Ramos AP, Zaniquelli MED, Baffa O (2011) Green synthesis of colloidal silver nanoparticles using natural rubber latex extracted from Hevea brasiliensis. Spectrochim Acta A Mol Biomol Spectrosc 82:140–145
Hart AE, Akers DB, Gorosh S, Kitchens CL (2013) Reverse micelle synthesis of silver nanoparticles in gas expanded liquids. J Supercrit Fluid 79:236–243
He L, Su Y, Lanhong J, Shi S (2015) Recent advances of cerium oxide nanoparticles in synthesis, luminescence and biomedical studies: a review. J Rare Earths 33(8):791–799
Heera P, Shanmugan S, Ramachandran J (2015) Green synthesis of copper nanoparticle using Gymnema sylvestre by different solvent extract. Int J Curr Res Acad Rev 3:268–275
Hoag GE, Collins JB, Holcomb JL, Hoag JR, Nadagouda MN, Varma RS (2009) Degradation of bromothymol blue by ‘greener’ nano-scale zero-valent iron synthesized using tea polyphenols. J Mater Chem 19(45):8671–8677. doi:10.1039/b909148c
Hong J, Peralta-Videa JR, Gardea-Torresdey JL (2013) Plant-based nanoparticle manufacturing. In: Lens PNL, Virkutyte J, Jegatheesan V, Kim S-H, Al-Abed S (eds) Nanotechnology for water wastewater treatment. Integrated environmental technology series, chapter 18. IWA Publishing, London, pp 409–435
Huang M-F, Kuo Y-C, Huang C-C, Chang H-T (2004) Separation of long double-stranded DNA by nanoparticle-filled capillary electrophoresis. Anal Chem 76:192–196. doi:10.1021/ac034908u
Huang Z, Cui F, Kang H, Chen J, Zhang X, Xia C (2008) Highly dispersed silica-supported copper nanoparticles prepared by precipitation–gel method: a simple but efficient and stable catalyst for glycerol hydrogenolysis. Chem Mater 20:5090–5099
Hulkoti NI, Taranath TC (2014) Biosynthesis of nanoparticles using microbes—a review. Colloid Surf B Biointerface 121:474–483. doi:10.1016/j.colsurfb.2014.05.027
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13:2638–2650. doi:10.1039/c1gc15386b
Iwase E (1928) The preparation of red-gold sols by using as reducing agents the extracts of fresh leaves and plants. Kolloid Z 44:42–43
Jang SJ, Jun YI, Tettery CO, Mo KK, Mook SH (2016) In-vitro anticancer activity of green synthesized silver nanoparticles on MCF-7 human breast cancer cells. Mater Sci Eng C. doi:10.1016/j.msec.2016.03.101
Jayandran M, Haneefa MM, Balasubramanian V (2015) Green synthesis of copper nanoparticles using natural reducer and stabilizer and an evaluation of antimicrobial activity. J Chem Pharm Res 7:251–259
Kaneko K, Inoke K, Freitag B, Hungria AB, Midgley PA, Hansen TW, Zhang J, Ohara S, Adschiri T (2007) Structural and morphological characterization of cerium oxide nanocrystals prepared by hydrothermal synthesis. Nano Lett 7(2):421–425
Keller AA, McFerran S, Lazareva A, Suh S (2013) Global life cycle releases of engineered nanomaterials. J Nanopart Res 15:1692. doi:10.1007/s11051-013-1692-4
Khan SB, Faisal M, Rahman MM, Jamal A (2011) Exploration of CeO2 nanoparticles as a chemi-sensor and photo-catalyst for environmental applications. Sci Total Environ 15:2987–2992
Kharissova OV, Rasika Dias HV, Dias R, Kharisov BI, Olvera Perez B, Jimenez Perez VM (2013) The greener synthesis of nanoparticles. Trends Biotechnol 31:240–248
Kidwai M, Bansal V, Saxena A, Aerry S, Mozumdar S (2006) Cu-nanoparticles: efficient catalysts for the oxidative cyclization of Schiffs’ bases. Tetrahedron Lett 46:8049–8053
Klaus T, Joerger R, Olsson E, Granqvist C-G (1999) Silver-based crystalline nanoparticles, microbially fabricated. Proc Natl Acad Sci USA 96:13611–13614
Klinkaewnarong J, Swatsitang E, Masingboon C, Seraphin S, Maensiri S (2010) Synthesis and characterization of nanocrystalline hap powders prepared by using Aloe vera plant extracted solution. Curr Appl Phys 10(2):521–525. doi:10.1016/j.cap.2009.07.014
Kołodziejczak-Radzimska A, Jesionowski T (2014) Zinc oxide—from synthesis to application: a review. Materials 7:2833–2881
