Abstract
Feeding behaviour of pest insects on host plants depends on the plant morphology and chemical composition. So far, leaf position was demonstrated important for estimation of thrips resistance in Capsicum, where resistant associations showed a higher resistance in the youngest leaves compared to old leaves. In the current study, the feeding behaviour of female Frankliniella occidentalis, a major pest of chrysanthemum, and the egg-laying activity was assessed in a non-choice experiment in the climate chamber. We hypothesize that the physiological status, i.e. the age of chrysanthemum leaves, is an important resistance factor influencing thrips silver damage and the number of offspring. In general, the results show significantly higher feeding damage on old (basal) leaves of susceptible cultivars compared to resistant cultivars on the abaxial side of the leaf. In contrast, we detected no significant differences on the adaxial side of the leaves neither for old nor for young leaves. However, feeding damage on different leaf positions is an important factor for resistance level determination. Additionally, we detected significant differences in reproductive activity, i.e. number of hatched larvae on old leaves compared to young leaves for all cultivars. Overall, on old leaves we detected more larvae. The comparison between the old leaves of all cultivars exhibited a significant difference between one resistant and one susceptible cultivar. Contrary to that, the comparison between the young leaves of all cultivars exhibited no significant differences. All findings are important for advancing future resistance screenings in chrysanthemum.
Similar content being viewed by others
Introduction
Western flower thrips (Frankliniella occidentalis (Pergande)) is a polyphagous insect and one of the major pests in vegetable and ornamental production due to direct and indirect damage. The thrips ingest the cell contents from epidermal cells as well as from palisade and spongy mesophyll cells (Fiene et al. 2013; Kindt et al. 2003; Krishna Kumar et al. 1995). This leads to empty, air-filled spaces. As the cells appear silvery to the human eye, this type of damage is called silver damage (De Jager et al. 1993; Reitz 2009). It can occur anywhere on aboveground plant parts, like leaves, flowers or fruits (Cloyd 2009; Morse and Hoddle 2006; Mound 2005), and it can lead to a considerable loss of cosmetic and economic value of the crop (Terry 1997). This type of damage represents a first sign of thrips on plants (Lopez et al. 2008; Reitz 2009), and hence a high focus is set to it.
F. occidentalis is abundant as serious pest in many field crops, e.g. cabbage, cucumber, cotton, pea, wheat, etc., and greenhouse crops like roses, potato, poinsettia, chrysanthemum, etc. (CABI 2020). Nonetheless where feeding takes place, control of thrips in greenhouse production becomes increasingly difficult and alternative strategies for thrips control are needed. Breeding of chrysanthemum cultivars with a focus on constitutive resistance against thrips is one possibility. Previously, the focus of breeding was on aesthetic values only, but the demand for resistance against pathogens and insects is increasing. Incorporation of resistance against F. occidentalis will gain importance in future chrysanthemum breeding due to the decreasing number of registered insecticides for this herbivore. The breeding process is very lengthy and does not necessarily show success. To determine as quickly and easily as possible whether a new cultivar is resistant or not, a rapid testing, i.e. resistance screening, would be desirable. For pathogens like Puccinia horiana, causing chrysanthemum white rust, first high-throughput bioassay resistance screenings were already developed (De Backer et al. 2011). For a good thrips resistance screening, however, basic knowledge must be available on expression of resistance, e.g. decreased silver damage on different plant parts, age or onthogenetic stage of the plant. It was shown that the youngest fully opened leaves of a resistant Capsicum accession are significantly more resistant to thrips larvae than older leaves during the non-flowering phase (Van Haperen et al. 2019). Contrary, Visschers et al. (2019) reported that leaf position within the onthogenetic stage did not affect thrips resistance in Capsicum, only ontogenetic development plays a role, as it was described for different ontogenetic states of chrysanthemum (Rogge and Meyhöfer in press). Consequently, it would be an important factor for a resistance screening, if a cultivar already shows resistance to thrips in the vegetative state and if this can be predicted by damage levels on specific leaves. Therefore, in the present study we investigated the feeding and oviposition behaviour of female F. occidentalis on leaves of chrysanthemum cultivars with different resistance level and hypothesize that the age of chrysanthemum leaves is an important factor for resistance screenings according to silver damage and egg laying behaviour of female F. occidentalis. Additionally, we hypothesize a feeding preference for the adaxial side of chrysanthemum leaves.
