Abstract
Sclerotinia stem rot, caused by Sclerotinia sclerotiorum (Lib.) de Bary, is a major disease in soybean in many parts of the world. Sustainable control measures to combat this pathogen can be better achieved by combining different available tools. One element to control fungal diseases could be changing biological activities by adding organic matter inputs, such as biochar and compost, to the soil. Other players are arbuscular mycorrhizal fungi (AMF); bioprotective effects have already been documented for them. In the present study, we assessed the effect of organic matter inputs, such as compost alone at the application rate of 20% of the total substrate (v/v) and/or green waste biochar at the application rate of 3% (v/v) in combination with or without arbuscular mycorrhizal fungi, against Sclerotinia sclerotiorum and their effect on plant growth characteristics in soybean. Substrates including compost resulted in a lower disease severity in both, plants inoculated and non-inoculated with AMF. The AMF root colonization was highest in plants grown in the control treatment and green waste biochar substrate inoculated with Sclerotinia sclerotiorum; the lowest colonization was found in plants grown in substrates containing compost. Soil substrates, especially compost, affected shoot dry matter production in soybean plants inoculated with Sclerotinia sclerotiorum and in non-inoculated plants; compost alone was superior in treatments with and without AMF. Root morphological traits were more strongly influenced by AMF than by the substrate. Our findings suggest that compost has a positive effect in terms of soybean growth and diseases suppression, which is more pronounced than that of biochar and AMF.
Similar content being viewed by others
Introduction
Soybean (Glycine max (L.) Merr.), one of the major crops providing protein and oil, can be affected by a number of plant diseases, which are causing serious yield losses worldwide. For example, in the USA annually 11% of the total soybean production get lost through soybean pathogens (Grau and Hartmann 2015). In Europe, Sclerotinia stem rot, caused by the ascomycetous fungus Sclerotinia sclerotiorum (Lib.) de Bary, is considered to be the most important soybean disease (Rüdelsheim and Smets 2012). The development of this pathogen is favored by the broad host range, the long-term viability of sclerotia in soils, even in the absence of a host, and its ability to reproduce vegetatively or generatively, depending on the host plant and environmental conditions (Boland and Hall 1994). Sclerotia, the resting structures of the fungus, arise through aggregation of vegetative hyphae and are characterized by a multicellular, tuberoid structure enclosed by a melanized rind layer (Li et al. 2018). They function as nutrient source during myceliogenic or carpogenic germination (Li and Rollins 2009). During myceliogenic germination, hyphal strands break out of the sclerotia and subsequent basal infections of roots, crowns and other parts of plants that touch the ground occur. So far, this type of infection is mainly attributed to a stimulation by root exudates of specific host plants and to small sclerotia types, such as formed by Sclerotinia minor, the fungus that causes Sclerotinia blight (Purdy 1979). However, soybeans are primarily infected via carpogenic germination (Grau et al. 1982; Cline and Jacobsen 1983). In this event, sclerotia, preconditioned in a cool and moist environment and located at the upper 2–3 cm soil layer, are functional in forming apothecia and already a single mature apothecium is able to release millions of ascospores which are spread with the wind for several kilometers and, if landing on susceptible host plants, germinate and infect the plants (Abawi and Grogan 1975; Grau and Hartman 2015). Requirements for a successful ascospore germination and the infection of the host plant are temperatures in the range from 10–30 °C and the presence of free water (Abawi and Grogan 1975). Furthermore, exogenous nutrient sources, such as dead flower parts, pollen grains, wounds or senescent tissues, are needed to enable ascospores to penetrate the host plant (Abawi and Grogan 1975, 1979; Stelfox et al. 1978). Although plants exhibit a range of multifaceted defense mechanisms (recently reviewed by Wang et al. 2019), these are not sufficient to reduce the infection level reliably under the damage threshold.
The control of Sclerotinia sclerotiorum is challenging. Environment-friendly plant protection relies on combinations of various approaches, such as preventive, physical and biological measures, to fight against plant pathogens. One element to control fungal diseases could be changing biological activities by adding organic matter inputs, such as biochar and compost, to the soil. Compost gained increasing interest for nearly 100 years (Diaz and de Bertoldi 2007). Meanwhile, as comprehensively reviewed (Martínez-Blanco et al. 2013; Mehta et al. 2014), multiple positive effects were attributed to compost, such as improvement of nutrient supply, carbon sequestration, soil resilience to erosion, soil moisture household, soil aggregation, biodiversity, crop yield and not least pest and disease suppression. Only in the recent years, biochar, a carbon-rich pyrolyzed biomass, has risen much attention. Biochar, i.e., charcoal produced from plant matter, is also considered to improve physical, chemical and biological soil properties. For example, biochar can improve the available water capacity (Abel et al. 2013; Liang et al. 2014) and has a positive effect on the saturated hydraulic conductivity of the topsoil (Asai et al. 2009) The addition of biochar to the soil results in higher nutrient retention and nutrient availability due to increased surface area, higher exchange capacity and direct nutrient addition (Glaser et al. 2002). Nutrient availability can be improved by soluble nutrients contained in the biochar (Sohi et al. 2010; Major et al. 2010), but also by the mineralization of organically bound nutrients (Lehmann and Joseph 2009). The resulting effect of biochar on plant growth and yield ranges from positive (e.g., Glaser et al. 2002; Lehmann et al. 2006) to negative (e.g., Van Zwieten et al. 2010; Crane-Droesch et al. 2013; Borchard et al. 2014). Biochar could provide benefits in terms of plant health too. The addition of biochars added at up to 3% has been shown to reduce several foliar pathogens (Elad et al. 2010; Harel et al. 2012) and root pathogens (Matsubara et al. 2002; Bonanomi et al. 2007; Elmer and Pignatello 2011; Akhter et al. 2015 and 2016), with the mechanism of action likely due to an induced systemic response in the plant (Harel et al. 2012). The suppressive effect of compost against plant pathogens has been shown for a wide range of fungal diseases (Coventry et al. 2005; Noble and Coventry 2005; Termorshuizen et al. 2006; Morauf and Steinkellner 2015). Good efficiencies were already achieved with a 20% compost rate (Morauf and Steinkellner 2015). Beneficial effects were also evident when combining compost and biochar. In this regard, Schulz and Glaser (2012) reported on an improved plant growth and Akhter et al. (2015) on a reduction in Fusarium oxysporum f.sp. lycopersici disease severity in tomato.