Kouvaris P, Delimitis A, Zaspalis V, Papadopoulos D, Tsipas SA, Michailidis N (2012) Green synthesis and characterization of silver nanoparticles produced using Arbutus unedo leaf extract. Mater Lett 76:18–20
Krishnaraj C, Muthukumaran P, Ramachandran R, Balakumaran MD, Kalaichelvan PT (2014) Acalypha indica Linn: biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol Rep 4:42–49
Kulkarni V, Suryawanshi S, Kulkarni P (2015) Biosynthesis of copper nanoparticles using aqueous extract of Eucalyptus sp. plant leaves. Curr Sci 109:255–257
Kumar B, Smita K, Cumbal L, Debut A (2014) Green approach for fabrication and applications of zinc oxide nanoparticles. Bioinorg Chem Appl. doi:10.1155/2014/523869
Kumar B, Angulo Y, Smita K, Cumbal L, Debut A (2015) Capuli cherry-mediated green synthesis of silver nanoparticles under white solar and blue LED light. Particuology. doi:10.1016/j.partic.2015.05.005
Kumar Das R, Brar SK, Verma M (2016) Checking the biocompatibility of plant-derived metallic nanoparticles: molecular perspective. Trends Biotechnol. doi:10.1016/j.tibtech.2016.02.005
Kumar JBP, Ramgopal G, Vidya YS, Anantharaju KS, Prasad BD, Sharma SC, Prashantha SC, Nagaswarupa HP, Kavyashree D, Nagabhushana H (2015) Green synthesis of Y2O3:Dy3+ nanophosphor with enhanced photocatalytic activity. Spectrochim Acta A 149:687–697. doi:10.1016/j.saa.2015.05.007
Kumar JBP, Ramgopal G, Vidya YS, Anantharaju KS, Prasad BD, Sharma SC, Prashantha SC, Premkumar HB, Nagabhushana H (2015) Bio-inspired synthesis of Y2O3:Eu3+ red nanophosphor for eco-friendly photocatalysis. Spectrochim Acta A 141:149–160. doi:10.1016/j.saa.2015.01.055
Lakshmi J, Sharath R, Chandraprabha MN, Neelufar E, Abhishikta H, Malyasree P (2012) Synthesis, characterization and evaluation of antimicrobial activity of zinc oxide nanoparticles. J Biochem Technol 3:S151–S154
Laokul P, Amornkitbamrung V, Seraphin S, Maensiri S (2011) Characterization and magnetic properties of nanocrystalline CuFe2O4, NiFe2O4, ZnFe2O4 powders prepared by the Aloe vera extract solution. Curr Appl Phys 11(1):101–108. doi:10.1016/j.cap.2010.06.027
Lazareva A, Keller AA (2014) Estimating potential life cycle releases of engineered nanomaterials from wastewater treatment plants. ACS Sustain Chem Eng 2(7):1656–1665
Lee H, Song J, Kim BS (2013) Biological synthesis of copper nanoparticles using Magnolia kobus leaf extract and their antibacterial activity. J Chem Technol Biotechnol 88:1971–1977
Liang H, Raitano JM, He G, Akey AJ, Herman IP, Zhang L, Chan S-W (2012) Aqueous co-precipitation of pd-doped cerium oxide nanoparticles: chemistry, structure, and particle growth. J Mater Sci 47(1):299–307
Li H, Wang G, Zhang F, Cai Y, Wang Y, Djerdj I (2012) Surfactant-assisted synthesis of CeO2 nanoparticles and their application in wastewater treatment. RSC Adv 2:12413–12423
Li G, Jin R (2013) Catalysis by gold nanoparticle: carbon–carbon coupling reactions. Nanotechnol Rev 2:529–549
Lin J, Chen R, Feng S, Pan J, Li Y, Chen G et al (2011) A novel blood plasma analysis technique combining membrane electrophoresis with silver nanoparticle-based SERS spectroscopy for potential applications in noninvasive cancer detection. Nanomed Nanotechnol Biol Med 7(5):655–663
Lin C, Tao K, Hua D, Ma Z, Zhou S (2013) Size effect of gold nanoparticles in catalytic reduction of p-nitrophenol with NaBH4. Molecules 18:12609–12620
Lingaraju K, Raja Naika H, Manjunath K, Basavaraj RB, Nagabhushana H, Nagaraju G, Suresh D (2015) Biogenic synthesis of zinc oxide nanoparticles using Ruta graveolens (L.) and their antibacterial and antioxidant activities. Appl Nanosci. doi:10.1007/s13204-015-0487-6
Luna C, Chávez VHG, Barriga-Castro ED, Núñez NO, Mendoza-Reséndez R (2015) Biosynthesis of silver fine particles and particles decorated with nanoparticles using the extract of Illicium verum (star anise) seeds. Spectrochim Acta A Mol Biomol Spectrosc 141:43–50
Machado S, Pinto SL, Grosso JP, Nouws HPA, Albergaria JT, Delerue-Matos C (2013) Green production of zero-valent iron nanoparticles using tree leaf extracts. Sci Total Environ 445:1–8. doi:10.1016/j.scitotenv.2012.12.033