Material and methods
Rearing of F. occidentalis and cultivation of plants
F. occidentalis rearing took place in a climate chamber (photoperiod 16:8 h L:D, 25 ± 1 °C and 50 ± 5 % relative humidity). They were mass reared on Phaseolus vulgaris L. in wooden cages covered with thrips safe gauze. Female F. occidentalis were collected prior to experiments in a box stored on ice, and three females were picked with a fine brush and transferred to an Eppendorf tube containing moist cotton wool. In total, 40 tubes containing thrips on moist cotton wool and 40 control tubes containing moist cotton wool only were prepared. Tubes remained in the laboratory at room temperature and thrips were starved for a 24 h period.
Chrysanthemum plants (N = 5) of 4 cultivars, i.e. two susceptible (Kanok, Mumbai Red) and two resistant [Colombo Apricot, Luzon Pink; Brandkamp, see (Rogge and Meyhöfer in press)], were cultivated in a greenhouse cabin at 22 ± 2 °C under long day conditions (16:8 h L:D) in Fruhstorfer soil substrate type P (Hawita Group, Vechta, Germany). Plants were 16 weeks old when the experiment started. They were frequently cut, with the last cutting 5 weeks before the experiment started. From each plant, two old leaves (basal, next to plant base) and two young leaves (apical, next to plant tip) were used for the experiment: one of each treated with thrips, the other one served as control without thrips. Control leaves were necessary to document basic infestation of host plants with thrips eggs prior to the experiments and impact on leaf quality after 11 d. To avoid a high thrips infestation in the greenhouse, beneficial insects (Orius laevigatus) were released biweekly.
Non-choice experiment assessing silver damage and egg laying activity
Eighty polystyrene boxes with a size of 154 × 138 × 59 mm (art.no.: 1738; Semadeni Plastics Group) were placed randomly in a climate chamber at environmental conditions of 22 ± 2 °C, 50% relative humidity and 16:8 h L:D. Each box contained a petri dish (90 × 15 mm) with 1.5% sloping water agar on one side enclosing the leaf petiole. Thrips had at any time access to both sides of the leaf.
All leaves were visually scored for damage symptoms before releasing thrips. Boxes were closed with a lid and sealed with Parafilm. On one side of the box, a hole was drilled with a silicone pipe inserted. An Eppendorf tube with three female thrips or without thrips (control) was attached to the pipe. Insects could walk through the pipe to the leaf in the box (Figure 1). 7 days after release, assessment of silver damage, i.e. silvery spots and patches with faeces on damaged cells, was done again visually in percentage of damaged area per leaf on the abaxial and adaxial side. The difference between both assessments (before and after thrips release) was calculated for each leaf for statistical analysis. To further correct data, the mean silver damage in the control treatment was calculated for each cultivar and subtracted from the calculated silver damage in the treatment for each cultivar. Negative values were set to zero (hereinafter referred to as adjusted silver damage). After silver damage assessment, females were counted and removed, and hatched larvae were counted 11 days after female release in each petri dish. The mean number of larvae in the control treatment was calculated and subtracted from the mean number of larvae in the treatment for each cultivar for statistical analysis (hereinafter referred to as adjusted number of larvae).
Schematic design of the arena in the non-choice bioassay. A box contained a petri dish with 1.5% water agar on one side, containing one leaf. On one side of the box, a silicone pipe was inserted. An Eppendorf tube with 3 female thrips (treatment) or without thrips (control) was attached to the pipe. The box was closed with a lid and sealed with Parafilm.