Other players, which could alter biological activities in soil and subsequently in the plant, are arbuscular mycorrhizal fungi (AMF). The majority of crop plants form mutualistic symbioses with AMF, which enhance the growth and bioprotection of numerous plant species. Abundant data illustrate the bioprotective effect of AMF against soil-borne and foliar fungal plant pathogens (Singh et al. 2000; Azcon- Aguilar et al. 2002; Whipps 2004; Xavier and Boyetchko 2004; Fritz et al. 2006; St-Arnaud and Vujanovic 2007; Hage-Ahmed et al. 2013).
Compost, biochar and AMF as possible soil amendments function individually and together in terms of plant response. To our knowledge, the combined bioprotective effect of compost, biochar and AMF against Sclerotinia sclerotiorum has not been investigated yet. Compatible combinations of soil amendments might be efficient tools in order to alleviate plant stresses (Ohsowski et al. 2018) and to establish more environmentally friendly and sustainable plant diseases management strategies. We addressed the question of whether the addition of organic matter inputs, such as compost and biochar, in combination with AMF reduces ascosporic infection in soybean and thus promotes plant health. The main objectives of this study were to assess the effect of biochar and compost in combination with or without AMF on Sclerotinia sclerotiorum disease suppression, growth of soybean and on changes in root morphological traits under a controlled greenhouse environment.
Materials and methods
Sclerotinia sclerotiorum inoculum production
Sclerotinia sclerotiorum was isolated from infected host plants (carrots, province Lower Austria, Austria) by surface sterilization of sclerotia with 50% household bleach (‘Dan Klorix,’ 2.8% NaOCl) for 4 min, followed by treatment with 70% ethanol for 4 min and subsequent washing with autoclaved distilled water three times for 2 min. The sclerotia were then bisected, placed on PDA (potato dextrose agar, Carl Roth GmbH + Co. KG, Karlsruhe, Germany) and incubated in dark at 24 °C. Sclerotia derived from this culture were stored in a refrigerator until further use. Inoculum production was done according to a slightly modified method of Clarkson et al. (2003). For that purpose, 25 g of wheat grains and 50 g distilled water were put in 500-ml flasks and autoclaved for 20 min at 121 °C. Afterward, this medium was inoculated with two agar plugs (approx. 8 mm2) from the edge of a one-week-old Sclerotinia sclerotiorum colony and incubated at 24 °C under dark conditions. Sclerotia emerged after 3–4 weeks of incubation and were then used for the production of apothecia.
To induce carpogenic germination, the sclerotia were collected from the flasks and carefully packed in cheesecloth (approximately 50–100 g/bag). The cheesecloth bags were hung in a plastic container filled with tap water and stored in a cold room at 4 °C under dark conditions for 8 weeks. During this conditioning process, air circulation was provided by an aquarium pump, and the water was replaced every week. Afterward, the sclerotia were washed with distilled water, dried at room temperature under a fume hood and stored in a refrigerator at 4 °C until further use.
For germination of sclerotia, transparent tissue culture boxes (Magenta vessel GA-7, Sigma-Aldrich, Vienna, Austria) filled with vermiculite up to a quarter were autoclaved at 121 °C for 20 min and afterward, 30 conditioned sclerotia, which were about the same diameter (approx. 5–8 mm), were evenly placed close together on the top of the vermiculite surface. Distilled water was added up to saturation of the vermiculite, and the boxes were sealed with a lid to avoid water evaporation. The boxes were kept in a growth chamber with vertical light supply at a day/night photoperiod of 14/10 h at 15 °C until apothecia matured. After 6–8 weeks, the apothecia were matured (Fig. 1a) and ready for harvesting ascospores. For that purpose, matured apothecia were cut very carefully with small cosmetic scissors under a fume hood. Five apothecia/boxes were sampled for three times (in total 15 apothecia/box within a period of six days. Five apothecia each were put in sterile 1.5-ml tubes filled with distilled water (1 ml per tube), closed, vortexed for 10 s and then stored at −80 °C. To avoid reducing ascospore germination ability and viability, this ascospore suspension (Fig. 1b) was used within 1 month after its preparation. For inoculation of the plants, the ascospore suspension was prepared by mixing the solution of tubes containing ascospores of three different harvests in a 50-ml falcon tube and adjusted to a final concentration of 106 ascospores ml−1.
Arbuscular mycorrhizal fungi inoculum production
For the AMF plant inoculation, a commercially available inoculum of Funneliformis mosseae (BEG 12, Biorize/Agrauxine, Quimper, France) multiplied in the host plant Sorghum sudanense cv. Piper was used. The start inoculum had been stored at 4 °C. Pots with a diameter of 14 cm were filled with an autoclaved substrate consisting of sand (Quarzsand 0.5–2.0 mm, Quarzwerke Österreich GmbH, Melk, Austria) and expanded clay (Liapor fit 1–4 mm, Lias Österreich GmbH, Fehring, Austria) (1:1 ratio v/v). Thereafter, the start inoculum (15 ml) was added and 5–7 sorghum seeds were sown and then covered with the substrate. The pots were cultivated in the greenhouse and watered with tap water according to plant demands. For preparing the inoculum, the plants were stopped watering and the sorghum shoots were cut off. Finally, the AMF inoculum was prepared by cutting the fresh roots of mycorrhizal Sorghum sudanense in small pieces of approx. 2–4 mm and mixing the root pieces with the colonized substrate (spores, hyphae, sand and expanded clay).
Organic soil amendments
The compost (Comp) was obtained from the municipal compost works in Klosterneuburg (Lower Austria). The compost was categorized as quality A + according to the Austrian compost regulation (BGBl. II Nr. 929/2001). The green waste biochar (GWB) was made from garden waste residues at pyrolysis temperature of 500 °C. The physiochemical parameters of compost and biochar are summarized in Table 1.
Greenhouse experiment
The experimental setup included four different substrates, those were control, green waste biochar (GWB), compost (Comp) and GWB + Comp, inoculated with Sclerotinia sclerotiorum (+ Scl) or without (− Scl) in the presence or absence of arbuscular mycorrhizal fungi (AMF). For that purpose, soybean seeds (Glycine max L. cv. Gallec, kindly provided by Raiffeisen Ware Austria AG) were surface-sterilized by soaking in 50% household bleach (2.8% NaOCl) for 5 min and subsequently washed three times with autoclaved distilled water for 2 min. Three soybean seeds were sown in each of a 1 L pot filled with four different substrates: I) an autoclaved (20 min at 121 °C) mixture of sand (Quarzsand 0.5–2.0 mm, Quarzwerke Österreich GmbH, Melk, Austria), soil (Aussaaterde, Gramoflor GmbH & Co. KG, Vechta, Germany; pH 5.2–6.2; 50–300 mg/l N; 80–300 mg/l P2O5; 80–400 mg/l K2O) and expanded clay (Liapor fit 1–4 mm, Lias Österreich GmbH, Fehring, Austria) (1:1:1, v/v/v) without additional amendments (‘Control’), the same mixture II) in combination with compost at 20% (v/v) (‘Comp’), III) in combination with green waste biochar at 3% (v/v) (‘GWB’) and IV) in combination with compost at 20% (v/v) and green waste biochar at 3% (v/v) (‘GWB + Comp’).