Macwan DP, Dave PN, Chaturvedi S (2011) A review on nano-TiO2 sol–gel type syntheses and its applications. J Mater Sci 46:3669–3686
Mädler L, Stark W, Pratsinis S (2002) Flame-made ceria nanoparticles. J Mater Res 17(06):1356–1362
Maensiri S, Laokul P, Klinkaewnarong J, Phokha S, Promarak V, Seraphin S (2008) Indium oxide (In2O3) nanoparticles using Aloe vera plant extract: synthesis and optical properties. J Optoelectron Adv Mater 10:161–165
Mahavinod Angrasan JKV, Subbaiya R (2014) Biosynthesis of copper nanoparticles by Vitis vinifera leaf aqueous extract and its antibacterial activity. Int J Curr Microbiol Appl Sci 3:768–774
Malleshappa J, Nagabhushana H, Prashantha SC, Sharma SC, Dhananjaya N, Shivakumara C, Nagabhushana BM (2014) Eco-friendly green synthesis, structural and photoluminescent studies of CeO2:Eu3+ nanophosphors using E. tirucalli plant latex. J Alloys Compd 612:425–434. doi:10.1016/j.jallcom.2014.05.101
Malleshappa J, Nagabhushana H, Kavyashree D, Prashantha SC, Sharma SC, Premkumar HB, Shivakumara C (2015) Shape tailored green synthesis of CeO2:HO3 + nanopowders, its structural, photoluminescence and gamma radiation sensing properties. Spectrochim Acta A 145:63–75. doi:10.1016/j.saa.2015.02.075
Malleshappa J, Nagabhushana H, Sharma SC, Vidya YS, Anantharaju KS, Prashantha SC, Prasad BD, Naika HR, Lingaraju K, Surendra BS (2015) Leucas aspera mediated multifunctional CeO2 nanoparticles: structural, photoluminescent, photocatalytic and antibacterial properties. Spectrochim Acta A 149:452–462. doi:10.1016/j.saa.2015.04.073
Manikandan A, Sridhar R, Antony SA, Ramakrishna S (2014) A simple Aloe vera plant-extracted microwave and conventional combustion synthesis: morphological, optical, magnetic and catalytic properties of CoFe2O4 nanostructures. J Mol Struct 1076:188–200. doi:10.1016/j.molstruc.2014.07.054
Marimuthu S, Rahuman AA, Jayaseelan C, Kirthi AV, Santhoshkumar T, Velayutham K, Siva C et al (2013) Acaricidal activity of synthesized titanium dioxide nanoparticles using Calotropis gigantea against Rhipicephalus microplus and Haemaphysalis bispinosa. Asian Pac J Trop Med 6(9):682–688
Maryanti E, Damayanti D, Gustian I, Yudha SS (2014) Synthesis of ZnO nanoparticles by hydrothermal method in aqueous rinds extracts of Sapindus rarak DC. Mater Lett 118:96–98
Masui T, Hirai H, Imanaka N, Adachi G, Sakata T, Mori H (2002) Synthesis of cerium oxide nanoparticles by hydrothermal crystallization with citric acid. J Mater Sci Lett 21(6):489–491
Mikami Y, Dhakshinamoorthy A, Alvaro M, García H (2013) Catalytic activity of unsupported gold nanoparticles. Catal Sci Technol 3:58–69
Mital GS, Manoj TA (2011) Review of TiO2 nanoparticles. Chin Sci Bull 56:639–1657
Mitsudome T, Arita S, Mori H, Mizugaki T, Jitsukawa K, Kaneda K (2008) Supported silver-nanoparticle-catalyzed highly efficient aqueous oxidation of phenylsilanes to silanols. Angew Chem 120:8056–8058
Molisch H (1921) The microchemistry of plants XVI. The reduction of silver by chlorophyll granules. Ber Botan Ges 39:136–139
Montes MO, Mayoral A, Deepak FL, Parsons JG, Jose-Yacaman M, Peralta-Videa JR, Gardea-Torresdey JL (2011) Anisotropic gold nanoparticles and gold plates biosynthesis using alfalfa extracts. J Nanopart Res 13:3113–3121. doi:10.1007/s11051-011-0230-5
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Ramani R, Parischa R, Ajayakumar PV, Alam M, Sastry M, Kumar R (2001) Reduction of AuCl4 − ions by the fungus Verticillium sp. and surface trapping of gold nanoparticles formed. Angew Chem Int Ed 40:3585
Mukherjee P, Senapati S, Mandal D, Ahmad A, Khan MI, Kumar R, Sastry M (2002) Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. ChemBioChem 5:461–463
Muthukumar H, Matheswaran M (2015) Amaranthus spinosus leaf extract mediated FeO nanoparticles: physicochemical traits, photocatalytic and antioxidant activity. ACS Sustain Chem Eng 3(12):3149–3156. doi:10.1021/acssuschemeng.5b00722