Statistical evaluation was done using RStudio Version 1.3.1073 (R Version 4.0.2). The adjusted silver damage and the adjusted number of larvae were analysed using Bayesian generalized linear model (Bayesian GLM) with quasipoisson distribution. The R package emmeans (version 1.4.7) was used to estimate the marginal mean and standard error (SE) of individual outcomes within leaf age for each cultivar [basic model structures: fit < -bayesglm(damage ~ cultivar*leaf age, data = data, family = quasipoisson) and emmeans (fit, specs = “leaf age”, by = c(“cultivar”), contr = “pairwise”)]. Pairwise comparison was conducted between the cultivars for the adjusted number of larvae and the number of recaptured females (alive) in arenas with old or young leaves (Supplemental Table 1 and 2).
Results
Silver damage on old and young chrysanthemum leaves on the abaxial and adaxial side
Overall, the abaxial side of the leaf was preferred over the adaxial side by thrips for feeding (Figures 2 and 3). Altogether, silver damage on the abaxial side of the leaf was 1.5–8.1 times higher compared to the adaxial side.
Box plot of adjusted silver damage (abaxial) on old and young leaves per cultivar 7 days after release of 3 female F. occidentalis per box. N = 5, Bayesian generalized linear model with quasipoisson distribution; different letters show significant differences between old and young leaves among the cultivars; circles indicate outliers.
Box plot of adjusted silver damage (adaxial) on old and young leaves per cultivar 7 days after release of 3 female F. occidentalis per box. N = 5, Bayesian generalized linear model with quasipoisson distribution; different letters show significant differences between old and young leaves among the cultivars; circles indicate outliers.
Abaxial side of the leaf
Within 7 days, feeding activity of female F. occidentalis on old leaves of the susceptible cultivars was significantly higher compared to young leaves (N = 5 with three females per box). For the susceptible cultivar Mumbai Red, feeding on old leaves was 13.3-times higher compared to young leaves. These differences were significant (estimate = 2.329, SE = 0.885, df = inf, z-ratio = 2.632, p = 0.0085). Similarly, for the susceptible cultivar Kanok, feeding on old leaves was 5.4-times higher and significant different (estimate = 1.629, SE = 0.513, df = inf, z-ratio = 3.179, p = 0.0015). For the resistant cultivars Colombo Apricot (estimate = 1.028, SE = 0.620, df = inf, z-ratio = 1.659, p = 0.0971) and Luzon Pink (estimate = 0.619, SE = 0.704, df = inf, z-ratio = 0.878, p = 0.379), we detected no significant differences in silver damage between old and young leaves (Figure 2).
Adaxial side of the leaf
The susceptible cultivar Mumbai Red exhibited slightly more (2.1-times) feeding damage on the old leaves (estimate = 0.645, SE = 0.926, df = inf, z-ratio = 0.696, p = 0.486), which was not significant and interestingly, the resistant cultivar Luzon Pink exhibited slightly more (3.3-times) feeding damage on the young leaves, which was also not significant (estimate = − 1.036, SE = 0.652, df = inf, z-ratio = − 1.589, p = 0.112). In addition, we detected no significant differences in silver damage on old leaves compared to young leaves for the susceptible cultivar Kanok (estimate = 0.612, SE = 0.729, df = inf, z-ratio = 0.840, p = 0.401) and the resistant cultivar Colombo apricot (estimate = 0.160, SE = 0.603, df = inf, z-ratio = 0.840, p = 0.791) (Figure 3).
Number of hatched larvae on old and young chrysanthemum leaves and number of recaptured females
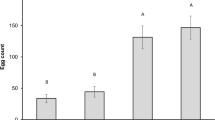
We showed significant differences in number of hatched larvae between old and young leaves among all cultivars. The number of hatched larvae on old leaves from the susceptible cultivar Mumbai Red was significantly higher (4.1-fold) compared to young leaves (estimate = 1.40, SE = 0.416, df = inf, z-ratio = 3.367, p = 0.0008) as it was for the susceptible cultivar Kanok (3-times higher, estimate = 1.09, SE = 0.479, df = inf, z-ratio = 2.275, p = 0.023), and the resistant cultivars Colombo Apricot (2.7-times higher, estimate = 1.01, SE = 0.474, df = inf, z-ratio = 2.138, p = 0.033) and Luzon Pink (4.3-times higher, estimate = 1.42, SE = 0.531, df = inf, z-ratio = 2.669, p = 0.008) (Figure 4). Supplemental table 1 compares the differences of hatched larvae between the cultivars on the one hand in old leaves and on the other hand in young leaves. The cultivars showed no significant differences in comparison of young leaves, but for the old leaves the resistant cultivar Colombo Apricot showed significant fewer hatched larvae compared to the susceptible cultivar Mumbai Red. Supplemental table 2 shows the summary statistics for the differences of recaptured females (alive) in the arenas between the cultivars. It is apparent from this table that we detected no significant differences in recaptured females between the cultivars neither in old nor in young leaves. The total number of hatched larvae and the number of recaptured females (dead and alive) are given in the supplemental table 3.