For AMF inoculation, the above-mentioned inoculum was mixed with sand (1:1 ratio v/v) and 30 ml of the mixture was added subsequently to the planting hole during the potting procedure. One week after sowing, the soybean plants were thinned from 3 to 1 even uniform plant per pot. After 3 weeks, the axillary bud tissue at the third node of the soybean plants was slightly scratched by a scalpel blade and inoculated by pipetting 10 µl of ascospore suspension (+ Scl) or water (−Scl) in the center of the scratched tissue. Each plant was carefully covered with a transparent plastic bag for 48 h to prevent a spread of ascospores to other plants and to maintain the moisture needed to germinate them. The plants were grown for 8 weeks in a greenhouse with a photoperiod of 14 h. The temperature ranged from 18 °C at night and 24 °C during the day, and the relative humidity was approximately 60%. Additional light was provided when outside photosynthetically active radiation (PAR) was below 367.43 µmol m−2 s−1 during the day. The plants were irrigated regularly according to their moisture requirement with a nutrient solution (Steinkellner et al. 2005). The experimental design was a completely randomized design, and each treatment consisted of six pots. The entire experiment was repeated three times (in total 18 pots/treatment).
Disease rating
The analysis of disease severity was performed in weeks 1–4 after inoculation. Sclerotinia disease severity was determined visually according to the following scale: (0) no symptoms, (1) first signs of symptoms through changing the color on the buds, (2) lesions on lateral branches only, (3) lesion on the main stem but little or only through changing the color, (4) lesions on main stem resulting in plant death, (5) dead plants. The formula given by Grau and Radke (1984) was used to calculate the disease rating as follows:
Diseases severity index (DSI) = Ʃ (severity class x number of plants per class) × 100/(total number of plants x total number of classes with symptoms).
Agronomic and physiological parameters
Eight weeks after sowing, the plants were removed from the substrate, gently rinsed under running tap water, taking care to preserve the root system, and dried between paper towels. The roots and shoots were separated, and their fresh weight, number of pods, shoot length and number of leaves were recorded. Thereafter, the roots were divided into two similar parts of 4 cm from taproots and maintained in 50-ml falcon tubes filled with 30% ethanol in 4 °C until further use. One part was used to determine the degree of mycorrhization. For that purpose, the roots were kept overnight and then clarified by boiling for 8 min in 10% KOH, rinsed three times with tap water and stained by boiling for 4 min in a 5% ink (Sheaffer black)–vinegar solution according to the method of Vierheilig et al. (1998). Then, the AMF root colonization was determined microscopically according to the counting procedure of Giovannetti and Mosse (1980). After that, both root parts were digitized by a modified flatbed scanner (Epson Perfection V700 Photo) and root morphological traits [root length (cm), root volume (cm3), root surface area (cm2) and average diameter (mm) of roots] were measured by means of the software WinRHIZO® Regular (Regent Instruments Inc. Ltd., Quebec, Canada). After imaging, root and shoot dry weight was calculated after drying the samples at 105 °C for 24 h.
Statistical analysis
The data analysis was performed using IBM SPSS Statistics V21.0.0 software. Analyses of variance (ANOVA) were carried out after a variance check by the Levene’s test. The data of plant growth and root morphological parameters were analyzed by two-way ANOVA with the main factors ‘Substrate’ (Control (I), GWB (II), Comp (III) and GWB + Comp (IV)) and ‘Scl’ (−Scl and + Scl). In addition, in the presence of significant interaction effects, a simple effect analysis test was performed. Moreover, AMF root colonization rate and disease severity index assessment data were analyzed by one-way ANOVA. Mean values were compared using Tukey’s post hoc test at the significance level of p ≤ 5%.
Results
Disease severity index
The assessment of Scl disease severity on soybean plants was performed in week 1–4 after inoculation (Table 2). In contrast to plants grown in the control and GWB substrate, plants grown in substrates including compost did not show any diseases symptoms 7 days after inoculation (DAI). The diseases severity rose up clearly between 7 and 14 DAI. ANOVA on mean disease severity (%) indicated a significant difference at 14 DAI (F (7, 16) = 34.85, p < 0.001). The disease severity was highest for plants grown in the control substrate and lowest in substrates including compost. The picture was similar for both −AMF- and + AMF-treated variants. This trend continued in the following weeks [21 DAI (F (7, 16) = 22.43, p < 0.001) and 28 DAI (F (7, 16) = 37.88, p < 0.001)]. 28 DAI, the disease severity in –AMF plants grown in the control substrate was 1.5 times higher than in plants grown in compost and 2.5 times higher than in plants grown in compost and GWB. Compost alone showed a tendency to lower diseases severity in + AMF plants compared to –AMF plants.
AMF root colonization rate
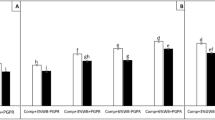
AMF root colonization for soybean plants grown in different soil substrates (F (7, 136) = 47.72, p < 0.001) is shown in Fig. 2. The mycorrhization was highest in plants grown in the control and GWB substrate inoculated with Sclerotinia sclerotiorum followed by non-inoculated plants grown on these substrates. The lowest colonization was found in plants grown in compost (22.61% in ‘Comp−Scl’ and 21.39% in ‘Comp + Scl’). There was only a tendency toward a higher AMF root colonization by combing compost and GWB.
Plant growth parameters
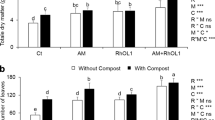
The soil substrates affected plant growth and biomass production in soybean plants (Tables 3 and 4). For all factors, except factor Scl in + AMF, and their interactions, a significant difference in shoot dry mass according to Tukey’s post hoc test (p < 0.05) has been observed. In the absence of Sclerotinia sclerotiorum (−Scl), the treatments including compost significantly increased the shoot dry mass compared to the control or GWB, where compost alone performed better than GWB + compost. In the presence of the pathogen (+ Scl), all treatments performed better than the control. Compost alone was superior both in treatments with and without AMF. The results of root dry mass showed significant differences between variants with or without Scl and between the different substrates in both + AMF and −AMF variants (no significant interactions). In the absence as well as in the presence of AMF, the amendment of compost with or without GWB increased the root dry mass compared to the control and GWB treatment. The effect of the different Scl inoculations and substrates on the number of leaves was not that pronounced. However, the sole compost treatment showed an increased number of leaves. Compost with and without GWB as soil amendment significantly improved the pod set in –AMF soybean plants. The picture looked different in the treatments + AMF; there were significant interactions between the substrates and pathogen.