Mystrioti C, Xanthopoulou TD, Papassiopi N, Xenidis A (2016) Comparative evaluation of five plant extracts and juices for nanoiron synthesis and application for hexavalent chromium reduction. Sci Total Environ 539:105–113. doi:10.1016/j.scitotenv.2015.08.091
Nadagouda MN, Castle AB, Murdock RC, Hussain SM, Varma RS (2010) In vitro biocompatibility of nanoscale zerovalent iron particles (NZVI) synthesized using tea polyphenols. Green Chem 12(1):114–122. doi:10.1039/b921203p
Nagai S (1951) The reduction of silver nitrate by plant cell. II. Nature and responsibility of substances which cause the reduction. J Osaka City Univ Inst Polytech Ser D Biol 2:1–8
Nagarajan S, Kuppusamy KA (2013) Extracellular synthesis of zinc oxide nanoparticle using seaweeds of gulf of Mannar, India. J Nanobiotechnol 11:39
Naika HR, Lingaraju K, Manjunath K, Kumar D, Nagaraju G, Suresh D, Nagabhushana H (2015) Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J Taibah Univ Sci 9:7–12
Nanjundaiah Ravishankar T, Ramakrishnappa T, Nagaraju G, Rajanaika H (2015) Synthesis and characterization of CeO2 nanoparticles via solution combustion method for photocatalytic and antibacterial activity studies. Chem Open 4:146–154
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci 156:1–13. doi:10.1016/j.cis.2010.02.001
Narayanan KB, Sakthivel N (2011) Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv Colloid Interface Sci 169:59–79
Naseem T, Farrukh MA (2015) Antibacterial activity of green synthesis of iron nanoparticles using Lawsonia inermis and Gardenia jasminoides leaves extract. J Chem. doi:10.1155/2015/912342
Natsuki J, Natsuki T, Hashimoto Y (2015) A review of silver nanoparticles: synthesis methods, properties and applications. Int J Mater Sci Appl 4:325–332
O’Carroll D, Sleep B, Krol M, Boparai H, Kocur C (2013) Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv Water Resour 51:104–122
Padil VVT, Cerník M (2013) Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int J Nanomed 8:889–898
Park Y, Hong YN, Weyers A, Kim YS, Linhardt RJ (2011) Polysaccharides and phytochemicals: a natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol 5:69–78
Parsons JG, Peralta-Videa JR, Dokken KM, Gardea-Torresdey JL (2009) Biological and biomaterials-assisted synthesis of precious metal nanoparticles. In: Kumar CSSR (ed) Metallic and metal oxide nanomaterials (Nanomaterials for life science, Vol. 1). Wiley-VCH, GmbH, KGaA, Weinheim, pp 461–491
Passos MLC, Costa D, Lima JLFC, Saraiva MLMFS (2015) Sequential injection technique as a tool for the automatic synthesis of silver nanoparticles in a greener way. Talanta 133:45–51
Patil KC, Aruna ST, Ekambaram S (1997) Combustion synthesis. Curr Opin Solid State Mater Sci 2:156–165
Patil KC, Aruna ST, Mimani T (2002) Combustion synthesis: an update. Curr Opin Solid State Mater Sci 6:507–512
Perez Espitia PJ, Ferreira Soares NF, dos Reis Coimbra JS, de Andrade NJ, Souza Cruz R, Alves Medeiros EA (2012) Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol 5:1447–1464
Pérez-Herrero E, Fernández-Medarde A (2015) Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm 93:52–79. doi:10.1016/j.ejpb.2015.03.018
Perkas N, Rotter H, Vradman L, Landau MV, Gedanken A (2006) Sonochemically prepared pt/CeO2 and its application as a catalyst in ethyl acetate combustion. Langmuir 22(16):7072–7077
Philip D (2009) Honey mediated green synthesis of gold nanoparticles. Spectrochim Acta A 73:650–653
Phoka S, Laokul P, Swatsitang E, Promarak V, Seraphin S, Maensiri S (2009) Synthesis, structural and optical properties of CeO2 nanoparticles synthesized by a simple polyvinyl pyrrolidone (PVP) solution route. Mater Chem Phys 1:423–428
Phumying S, Labuayai S, Swatsitang E, Amornkitbamrung V, Maensiri S (2013) Nanocrystalline spinel ferrite (mFe2O4, m = ni, co, mn, mg, zn) powders prepared by a simple Aloe vera plant-extracted solution hydrothermal route. Mater Res Bull 48(6):2060–2065. doi:10.1016/j.materresbull.2013.02.042