Box plot with adjusted number of hatched larvae on old and young leaves per cultivar 11 days after release of 3 female F. occidentalis (removed after 7 days) per box. N = 5, Bayesian generalized linear model with quasipoisson distribution; different letters show significant differences between old and young leaves among the cultivars; circles indicate outliers.
Discussion
For a clear discrimination of host plant resistance against the piercing and sucking Western flower thrips, it is important to know the preferred feeding side of a host plant leaf. It is known that thrips feed on both leaf sides. However, different studies suggested a preference for one side of the leaf. As described by Van Dijken (1992) and Rogge and Meyhöfer (in press), we hypothesized higher silver damage on the adaxial side of chrysanthemum leaves. In the current study, we assessed the silver damage on the adaxial and abaxial side of leaves of resistant and susceptible chrysanthemum cultivars. Results show that thrips feed more on the abaxial side of the leaf, independent of the resistance status of the chrysanthemum cultivar. For F. occidentalis on cotton leaves and Heliothrips haemorrhoidalis on Rhododendron simsii, more feeding was also recorded on abaxial leaf side (Fiene et al. 2013; Scott-Brown et al. 2016). Compared to Rogge and Meyhöfer (in press), where whole plants were investigated in blocks in greenhouses, the detached leaves in our current laboratory bioassay were directly exposed to roof lights without shading from neighbouring plants, which may have caused a preference for the shaded areas on the abaxial side. In field studies with whole cowpea plants in a choice test, it was also described for Megalurothrips sjostedt that the insects preferred most likely the unshaded plants (Kyamanywa and Ampofo 1988). Similar results were shown in other field trials, where the order Thysanoptera preferred unshaded over shaded field margins (Cauwer et al. 2006). Both studies only mention the presence of thrips, but not more details on which leaf side they were found, because traps were used in both studies rather than on plant observations. According to this, thrips seems to have in general no problem with feeding on light exposed plants. Additionally, own follow-up experiments show that light exposure is only of minor importance for selection of feeding sites for F. occidentalis (unpublished data). We suggest other factors like morphology or trichome density as more important for foraging decisions by Western flower thrips.
In the present study, old and young leaves were taken from plants in the vegetative state. Two of the investigated cultivars were characterized as susceptible (Kanok, Mumbai Red), and two as resistant (Luzon Pink, Colombo Apricot) in generative state. However, a constitutive resistance in these cultivars is given also in the vegetative state (Rogge and Meyhöfer in press). Moreover in the present study, female thrips showed significantly reduced feeding activity on old leaves of resistant cultivars compared to susceptible cultivars. For young leaves, this result is not consistent. De Kogel et al. (1997) described a feeding preference of F. occidentalis to younger leaves in cucumber. However, Kos et al. (2014) described silver damage mainly occurring on old leaves and observed a growth depression on young chrysanthemum leaves. Visschers et al. (2019) investigated resistance varying with onthogenetic stage in Capsicum. Based on larval feeding, this study showed similar thrips resistance on all leaf positions within an ontogenetic stage. According to our results consequently, we recommend to distinguish carefully between leaf age respectively leaf position in resistance screenings with chrysanthemum plants and to select leaves in the same position for meaningful comparisons.