Root morphological traits
The two-way ANOVA revealed a significant effect of the substrate on root length and root surface area in the absence of AMF. Here, compost alone lowered these root traits compared to the control. Furthermore, a significant effect of Scl and significant interactions between the main factors in root average diameter in the absence of AMF were found. In treatments + AMF neither the pathogen nor the substrate affected the root morphological traits significantly (Tables 5 and 6).
Discussion
In this study, we analyzed for the first time the simultaneous use of compost, biochar and AMF on Sclerotinia disease caused by foliar infection of soybeans. Our data show that the amendment with both, compost and biochar, can cause a delay in symptom development and is able to reduce the susceptibility to Sclerotinia sclerotiorum in soybean. This beneficial impact was even more pronounced, when the plants were grown in compost, and the combination of both even showed a tendency to improve the performance of compost alone. The microbial communities present in compost are the most frequently reported drivers in diseases suppression (Mehta et al. 2014) which has been documented for numerous soil-borne pathogens (Termorshuizen et al. 2006). Of course, the amendment with compost and biochar significantly altered the environment for microbes in our soil substrate, in particular in consideration of the autoclaved control substrate. However, as we inoculated above ground plant parts with the pathogen, a direct impact on Sclerotinia sclerotiorum based on the main modes of action of beneficial microbes, such as competition, antibiosis, hyperparasitism or ineffective pathogen proliferation (Termorshuizen et al. 2006; Mehta et al. 2014), is unlikely. A recent study (Aggeli et al. 2020) found clear evidence for an early triggering of salicylic acid (SA), jasmonate- or ethylene-dependent plant defenses for Sclerotina sclerotiorum in lettuce subjected to plant biocontrol strains isolated from a diseases suppressive compost. Therefore, although we did not address the microbial communities in detail, this mechanism might to some extend explain our results.
In fact, the addition of compost to the soil substrate has led to a higher nutrient input compared to biochar and thus might have improved more the plant’s nutritional status. It is generally thought that a high nutrient availability in the soil, achieved by nitrogen-rich manures and fertilizers, alters the plant growth and accelerates the canopy closure and thereby benefits the pathogen’s development (Wallace et al. 1990; Schmidt et al. 2001). However, in our experimental setup diseases promoting environmental conditions due to canopy closure play a minor role. Furthermore, specific data regarding host nutrition in Sclerotinia symptom development are lacking or conflicting. Couper (2001) reported on a reduction of Sclerotinia in carrot upon low nitrogen supply, while the opposite trend was shown for oil seed rape (Söchting and Verreet 2004). Recent data pointed out the importance of Zn (Helferstein et al. 2015). In their study, a significant reduction in Sclerotinia sclerotiorum has been reported for soybeans grown with a normal or increased Zn-fertilization compared to plants grown without or reduced Zn-status. In our study, the compost used was characterized by a high Zn-content. This might have contributed to the lower disease development in compost-treated plots, although we did not directly focus on specific nutrients.
In a study on soybean charcoal root rot, caused by Macrophomina phaseolina (Spagnoletti et al. 2020), it has been concluded that AMF might limit soybean diseases, even when the disease development is promoted by an increased nitrogen fertilization. This is in contrast to our data, as we could neither find an increased risk of diseases upon an improved nutrient status nor a sustainable bioprotective AMF effect, since the significant disease reduction in the control treatment by + AMF two weeks after inoculation was already compensated four weeks later. For all other substrates, we found no or only a tendency for a lower diseases severity index in + AMF plants compared to –AMF plants.
Compared to the pathogen effect, our data showed a reverse soil substrate effect regarding AMF root colonization. Here, compost decreased mycorrhization, while GWB kept it stable on the level of the control substrate. This is contrasting to other studies on compost or biochar and shows the complexity of plant–microbe interactions. Most frequently, adding biochar to the soil substrate is attributed to an enhanced AMF activity (Ishii and Kadoy 1994; Solaiman et al. 2010; Rillig et al. 2010). However, other studies found this effect only under specific conditions. These are, for instance, the use of grounded biochar as shown in marigold (Ezawa et al. 2002), specific growth stages and fertilization rates in soybean (Saito 1990) or a certain threshold of biochar application (Warnock et al. 2010). Nevertheless, our study revealed that the 3% addition of biochar to the soil substrates could not affect the mycorrhization rate. This underlines the observations that biochar can show its AMF-stimulating potential mainly at higher rates (Solaiman et al. 2010; Rillig et al. 2010). Furthermore, there is evidence that the AMF-promoting effect of biochar can only be utilized at high N levels in the soil substrate (Le Croy et al. 2013). This is in turn contrary to our data, as we found a lower AMF root colonization in the GWB + comp treatment compared to GWB alone.
On the other hand, we found a reduction of mycorrhization in the treatments containing compost. Our data on AMF root colonization are contrary to a recent study on soybean (Yang et al. 2018) which reported no effect of low compost levels but increasing mycorrhization with increasing compost levels. Therefore, our findings are in line with a comparatively small number of studies, as according to Cavagnaro (2015) only 8% of studies report on such detrimental effects of compost on AMF. On the one hand, this has been attributed to the larger supply of P and N through compost (Baon et al. 1992). On the other hand, under low P availability an increase in AMF root colonization is well described (Smith et al. 2011; Ryan et al. 2016; Wen et al. 2020). Unfortunately, the specific P content in our compost was not available to us. However, in general, P contents in commercial composts from biogenous waste are in the range of 0.09–0.22% in dry matter (Baumgarten et al. 2010), and thus, a distinct phosphorus effect in our study seems to be unlikely, even more so as all plants were watered regularly with the same low dosage of nutrient solution. With 2.2%, the N content in the compost we used was in the upper range for composts (0.6–2.3% in dry matter, according to Baumgarten et al. 2010).
Another interesting point in our experiment was the increasing AMF colonization after shoot inoculation with Sclerotinia sclerotiorum only in the control and GWB treatment. Both treatments were also characterized by a higher diseases severity compared to the compost treatments. This indicates an alteration in the host plant physiology upon pathogen infection at lower nutrient supply. Here, we can only speculate on possible changes in nutrient availability and/or the release of diverse inorganic and organic compounds that the plants exude via the roots into the rhizosphere in response to biotic and abiotic stresses (Bais et al. 2006; Toljander et al. 2007) which in turn promote root colonization by AMF in our study.