Phumying S, Labuayai S, Thomas C, Amornkitbamrung V, Swatsitang E, Maensiri S (2013) Aloe vera plant-extracted solution hydrothermal synthesis and magnetic properties of magnetite (Fe2O4) nanoparticles. Appl Phys A Mater 111(4):1187–1193. doi:10.1007/s00339-012-7340-5
Prasannakumar JB, Vidya YS, Anantharaju KS, Ramgopal G, Nagabhushana H, Sharma SC, Prasad BD, Prashantha S, Basavaraj RB, Rajanaik H, Lingaraju K, Prabhakara KR, Nagaswarupa HP (2015) Bio-mediated route for the synthesis of shape tunable Y2O3:Tb3+ nanoparticles: photoluminescence and antibacterial properties. Spectrochim Acta A 151:131–140. doi:10.1016/j.saa.2015.06.081
Qu J, Yuan X, Wang X, Shao P (2011) Zinc accumulation and synthesis of ZnO nanoparticles using Physalis alkekengi L. Environ Pollut 159:1783–1788
Rafaie HA, Samat NA, Nor RM (2014) Effect of pH on the growth of zinc oxide nanorods using Citurs aurantifolia extracts. Mater Lett 137:297–299
Rajakumar G, Rahuman AA, Priyamvada B, Khanna VG, Kumar DK, Sujin PJ (2012) Eclipta prostrata leaf aqueous extract mediated synthesis of titanium dioxide nanoparticles. Mater Lett 68:115–117
Rajakumar G, Rahuman AA, Jayaseelan C, Santhoshkumar T, Marimuthu S, Kamaraj C, Arora P (2014) Solanum trilobatum extract-mediated synthesis of titanium dioxide nanoparticles to control Pediculus humanus capitis, Hyalomma anatolicum anatolicum and Anopheles subpictus. Parasitol Res 113(2):469–479
Ramesh M, Anbuvannan M, Viruthagiri G (2015) Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim Acta A 136:864–870
Ranu BC, Dey R, Chatterjee T, Ahammed S (2012) Copper nanoparticle-catalyzed carbon carbon and carbon heteroatom bond formation with a greener perspective. ChemSusChem 1:22–44
Reddy V, Torati RS, Oh S, Kim CG (2013) Biosynthesis of gold nanoparticles assisted by Sapindus mukorossi Gaertn. Fruit pericarp and their catalytic application for the reduction of p-nitroaniline. Ind Eng Chem Res 52:556–564
Roopan SM, Bharathi A, Prabhakarn A, Rahuman AA, Velayutham K, Rajakumar G, Madhumitha G (2012) Efficient phyto-synthesis and structural characterization of rutile TiO2 nanoparticles using Annona squamosa peel extract. Spectrochim Acta A Mol Biomol Spectrosc 98:86–90
Ruparelia JP, Kumar Chatterjee A, Duttagupta SP, Mukherji S (2008) Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 3:707–716
Sabir S, Arshad M, Chaudhari SK (2014) Zinc oxide nanoparticles for revolutionizing agriculture: synthesis and applications. Sci World J. doi:10.1155/2014/925494
Saha K, Agasti SS, Kim C, Li X, Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem Rev 112:2739–2779
Samat NA, Nor RM (2013) Sol–gel synthesis of zinc oxide nanoparticles using Citrus aurantifolia extracts. Ceram Int 39:S545–S548
Sankar V, SalinRaj P, Athira R, Soumya RS, Raghu KG (2015) Cerium nanoparticles synthesized using aqueous extract of Centella asiatica: characterization, determination of free radical scavenging activity and evaluation of efficacy against cardiomyoblast hypertrophy. RSC Adv 5(27):21074–21083. doi:10.1039/c4ra16893c
Santhoshkumar T, Rahuman AA, Jayaseelan C, Rajakumar G, Marimuthu S, Kirthi AV, Kim SK et al (2014) Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac J Trop Med 7(12):968–976
Santi M, Sarawuth L, Paveena L, Jutharatana K, Ekaphan S (2014) Structure and optical properties of CeO2 nanoparticles prepared by using lemongrass plant extract solution. Jpn J Appl Phys 53(6S):06JG14
Saranyaadevi K, Subha V, Ravindran RSE, Renganathan S (2014) Synthesis and characterization of copper nanoparticle using Capparis zeylanica leaf extract. Int J Chem Tech Res 6:4533–4541
Saravanakumar A, Ganesh M, Jayaprakash J, Jang HT (2015) Biosynthesis of silver nanoparticles using Cassia tora leaf extract and its antioxidant and antibacterial activities. J Ind Eng Chem 28:277–281