Van Haperen et al. (2019) mentioned that significantly more larvae emerged from young Capsicum leaves, although the number of emerged larvae is not high. At temperatures ranging from 20 to 23 °C, female thrips lay in general 5–6 eggs per 24 h (Gaum et al. 1994; Teulon and Penman 1991). Contrary to that, we showed a higher number of hatched larvae on old chrysanthemum leaves compared to young leaves. Nevertheless, leaves of the susceptible cultivars Kanok and Mumbai Red seem to be optimal substrates for thrips egg laying, since up to 1.9-times more larvae compared to the other chrysanthemum cultivars were recorded in the current study. However, for future resistance experiments, we recommend to distinguish between oviposition rate (number of eggs in the leaf tissue), the number of hatched larvae and the number of surviving larvae.
In summary, thrips feeding and egg-laying behaviour differs on old compared to young leaves. Different authors mentioned a higher resistance of host plants to e.g. Bemisia tabaci (Van den Oever-van den Elsen et al. 2016), Aleyrodes proletella (Broekgaarden et al. 2012) or F. occidentalis (Van Haperen et al. 2019) which increased with plant age. But so far it is not clear, if the difference is based on morphological or metabolomic differences. For Stenchaetothrips biformis, it is known that trichome density on rice leaves is positively correlated with the resistance of the rice plant (Wickramasinghe et al. 2007) and on Rhododendron simsii, the area of feeding damage increased as trichome density decreased in a study with Heliothrips haemorrhoidalis (Scott-Brown et al. 2016). However, leaf hairiness, leaf hardness and leaf shape had no effect on thrips feeding on different Gossypium species (Miyazaki et al. 2017), and only plant height and number of leaves in chrysanthemum were important for thrips resistance so far (De Jager et al. 1995). With the human eye, we detected no differences in trichome density between the cultivars or between old and young leaves. However, follow-up experiments will give us a better differentiation. Size of the leaves differed between the cultivars, ranging from some small leaves for cultivar Kanok (smaller than 4 cm in length with petiole) up to twice as large leaves for cultivar Mumbai Red (larger 6 cm in length with petiole). But due to evaluation of silver damage using a percentagewise scoring, the potential effect of leaf size in our current study can be neglected.
F. occidentalis, as a polyphagous insect, is very flexible in feeding on different host plants (CABI 2020). It is very eager to find a new host, if the present host is not suitable. In the current study, we recognized that females escaped from some of our arenas, e.g. when the sealing around the box was broken. Seemingly the thrips tried to find another host. Some treatment arenas with slightly broken sealing showed no females anymore after 7 days. However, the only available hosts in our chamber were other chrysanthemum leaves in the boxes. The susceptible cultivar Mumbai Red served as good host for feeding and egg laying and seemed to be one choice for female thrips. In four control treatments, females found ways to colonize the thrips-free Mumbai Red control treatments and in one case a thrips-free Luzon Pink control treatment. It is not clear at which time point the animals escaped from the arenas. However, in some arenas with unbroken sealing we could not find the initially released three female thrips. One reason could have been the opening of the lids and the escape of the females. A visual perception of the flying thrips is not possible by human eye, and accordingly, they are not taken into account. No significant differences were detected for the number of recaptured living females between all cultivars. We assume accordingly that the number of living females had no influence on the percentage of silver damage or number of laid eggs for all cultivars.
Future research about resistance in chrysanthemum against F. occidentalis should always consider the difference between old (basal) and young (apical) leaves. For a clearer determination of the resistance levels in bioassay setups, we recommend the use of basal leaves of chrysanthemums. If only one side of a leaf is available, we recommend to select the abaxial side of the leaf. Plants in the current study were 16 weeks old. It should be tested whether younger plants, e.g. 8 weeks old, could also be suitable for characterization of the resistance level using old leaves.