In our study, the shoot growth of soybean was clearly improved in soil substrates amended with compost. In fact, the quality and characteristics of organic amendments such as biochar and compost clearly depend on feedstock and production conditions used. For example, Al-Wabel et al. (2013) reported that biochar produced at higher pyrolysis temperature has a higher carbon and nutrient content than biochar produced at lower pyrolysis temperatures. Furthermore, the feedstock can modify the soil organic matter and nutrient status (Mukherjee and Zimmerman 2013; Kloss et al. 2014). In our study, the plants were subjected to a regular, low-dose fertilization. The amendment of compost is known to enhance plant nutrient availability (Epstein 1997; Kawasaki et a1. 2008) and thus exhibited a growth-promoting effect. A short-term immobilization of N in substrates, as known for higher C/N ratios (Scherer et al. 1996), is unlikely in our study. The compost we used was characterized by a relatively low C/N ratio and thus makes the sufficient N-availability plausible. Our data suggest that under a lower nutrient availability, Sclerotinia sclerotiorum boosts the mycorrhization of soybean roots which in turn compensates the negative impact on plant growth, whereas in general, the plant growth effects were more pronounced in the shoot compared to the root.
In a study on sorghum, Le Croy et al. (2013) found that biochar and AMF did not alter the shoot and root biomass under low N levels. Furthermore, the application of biochar at a rate of 2.6% and/or AMF did not affect the plant growth, but at higher N rates the combination of biochar and AMF lowered the growth-promoting N-effect. This is similar to our data on shoot and root growth of soybean under lower nutrient supply (control and GWB). However, it could not be confirmed under higher nutrient supply as given by the use of compost in our study. Here, we found only a slight trend toward a growth reduction due to AMF and biochar. Another factor, which might have contributed to our outcome, is the soil P availability. The addition of biochar might have led to a decline in P availability and thus the plant growth parameters (Clark and Zeto 1996; Warnock et al. 2010), although, contrary to Warnock et al. (2010) in our study, the AMF root colonization was not reduced in GWB compared to the control treatment.
Similarly, to the shoot dry weight, the number of pods/plant was altered by the addition of compost to the soil substrate in –AMF treatments. Besides of nutrient effects, this could also be attributed to the microbial community in compost. As mentioned above, the beneficial microbes in compost might be a trigger for a plant hormone-regulated responses (Aggeli et al. 2020), which may not only affect plant defense but also may be involved in the development of plant parts. This has at least been reported for Arabidopsis NahG transgenic lines and sid2 mutants (Abreu and Munné-Bosch 2009), where decreasing SA concentrations improved the number of seeds and pods. However, our study was characterized by significant substrate x Scl effects in + AMF treatments. This indicates that AMF intervenes differently here, but the exact role of the AMF remains unclear.
Observations in tomato showed that the substrate and a pathogen infection can cause a decrease in root morphological traits (Morauf and Steinkellner 2015). Our data suggest that AMF can compensate the negative effects of the pathogen. In our study, root length and root surface area were lowered in –AMF treatments, but in + AMF treatments morphological traits were not affected neither by the soil substrate nor by the pathogen. However, this is in contrast to Wang et al. (2011) who reported that AMF significantly decreased these variables in two soybean genotypes under low P and N-levels, but even at higher nutrient levels they found only a neutral response in terms of these root traits. Similar data are also available for other plants, e.g., for Plantago lanceolata (Forbes et al. 1996) or tomato (Trotta et al. 1996). Generally, such modifications in root traits can allow for an improved P acquisition (Lambers et al. 2006; Shen et al. 2011), but as shown by Wen et al. (2020) this response is highly dependent on the plant genotype. In particular, thick rooted genotypes with large amounts of rhizosheath carboxylates are considered to respond less markedly to the P availability in soil. In our study, under conditions providing a low-dose fertilization, AMF were able to increase root length, root surface area and root volume, in particular in substrates amended with compost and/or biochar. Our data are in agreement with some other AMF–plant interactions. For instance, enhanced lateral root development upon AMF inoculation has been shown for mycorrhizal rice plants (Oryza sativa) (Gutjahr et al. 2009), peanut (Archis hypogaea) or pigeon pea (Cajanus cajan) (Yano et al. 1996). The latter assumed that nutrient-poor conditions might foster mycorrhizal roots and subsequently trigger local modifications in root traits. However, our data reveal a trend toward a decrease in the mentioned root traits in soil substrates including compost and/or biochar. There is therefore much to suggest that AMF can compensate a potential impact of possibly growth-inhibiting microbes introduced via these amendments. However, our findings suggest that compost has a positive effect in terms of soybean growth and diseases suppression, which is more pronounced than that of biochar and AMF.