Sarkar A, Mukherjee T, Kapoor S (2008) PVP-stabilized copper nanoparticles: a reusable catalyst for “click” reaction between terminal alkynes and azides in nonaqueous solvents. J Phys Chem C 112:3334–3340
Sathyamurthy S, Leonard KJ, Dabestani RT, Paranthaman MP (2005) Reverse micellar synthesis of cerium oxide nanoparticles. Nanotechnology 16(9):1960–1964. doi:10.1088/0957-4484/16/9/089
Sau TK, Murphy CJ (2004) Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J Am Chem Soc 126:8648–8649
Shankar SS, Rai A, Ankamwar B, Singh A, Ahmad A, Sastry M (2004) Biological synthesis of triangular gold nanoprisms. Nat Mater 3:482–488
Shankar SS, Rai A, Ahmad A, Sastry M (2005) Controlling the optical properties of lemongrass extract synthesized gold nanotriangles and potential application in infrared-absorbing optical coatings. Chem Mater 17:566–572
Sharma N, Sahi S, Nath S, Parsons JG, Gardea-Torresdey JL, Pal T (2007) Synthesis of plant-mediated gold nanoparticles and catalytic role of biomatrix-embedded nanomaterials. Environ Sci Technol 41:5137–5142
Sharma JK, Srivastava P, Akhtar MS, Singh G, Ameen S (2015) Alpha-Fe2O3 hexagonal cones synthesized from the leaf extract of Azadirachta indica and its thermal catalytic activity. New J Chem 39(9):7105–7111. doi:10.1039/c5nj01344e
Sinha T, Ahmaruzzaman M (2015) Biogenic synthesis of Cu nanoparticles and its degradation behavior for methyl red. Mater Lett 159:168–171
Smitha SL, Philip D, Gopchandran KG (2009) Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth. Spectrochim Acta A 74:735–739
Sone BT, Manikandan E, Gurib-Fakim A, Maaza M (2015) Sm2O3 nanoparticles green synthesis via Callistemon viminalis’ extract. J Alloys Compd 650:357–362. doi:10.1016/j.jallcom.2015.07.272
Soomro RA, Sherazi STH, Sirajuddin Memon N, Shah MR, Kalwar NH, Hallam KR, Shah A (2014) Synthesis of air stable copper nanoparticles and their use in catalysis. Adv Mater Lett 5:191–198
Sreekanth TVM, Jung M-J, Eom I-Y (2016) Green synthesis of silver nanoparticles, decorated on graphene oxide nanosheets and their catalytic activity. Appl Surf Sci 361:102–106
Sreeremya TS, Thulasi KM, Krishnan A, Ghosh S (2011) A novel aqueous route to fabricate ultrasmall monodisperse lipophilic cerium oxide nanoparticles. Ind Eng Chem Res 51(1):318–326
Strunk J, Kahler K, Xia X, Muhler M (2009) The surface chemistry of ZnO nanoparticles applied as heterogeneous catalysts in methanol synthesis. Surf Sci 603:1776–1783
Suarez-Cerda J, Alonso-Nuñez G, Espinoza-Gómez H, Flores-López LZ (2015) Synthesis, kinetics and photocatalytic study of “ultra-small” Ag-NPs obtained by a green chemistry method using an extract of Rosa ‘Andeli’ double delight petals. J Colloid Interface Sci 458:169–177
Subbaiya R, Selvam MM (2015) Green synthesis of copper nanoparticles from Hibicus rosa-sinensis and their antimicrobial, antioxidant activities. Res J Pharm Biol Chem Sci 6:1183–1190
Subhankari I, Nayak PL (2013) Synthesis of copper nanoparticles using Syzygium aromaticum (Cloves) aqueous extract by using green chemistry. World J Nano Sci Technol 2:14–17
Suman TY, Ravindranath RRS, Elumalai D, Kaleena PK, Ramkumar R, Perumal P, Chitrarasu PS et al (2015) Larvicidal activity of titanium dioxide nanoparticles synthesized using Morinda citrifolia root extract against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus and its other effect on non-target fish. Asian Pac J Trop Dis 5(3):224–230
Sundrarajan M, Gowri S (2011) Green synthesis of titanium dioxide nanoparticles by Nyctanthes arbor-tristis leaves extract. Chalcogenide Lett 8(8):447–451
Sundrarajan M, Jegatheeswaran S, Selvam S, Sanjeevi N, Balaji M (2015) The ionic liquid assisted green synthesis of hydroxyapatite nanoplates by Moringa oleifera flower extract: a biomimetic approach. Mater Des 88:1183–1190. doi:10.1016/j.matdes.2015.09.051
Sutradhar P, Saha M, Maiti D (2014) Microwave synthesis of copper oxide nanoparticles using tea leaf and coffee powder extracts and its antibacterial activity. J Nanostruct Chem 4:86