Change history
19 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s41348-021-00542-y
References
Broekgaarden C, Riviere P, Steenhuis G, del SolCuenca M, Kos M, Vosman B (2012) Phloem-specific resistance in Brassica oleracea against the whitefly Aleyrodes proletella. Entomol Exp et Appl 142:153–164. https://doi.org/10.1111/j.1570-7458.2011.01210.x
CABI (2020) Invasive Species Compendium. www.cabi.org/isc
Cloyd RA (2009) Western flower thrips (Frankliniella occidentalis) management on ornamental crops grown in greenhouses: have we reached an impasse. Pest Technol 3:1–9
De Backer M, Alaei H, van Bockstaele E, Roldan-Ruiz I, van der Lee T, Maes M, Heungens K (2011) Identification and characterization of pathotypes in Puccinia horiana, a rust pathogen of Chrysanthemum x morifolium. Eur J Plant Pathol 130:325–338. https://doi.org/10.1007/s10658-011-9756-8
De Cauwer B, Reheul D, de Laethauwer S, Nijs I, Milbau A (2006) Effect of light and botanical species richness on insect diversity. Agron Sustain Dev 26:35–43. https://doi.org/10.1051/agro:2005058
De Jager CM, Butôt RPT, Jong TJ, Klinkhamer PGL, Meijden E (1993) Population growth and survival of western flower thrips Frankliniella occidentalis Pergande (Thysanoptera, Thripidae) on different chrysanthemum cultivars: two methods for measuring resistance. J Appl Entomol 115:519–525. https://doi.org/10.1111/j.1439-0418.1993.tb00422.x
De Jager CM, Butôt RPT, Klinkhamer PGL, Jong TJ, Wolff K, Meijden E (1995) Genetic variation in chrysanthemum for resistance to Frankliniella occidentalis. Entomol Exp et Appl 77:277–287. https://doi.org/10.1111/j.1570-7458.1995.tb02325.x
De Kogel W, van der Hoek M, Mollema C (1997) Oviposition preference of western flower thrips for cucumber leaves from different positions along the plant stem. Entomol Exp et Appl 82:283–288. https://doi.org/10.1046/j.1570-7458.1997.00142.x
Den Oever-van Van, den Elsen F, Lucatti AF, van Heusden S, Broekgaarden C, Mumm R, Dicke M, Vosman B (2016) Quantitative resistance against Bemisia tabaci in Solanum pennellii: genetics and metabolomics. J Integr Plant Biol 58:397–412. https://doi.org/10.1111/jipb.12449
Fiene J, Kalns L, Nansen C, Bernal J, Harris M, Sword GA (2013) Foraging on individual leaves by an intracellular feeding insect is not associated with leaf biomechanical properties or leaf orientation. PLoS One 8:e80911. https://doi.org/10.1371/journal.pone.0080911
Gaum WG, Giliomee JH, Pringle KL (1994) Life history and life tables of western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), on english cucumbers. BER 84:219–224. https://doi.org/10.1017/S0007485300039729
Kindt F, Joosten NN, Peters D, Tjallingii WF (2003) Characterisation of the feeding behaviour of western flower thrips in terms of electrical penetration graph (EPG) waveforms. J Insect Physiol 49:183–191. https://doi.org/10.1016/S0022-1910(02)00255-X
Kos SP, Klinkhamer PGL, Leiss KA (2014) Cross-resistance of chrysanthemum to western flower thrips, celery leafminer, and two-spotted spider mite. Entomol Exp et Appl 151:198–208
Krishna Kumar NK, Ullman DE, Cho JJ (1995) Resistance among lycopersicon species to Frankliniella occidentalis (Thysanoptera: Thripidae). J Econ Entomol 88:1057–1065. https://doi.org/10.1093/jee/88.4.1057
Kyamanywa S, Ampofo JKO (1988) Effect of cowpea/maize mixed cropping on the incident light at the cowpea canopy and flower thrips (Thysanoptera: Thripidae) population density. Crop Prot 7:186–189. https://doi.org/10.1016/0261-2194(88)90068-3
Lopez Jr JD, Fritz BK, Latheef MA, Lan Y, Martin DE, Hoffmann C (2008) Evaluation of toxicity of selected insecticides against thrips on cotton in laboratory bioassays. J Cotton Sci 2008 12(3):188
Miyazaki J, Stiller WN, Wilson LJ (2017) Sources of plant resistance to thrips: a potential core component in cotton IPM. Entomol Exp Appl 162:30–40. https://doi.org/10.1111/eea.12501