References
Abawi GS, Grogan RG (1975) Source of primary inoculum and effects of temperature and moisture on infection of beans by Whetzelinia sclerotiorum. Phytopathology 65:300–309. https://doi.org/10.1094/Phyto-65-300
Abawi GS, Grogan RG (1979) Epidemiology of diseases caused by Sclerotinia species. Phytopathology 69:899–904. https://doi.org/10.1094/Phyto-69-899
Abel S, Peters A, Trink S, Schonsky H, Facklam M, Wessolek G (2013) Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 202:183–191. https://doi.org/10.1016/j.geoderma.2013.03.003
Abreu ME, Munne-Bosch S (2009) Salicylic acid deficiency in NahG transgenic lines and sid2 mutants increases seed yield in the annual plant Arabidopsis thaliana. J Exp Bot 60:1261–1271. https://doi.org/10.1093/jxb/ern363
Aggeli F, Ziogas I, Gkizi D, Fragkogeorgi GA, Tjamos SE (2020) Novel biocontrol agents against Rhizoctonia solani and Sclerotinia sclerotiorum in lettuce. Biocontrol 65:763–773. https://doi.org/10.1007/s10526-020-10043-w
Akhter A, Hage-Ahmed K, Soja G, Steinkellner S (2015) Compost and biochar alter mycorrhization, tomato root exudation, and development of Fusarium oxysporum f. sp lycopersici. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00529
Akhter A, Hage-Ahmed K, Soja G, Steinkellner S (2016) Potential of Fusarium wilt- inducing chlamydospores, in vitro behaviour in root exudates and physiology of tomato in biochar and compost amended soil. Plant Soil 406:425–440
Al-Wabel MI, Al-Omran A, El-Naggar AH, Nadeem M, Usman ARA (2013) Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour Technol 131:374–379. https://doi.org/10.1016/j.biortech.2012.12.165
Asai H, Samson BK, Stephan HM, Songyikhangsuthor K, Homma K, Kiyono Y, Inoue Y, Shiraiwa T, Horie T (2009) Biochar amendment techniques for upland rice production in Northern Laos 1. Soil physical properties, leaf SPAD and grain yield. Field Crops Res 111(1–2):81–84. https://doi.org/10.1016/j.fcr.2008.10.008
Azcón-Aguilar C, Jaizme-Vega MC, Calvet C (2002) The contribution of arbuscular mycorrhizal fungi to the control of soil-borne plant pathogens. In: Gianinazzi S, Schüepp H, Barea JM, Haselwandter K (eds) Mycorrhizal technology in agriculture: from genes to bioproducts. Birkhäuser Verlag, Boston, pp 187–198
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Ann Rev Plant Biol 57:233–266. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Baon JB, Smith SE, Alston AM, Wheeler RD (1992) Phosphorus efficiency of 3 cereals as related to indigenous mycorrhizal infection. Aust J Agr Res 43:479–491. https://doi.org/10.1071/ar9920479
Baumgarten A, Müller H, Spatny N, Feichtinger F, Tulnik R, Strebl H, Humer J (2010) Swoboda M (2010) Richtlinie für die Anwendung von Kompost aus biogenen Abfällen in der Landwirtschaft. Fachbeirat für Bodenfruchtbarkeit und Bodenschutz beim Bundesministerium für Land- und Forstwirtschaft, Wien
Boland GJ, Hall R (1994) Index of plant hosts of Sclerotinia sclerotiorum. Can J Plant Pathol 16:93–108
Bonanomi G, Antignani V, Pane C, Scala E (2007) Suppression of soilborne fungal diseases with organic amendments. J Plant Pathol 89:311–324
Borchard N, Siemens J, Ladd B, Moller A, Amelung W (2014) Application of biochars to sandy and silty soil failed to increase maize yield under common agricultural practice. Soil Tillage Res 144:184–194. https://doi.org/10.1016/j.still.2014.07.016
Cavagnaro TR (2015) Biologically regulated nutrient supply systems: compost and arbuscular mycorrhizas-a review. Adv Agron 129:293–321. https://doi.org/10.1016/bs.agron.2014.09.005
Clark RB, Zeto SK (1996) Growth and root colonization of mycorrhizal maize grown on acid and alkaline soil. Soil Biol Biochem 28:1505–1511. https://doi.org/10.1016/s0038-0717(96)00164-2
Clarkson JP, Staveley J, Phelps K, Young CS, Whipps JM (2003) Ascospore release and survival in Sclerotinia sclerotiorum. Mycol Res 107:213–222. https://doi.org/10.1017/s0953756203007159
Cline MN, Jacobsen BJ (1983) Methods for evaluating soybean cultivars for resistance to Sclerotinia sclerotiorum. Plant Dis 67:784–786. https://doi.org/10.1094/pd-67-784
Couper G (2001) The biology, epidemiology and control of Sclerotinia sclerotiorum on carrots in north east Scotland. Ph.D. thesis, University of Aberdeen, Aberdeen, Scotland
Coventry E, Noble R, Mead A, Whipps JM (2005) Suppression of allium white rot (Sclerotium cepivorum) in different soils using vegetable wastes. Eur J Plant Pathol 111:101–112. https://doi.org/10.1007/s10658-004-1420-0
Crane-Droesch A, Abiven S, Jeffery S, Torn MS (2013) Heterogeneous global crop yield response to biochar: a meta-regression analysis. Environ Res Lett. https://doi.org/10.1088/1748-9326/8/4/044049
Diaz LF, de Bertoldi M (2007) History of composting. In: Diaz LF, de Bertoldi M, Bidlingmaier W (eds) Compost science and technology, waste management series 8. Elsevier, Amsterdam, pp 7–24
Elad Y, David DR, Harel YM, Borenshtein M, Ben Kalifa H, Silber A, Graber ER (2010) Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent. Phytopathology 100:913–921. https://doi.org/10.1094/phyto-100-9-0913
Elmer WH, Pignatello JJ (2011) Effect of biochar amendments on mycorrhizal associations and Fusarium crown and root rot of asparagus in replant soils. Plant Dis 95:960–966. https://doi.org/10.1094/pdis-10-10-0741
Epstein E (1997) Science of composting. Technomic publishing company, Lancaster
Ezawa T, Yamamoto K, Yoshida S (2002) Enhancement of the effectiveness of indigenous arbuscular mycorrhizal fungi by inorganic soil amendments. Soil Sci Plant Nutr 48:897–900. https://doi.org/10.1080/00380768.2002.10408718
Forbes PJ, Ellison CH, Hooker JE (1996) The impact of arbuscular mycorrhizal fungi and temperature on root system development. Agron J 16:617–620
Fritz M, Jakobsen I, Lyngkjaer MF, Thordal-Christensen H, Pons-Kuhnemann J (2006) Arbuscular mycorrhiza reduces susceptibility of tomato to Alternaria solani. Mycorrhiza 16:413–419. https://doi.org/10.1007/s00572-006-0051-z