Sutradhar P, Saha M (2015) Green synthesis of zinc oxide nanoparticles using tomato (Lycopersicon esculentum) extract and its photovoltaic application. J Exp Nanosci. doi:10.1080/17458080.2015.1059504
Tamilvanan A, Balamurugan K, Ponappa K, Madhan Kumar B (2014) Copper nanoparticles: synthetic strategies, properties and multifunctional application. Int J Nanosci 13:1430001
Terenteva EA, Apyari VV, Dmitrienko SG, Zolotov YA (2015) Formation of plasmonic silver nanoparticles by flavonoid reduction: a comparative study and application for determination of these substances. Spectrochim Acta A Mol Biomol Spectrosc 151:89–95
The Royal Society (2004) Nanoscience and nanotechnologies. London, pp 25–33. https://royalsociety.org/~/media/Royal_Society_Content/policy/publications/2004/9693.pdf
Thema FT, Beukes P, Gurib-Fakim A, Maaza M (2015) Green synthesis of monteponite CdO nanoparticles by Agathosma betulina natural extract. J Alloys Compd 646:1043–1048. doi:10.1016/j.jallcom.2015.05.279
Thema FT, Manikandan E, Dhlamini MS, Maaza M (2015) Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mater Lett 161:124–127
Thema FT, Manikandan E, Gurib-Fakim A, Maaza M (2016) Single phase bunsenite NiO nanoparticles green synthesis by Agathosma betulina natural extract. J Alloys Compd 657:655–661. doi:10.1016/j.jallcom.2015.09.227
Thovhogi N, Diallo A, Gurib-Fakim A, Maaza M (2015) Nanoparticles green synthesis by Hibiscus sabdariffa flower extract: main physical properties. J Alloys Compd 647:392–396. doi:10.1016/j.jallcom.2015.06.076
Thovhogi N, Park E, Manikandan E, Maaza M, Gurib-Fakim A (2016) Physical properties of CdO nanoparticles synthesized by green chemistry via Hibiscus sabdariffa flower extract. J Alloys Compd 655:314–320. doi:10.1016/j.jallcom.2015.09.063
Tiwari PM, Vig K, Dennis VA, Singh SR (2011) Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 1:31–63
Tobiszewski M, Marc M, Galuszka A, Namiesnik J (2015) Green chemistry metrics with special reference to green analytical chemistry. Molecules 20:10928–10946
Trujillo-Reyes J, Peralta-Videa JR, Gardea-Torresdey JL (2014) Supported and unsupported nanomaterials for water and soil remediation: are they a useful solution for worldwide pollution? J Hazard Mater 280:487–503
Tu C-H, Wang A-Q, Zheng M-Y, Wang X-D, Zhang T (2006) Factors influencing the catalytic activity of SBA-15-supported copper nanoparticles in CO oxidation. Appl Catal A 4:40–47
Turkevich J (1985) Collodial gold part I: historical and preparative aspects, morphology and structure. Gold Bull 18:86–91
Turkevich J (1985) Collodial gold part II: colour, coagulation, adhesion, alloying and catalytic properties. Gold Bull 18:125–131
Vanathi P, Rajiv P, Narendhran S, Rajeshwari S, Rahman PKSM, Venckatesh R (2014) Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: a green chemistry approach. Mater Lett 134:13–15
Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MF Jr, Rejeski D, Hull MS (2015) Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol 6:1769–1780. doi:10.3762/bjnano.6.181
Velayutham K, Rahuman AA, Rajakumar G, Santhoshkumar T, Marimuthu S, Jayaseelan C, Elango G et al (2012) Evaluation of Catharanthus roseus leaf extract-mediated biosynthesis of titanium dioxide nanoparticles against Hippobosca maculata and Bovicola ovis. Parasitol Res 111(6):2329–2337
Velmurugan P, Sivakumar S, Young-Chae S, Seong-Ho J, Pyoung-In Y, Jeong-Min S, Sung-Chul H (2015) Synthesis and characterization comparison of peanut shell extract silver nanoparticles with commercial silver nanoparticles and their antifungal activity. J Ind Eng Chem 31:51–54
Vidya YS, Anantharaju KS, Nagabhushana H, Sharma SC (2015) Euphorbia tirucalli mediated green synthesis of rose like morphology of Gd2O3:Eu3+ red phosphor: structural, photoluminescence and photocatalytic studies. J Alloys Compd 619:760–770. doi:10.1016/j.jallcom.2014.09.050