Morse JG, Hoddle MS (2006) Invasion biology of thrips. Annu Rev Entomol 51:67–89. https://doi.org/10.1146/annurev.ento.51.110104.151044
Mound LA (2005) Thysanoptera: diversity and interactions. Annu Rev Entomol 50:247–269. https://doi.org/10.1146/annurev.ento.49.061802.123318
Reitz Stuart R (2009) Biology and ecology of the western flower thrips (Thysanoptera: Thripidae): the making of a pest. Fla Entomol 92:7–13. https://doi.org/10.1653/024.092.0102
Rogge SA, Meyhöfer R (in press) The role of plant physiology and cultivar of chrysanthemum in the resistance against Western flower thrips. Entomol Exp Appl
Scott-Brown AS, Gregory T, Farrell IW, Stevenson PC (2016) Leaf trichomes and foliar chemistry mediate defence against glasshouse thrips; Heliothrips haemorrhoidalis (Bouché) in Rhododendron simsii. Funct Plant Biol 43:1170. https://doi.org/10.1071/FP16045
Terry IL (1997) Host selection, communication and reproductive behaviour. In: Lewis T (ed) Thrips as crop pests. CAB Internat, Wallingford
Teulon DAJ, Penman DR (1991) Effects of temperature and diet on oviposition rate and development time of the New Zealand flower thrips, thrips obscuratus. Entomol Exp et Appl 60:143–155. https://doi.org/10.1111/j.1570-7458.1991.tb01533.x
Van Dijken FR (1992) Measurements of host-plant resistance in Chrysanthemum to Frankliniella occidentalis. In: Menken SBJ, Visser JH, Harrewijn P (eds) Proceedings of the international symposium on insect-plant relationships. Springer, Netherlands, Dordrecht, pp 258–260
Van Haperen P, Voorrips RE, van Loon JJA, Vosman B (2019) The effect of plant development on thrips resistance in Capsicum. Arthropod-Plant Interact 13:11–18. https://doi.org/10.1007/s11829-018-9645-6
Visschers IGS, Peters JL, van de Vondervoort JAH, Hoogveld RHM, van Dam NM (2019) Thrips resistance screening is coming of age: leaf position and ontogeny are important determinants of leaf-based resistance in pepper. Front Plant Sci 10:510. https://doi.org/10.3389/fpls.2019.00510
Wickramasinghe KS, Nugaliyadde L, Samarajeewa PK, Rajapakse RT, Ahangama D (2007) Effect of leaf trichomes to resistance in rice. Annual Symposium of Department of Agriculture, 9: 189–199
Acknowledgements
We thank Christina Paul for her help in the greenhouse and climate chamber work. Chrysanthemum plants were kindly provided by Brandkamp company (Germany).
Funding
Open Access funding enabled and organized by Projekt DEAL. The project was supported by funds of the Federal Ministry of Food and Agriculture (BMEL) based on a decision of the Parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE) under the innovation support program (ptble).
Author information
Authors and Affiliations
Contributions
S.A.R. was involved in methodology, validation, formal analysis, data curation, investigation, visualization, and writing—original draft; R.M. and S.A.R. were involved in conceptualization, writing—review and editing; R.M was involved in resources, supervision, project administration, and funding acquisition. All authors have read and agree to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest and confirm that there are no disputes over the ownership of the data resented and all contributions have been attributed appropriately.
Data Availability
Please find here the data from the submitted experiment: Sina Alexandra Rogge, Rainer Meyhöfer (2020). Dataset: Leaf age is important for assessment of resistance in chrysanthemum against Frankliniella occidentalis. https://doi.org/10.25835/0003286
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rogge, S.A., Meyhöfer, R. Leaf age is important for assessment of resistance in chrysanthemum against Frankliniella occidentalis. J Plant Dis Prot 128, 511–516 (2021). https://doi.org/10.1007/s41348-020-00402-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-020-00402-1