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol 84:489–500. https://doi.org/10.1111/j.1469-8137.1980.tb04556.x
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal-a review. Biol Fertil Soil 35:219–230. https://doi.org/10.1007/s00374-002-0466-4
Grau CR, Hartman GL (2015) In: Hartman GL, Rupe JC, Sikora EJ, Domier LL, Davis JA, Steffey KL (eds) Compendium of soybean diseases and pests, 5th edn. The American Phytopathological Society, St. Paul
Grau CR, Radke VL (1984) Effects of cultivars and cultural practices on Sclerotinia stem rot of soybean. Plant Dis 68:56–58. https://doi.org/10.1094/pd-69-56
Grau CR, Radke VL, Gillespie FL (1982) Resistance of soybean cultivars to Sclerotinia sclerotiorum. Plant Dis 66:506–508. https://doi.org/10.1094/pd-66-506
Gutjahr C, Casieri L, Paszkowski U (2009) Glomus intraradices induces changes in root system architecture of rice independently of common symbiosis signaling. New Phytol 182:829–837. https://doi.org/10.1111/j.1469-8137.2009.02839.x
Hage-Ahmed K, Krammer J, Steinkellner S (2013) The intercropping partner affects arbuscular mycorrhizal fungi and Fusarium oxysporum f. sp lycopersici interactions in tomato. Mycorrhiza 23:543–550. https://doi.org/10.1007/s00572-013-0495-x
Harel YM, Elad Y, Rav-David D, Borenstein M, Shulchani R, Lew B, Graber ER (2012) Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil 357:245–257. https://doi.org/10.1007/s11104-012-1129-3
Helferstein J, Pawlowski ML, Hill CB, Stewart J, Lagos-Kutz D, Bowen CR, Frossard E, Hartman GL (2015) Zinc deficiency alters soybean susceptibility to pathogens and pests. Jsoil Sci Plant Nutr 178:896–903. https://doi.org/10.1002/jpln.201500146
Ishii T, Kadoya K (1994) Effects of charcoal as a soil conditioner on citrus growth and vesicular-arbuscular mycorrhizal development. J Jap Soc Hortic Sci 63:529–535
Kawasaki S, Maie N, Kitamura S, Watanabe A (2008) Effect of organic amendment on amount and chemical characteristics of humic acids in upland field soils. Eur J Soil Sci 59:1027–1037. https://doi.org/10.1111/j.1365-2389.2008.01076.x
Kloss S, Zehetner F, Wimmer B, Buecker J, Rempt F, Soja G (2014) Biochar application to temperate soils: effects on soil fertility and crop growth under greenhouse conditions. J Soil Sci Plant Nutr 177:3–15. https://doi.org/10.1002/jpln.201200282
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713. https://doi.org/10.1093/aob/mcl114
LeCroy C, Masiello CA, Rudgers JA, Hockaday WC, Silberg JJ (2013) Nitrogen, biochar, and mycorrhizae: alteration of the symbiosis and oxidation of the char surface. Soil Biol Biochem 58:248–254. https://doi.org/10.1016/j.soilbio.2012.11.023
Lehmann J, Joseph S (2009) Biochar for environmental management: science and technology, Earthscan, London, pp 1–12
Lehmann J, Gaunt J, Rondon M (2006) Biochar sequestration in terrestrial ecosystems a review. Mitig Adapt Strat Glob Change 11:403–427
Li M, Rollins JA (2009) The development-specific protein (Ssp1) from Sclerotinia sclerotiorum is encoded by a novel gene expressed exclusively in sclerotium tissues. Mycologia 101:34–43. https://doi.org/10.3852/08-114
Li J, Zhang X, Li L, Liu J, Zhang Y, Pan H (2018) Proteomics analysis of SsNsd1-mediated compound appressoria formation in Sclerotinia sclerotiorum. Int J Mol Sci. https://doi.org/10.3390/ijms19102946
Liang F, Li GT, Lin QM, Zhao XR (2014) Crop yield and soil properties in the first 3 years after biochar application to a calcareous soil. J Integr Agric 13:525–532. https://doi.org/10.1016/s2095-3119(13)60708-x
Major J, Rondon M, Molina D, Riha SJ, Lehmann J (2010) Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 333:117–128. https://doi.org/10.1007/s11104-010-0327-0
Martinez-Blanco J, Lazcano C, Christensen TH, Munoz P, Rieradevall J, Moller J, Anton A, Boldrin A (2013) Compost benefits for agriculture evaluated by life cycle assessment. A Rev Agron Sustain Dev 33:721–732. https://doi.org/10.1007/s13593-013-0148-7
Matsubara Y, Hasegawa N, Fukui H (2002) Incidence of Fusarium root rot in asparagus seedlings infected with arbuscular mycorrhizal fungus as affected by several soil amendments. J Jap Soc Hortic Sci 71:370–374
Mehta CM, Palni U, Franke-Whittle IH, Sharma AK (2014) Compost: Its role, mechanism and impact on reducing soil-borne plant diseases. Waste Manage 34:607–622. https://doi.org/10.1016/j.wasman.2013.11.012
Morauf C, Steinkellner S (2015) Fusarium oxysporum f. sp lycopersici and compost affect tomato root morphology. Eur J Plant Pathol 143:385–398. https://doi.org/10.1007/s10658-015-0691-y
Mukherjee A, Zimmerman AR (2013) Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar-soil mixtures. Geoderma 193:122–130. https://doi.org/10.1016/j.geoderma.2012.10.002
Noble R, Coventry E (2005) Suppression of soil-borne plant diseases with composts: a review. Biocontrol Sci Technol 15:3–20. https://doi.org/10.1080/09583150400015904
Ohsowski BM, Dunfield K, Klironomos JN, Hart MM (2018) Plant response to biochar, compost, and mycorrhizal fungal amendments in post-mine sandpits. Restor Ecol 26:63–72. https://doi.org/10.1111/rec.12528
Purdy LH (1979) Sclerotinia sclerotiorum history, diseases and symptomatology, host range, geographic distribution, and impact. Phytopathology 69:875–880. https://doi.org/10.1094/Phyto-69-875
Rillig MC, Wagner M, Salem M, Antunes PM, George C, Ramke HG, Titirici MM, Antonietti M (2010) Material derived from hydrothermal carbonization: effects on plant growth and arbuscular mycorrhiza. Appl Soil Ecol 45:238–242. https://doi.org/10.1016/j.apsoil.2010.04.011
Rüdelsheim P, Smets G (2012) Baseline information on agricultural practices in the EU Soybean (Glycine max (L.) Merr.). EuropaBio AISBL, https://www.europabio.org/sites/default/files/120526_report_eu_farming_practices_soybean.pdf (retrieved 13.07.2020).