Villanueva-Ibáñez M, Yañez-Cruz MG, Álvarez-García R, Hernández-Pérez MA, Flores-González MA (2015) Aqueous corn husk extract-mediated green synthesis of AgCl and Ag nanoparticles. Mater Lett 152:166–169
Vinothkannan M, Karthikeyan C, Kumar GG, Kim AR, Yoo DJ (2015) One-pot green synthesis of reduced graphene oxide (rGo)/Fe3O4 nanocomposites and its catalytic activity toward methylene blue dye degradation. Spectrochim Acta A 136:256–264. doi:10.1016/j.saa.2014.09.031
Virkutyte J, Varma RS (2015) Green synthesis of nanomaterials: environmental aspects? In: Shamin N, Sharma VK (eds) Sustainable nanotechnology and the environment: advances and achievements. ACS symposium series vol 1124, pp 11–39. doi:10.1021/bk-2013-1124
Walkey C, Das S, Seal S, Erlichman J, Heckman K, Ghibelli L, Traversa E, McGinnis JF, Self WT (2015) Catalytic properties and biomedical applications of cerium oxide nanoparticles. Environ Sci Nano 2(1):33–53. doi:10.1039/C4EN00138A
Wang H, Zhu J-J, Zhu J-M, Liao X-H, Xu S, Ding T, Chen H-Y (2002) Preparation of nanocrystalline ceria particles by sonochemical and microwave assisted heating methods. Phys Chem Chem Phys 4(15):3794–3799
Wang Y, He X, Wang K, Zhang X, Tan W (2009) Barbated Skullcup herb extract-mediated biosynthesis of gold nanoparticles and its primary application in electrochemistry. Colloid Surf B Biointerfaces 73:75–79
Wang Z, Fang C, Megharaj M (2014) Characterization of iron–polyphenol nanoparticles synthesized by three plant extracts and their fenton oxidation of azo dye. ACS Sustain Chem Eng 2(4):1022–1025. doi:10.1021/sc500021n
Wu C-C, Chen D-H (2010) Facile green synthesis of gold nanoparticles with gum Arabic as a stabilizing agent and reducing agent. Gold Bull 43:234–240
Xiong J, Wang Y, Xue Q, Wu X (2011) Synthesis of highly stable dispersions of nanosized copper particles using l-ascorbic acid. Green Chem 13:900
Xu H, Zeigera BW, Suslick KS (2013) Sonochemical synthesis of nanomaterials. Chem Soc Rev 42(7):2555–2567
Xu C, Qu X (2014) Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater 6:e90
Yang H, Huang C, Tang A, Zhang X, Yang W (2005) Microwave-assisted synthesis of ceria nanoparticles. Mater Res Bull 40(10):1690–1695
Yu T, Joo J, Park YI, Hyeon T (2005) Large-scale nonhydrolytic sol–gel synthesis of uniform-sized ceria nanocrystals with spherical, wire, and tadpole shapes. Angew Chem 117(45):7577–7580
Zhang J, Sunkara B, Tang J, Wang Y, He J, McPherson GL, John VT (2011) Carbothermal synthesis of aerosol-based adsorptive-reactive iron–carbon particles for the remediation of chlorinated hydrocarbons. Ind Eng Chem Res 50(23):13021–13029
Acknowledgments
This material is based upon work supported by the National Science Foundation and the Environmental Protection Agency under Cooperative Agreement Number DBI-1266377. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation or the Environmental Protection Agency. This work has not been subjected to EPA review, and no official endorsement should be inferred. The authors also acknowledge the USDA Grants 2011-38422-30835 and 2016-67021-24985 and the NSF Grants # CHE-0840525 and DBI-1429708. Partial funding was provided by the NSF ERC on Nanotechnology-Enabled Water Treatment (EEC-1449500). This work was also supported by Grant 2G12MD007592 from the National Institutes on Minority Health and Health Disparities (NIMHD), a component of the National Institutes of Health (NIH). J. L. Gardea-Torresdey acknowledges the Dudley family for the Endowed Research Professorship and the Academy of Applied Science/US Army Research Office, Research and Engineering Apprenticeship program (REAP) at UTEP, Grant #W11NF-10-2-0076, Sub-Grant 13-7. N.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Peralta-Videa, J.R., Huang, Y., Parsons, J.G. et al. Plant-based green synthesis of metallic nanoparticles: scientific curiosity or a realistic alternative to chemical synthesis?. Nanotechnol. Environ. Eng. 1, 4 (2016). https://doi.org/10.1007/s41204-016-0004-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41204-016-0004-5