Ryan MH, Kidd DR, Sandral GA, Yang ZJ, Lambers H, Culvenor RA, Stefanski A, Nichols PGH, Haling RE, Simpson RJ (2016) High variation in the percentage of root length colonised by arbuscular mycorrhizal fungi among 139 lines representing the species subterranean clover (Trifolium subterraneum). Appl Soil Ecol 98:221–232. https://doi.org/10.1016/j.apsoil.2015.10.019
Saito M (1990) Charcoal as a micro habitat for VA mycorrhizal fungi, and its practical application. Agric Ecosyst Environ 29:341–344
Scherer H, Werner W, Neumann A (1996) N-Nachlieferung und N-Immobilisierung von Komposten mit unterschiedlichem Ausgangsmaterial, Rottegrad und C/N-Verhältnis. Agrobiol Res 49:120–129
Schmidt JP, Lamb JA, Schmitt MA, Randall GW, Orf JH, Gollany HT (2001) Soybean varietal response to liquid swine manure application. Agron J 93:358–363. https://doi.org/10.2134/agronj2001.932358x
Schulz H, Glaser B (2012) Effects of biochar compared to organic and inorganic fertilizers on soil quality and plant growth in a greenhouse experiment. J Soil Sci Plant Nutr 175:410–422. https://doi.org/10.1002/jpln.201100143
Shen JB, Yuan LX, Zhang JL, Li HG, Bai ZH, Chen XP, Zhang WF, Zhang FS (2011) Phosphorus dynamics: from soil to plant. Plant Physiol 156:997–1005. https://doi.org/10.1104/pp.111.175232
Singh A, Sharma J, Rexer KH, Varma A (2000) Plant productivity determinants beyond minerals, water and light: Piriformospora indica - a revolutionary plant growth promoting fungus. Curr Sci 79:1548–1554
Smith SE, Jakobsen I, Gronlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156:1050–1057. https://doi.org/10.1104/pp.111.174581
Söchting HP, Verreet A-J (2004) Effects of different cultivation systems (soil management, nitrogen fertilization) on the epidemics of fungal diseases in oilseed rape (Brassica napus L. var. napus). J Plant Dis Prot 111:1–29
Sohi SP, KrullLopez-Capel EE, Bol R (2010) A review of biochar and its use and function in soil. Adv Agron 105:47–82. https://doi.org/10.1016/s0065-2113(10)05002-9
Solaiman ZM, Blackwell P, Abbott LK, Storer P (2010) Direct and residual effect of biochar application on mycorrhizal root colonisation, growth and nutrition of wheat. Aust J Soil Res 48:546–554. https://doi.org/10.1071/sr10002
Spagnoletti FN, Cornero M, Chiocchio V, Lavado RS, Roberts IN (2020) Arbuscular mycorrhiza protects soybean plants against Macrophomina phaseolina even under nitrogen fertilization. Eur J Plant Pathol 156:839–849. https://doi.org/10.1007/s10658-020-01934-w
St-Arnaud M, Vujanovic V (2007) Effect of the arbuscular mycorrhizal symbiosis on plant diseases and pests. In: Hamel C, Plenchette C (eds) Mycorrhizae in crop production. Haworth, Binghamton, pp 67–122
Steinkellner S, Mammerler R, Vierheilig H (2005) Microconidia germination of the tomato pathogen Fusarium oxysporum in the presence of root exudates. J Plant Interact 1:23–30. https://doi.org/10.1080/17429140500134334
Stelfox D, Williams JR, Soehngen U, Topping RC (1978) Transport of Sclerotinia sclerotiorum ascospores by rapeseed pollen in Alberta. Plant Dis Rep 62:576–579
Termorshuizen AJ, van Rijn E, van der Gaag DJ, Alabouvette C, Chen Y, Lagerlof J, Malandrakis AA, Paplomatas EJ, Ramert B, Ryckeboer J, Steinberg C, Zmora-Nahum S (2006) Suppressiveness of 18 composts against 7 pathosystems: variability in pathogen response. Soil Biol Biochem 38:2461–2477. https://doi.org/10.1016/j.soilbio.2006.03.002
Toljander JF, Lindahl BD, Paul LR, Elfstrand M, Finlay RD (2007) Influence of arbuscular mycorrhizal mycelial exudates on soil bacterial growth and community structure. Fems Microbiol Ecol 61:295–304. https://doi.org/10.1111/j.1574-6941.2007.00337.x
Trotta A, Varese GC, GnaviE., Fusconi A, Sampo S, Berta G, (1996) Interaction between the soil-borne root pathogen Phytophthora nicotianae var. parasitica and the arbuscular mycorrhizal fungus Glomus mosseae in tomato plants. Plant Soil 185:199–209
Van Zwieten L, Kimber S, Morris S, Chan KY, DownieA RJ, Joseph S, Cowie A (2010) Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 327:235–246. https://doi.org/10.1007/s11104-009-0050-x
Vierheilig H, Coughlan AP, Wyss U, Piche Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Env Microbiol 64:5004–5007
Wallace SU, Blanchet R, Bouniols A, Gelfi N (1990) Influence of nitrogen fertilization on morphological development of indeterminate and determinate soybeans. J Plant Nutrit 13:1523–1537. https://doi.org/10.1080/01904169009364173
Wang XR, Pan QA, Chen FX, Yan XL, Liao H (2011) Effects of co-inoculation with arbuscular mycorrhizal fungi and rhizobia on soybean growth as related to root architecture and availability of N and P. Mycorrhiza 21:173–181. https://doi.org/10.1007/s00572-010-0319-1
Wang Z, Ma LY, Cao J, Li YL, Ding L, Zhu KM, Yang YH, Tan XL (2019) Recent advances in mechanisms of plant defense to Sclerotinia sclerotiorum. Front Plant Sci. https://doi.org/10.3389/fpls.2019.01314
Warnock DD, Mummey DL, McBride B, Major J, Lehmann J, Rillig MC (2010) Influences of non-herbaceous biochar on arbuscular mycorrhizal fungal abundances in roots and soils: results from growth-chamber and field experiments. Appl Soil Ecol 46:450–456. https://doi.org/10.1016/j.apsoil.2010.09.002
Wen Z, Pang J, Tueux G, Liu YF, Shen JB, Ryan MH, Lambers H, Siddique KHM (2020) Contrasting patterns in biomass allocation, root morphology and mycorrhizal symbiosis for phosphorus acquisition among 20 chickpea genotypes with different amounts of rhizosheath carboxylates. Funct Ecol 34:1311–1324. https://doi.org/10.1111/1365-2435.13562
Whipps JM (2004) Prospects and limitations for mycorrhizas in biocontrol of root pathogens. Can J Bot 82:1198–1227. https://doi.org/10.1139/b04-082
Xavier L, Boyetchko S (2004) Arbuscular mycorrhizal fungi in plant disease control. In: Arora D, Bridge P, Bhatnagar D (eds) Fungal biotechnology in agricultural, food, and environmental applications. Marcel Dekker Inc, New York
Yang W, Gu S, Xin Y, Bello A, Sun W, Xu X (2018) Compost addition enhanced hyphal growth and sporulation of arbuscular mycorrhizal fungi without affecting their community composition in the soil. Front Microbiol. https://doi.org/10.3389/fmicb.2018.00169
Yano K, Yamauchi A, Kono Y (1996) Localized alteration in lateral root development in roots colonized by an arbuscular mycorrhizal fungus. Mycorrhiza 6:409–415. https://doi.org/10.1007/s005720050140
Acknowledgements
We thank Karin Baumgartner and Sabine Daxböck-Horvath for their excellent technical assistance.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). This research was not funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Safaei Asadabadi, R., Hage-Ahmed, K. & Steinkellner, S. Biochar, compost and arbuscular mycorrhizal fungi: a tripartite approach to combat Sclerotinia sclerotiorum in soybean. J Plant Dis Prot 128, 1433–1445 (2021). https://doi.org/10.1007/s41348-021-00495-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-021-00495-2