Abstract
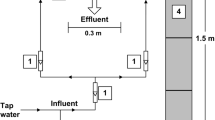
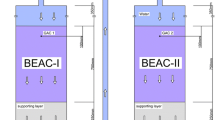
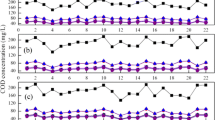
To cut down aeration power for nitrification, we constructed a biological nitrification–denitrification process with a trickling filter for nitrification and an anaerobic biological filter for denitrification. The influences of temperature and chemical oxygen demand (COD) loading on the nitrification and denitrification performance of the process are discussed in this paper through experimental and empirical modeling approaches. The model constants were determined by the experimental data of the process using municipal wastewater in Ryukoku University. Then, the influences of temperature and COD loading were estimated by the model. The COD levels required for NO3–N removal depended on both, the temperature and influent COD/NO3–N ratio. A lower temperature and higher influent COD/NO3–N ratio increased the COD requirement, because of the different responses between denitrifying bacteria and heterotrophic bacteria against temperature, COD, and NO3–N concentrations. In addition, our experimental system could satisfy the Japanese effluent standard at temperatures higher than 12 °C. The dissolved nitrogen (DN) concentration in the final effluent was more strongly affected by the NH4–N discharged from the trickling filter than it was by the residual NO3–N in the effluent from the denitrification tank. Therefore, the enhancement of the nitrification efficiency in the trickling filter was inferred to enhance the nitrogen removal efficiency. To prevent a low nitrogen removal efficiency at temperatures lower than 15 °C, it was necessary to set the low hydraulic loading.











Similar content being viewed by others
Abbreviations
- \(C_{{{\text{NO}}_{ 3} - {\text{N}}}}\) :
-
NO3–N concentration (mg-N L−1)
- \(C_{{{\text{inNO}}_{ 3} {\text{ - N}}}}\) :
-
NO3–N concentration in the influent (mg-N L−1)
- \(C_{{{\text{NO}}_{ 3} - {\text{N}}}}^{'}\) :
-
NO3–N concentration in effluent from the trickling filter (mg-N L−1)
- \(C_{\text{in,WW}}\) :
-
NO3–N concentration in influent wastewater (mg-N L−1)
- \(C_{\text{in NL}}\) :
-
NO3–N concentration in the nitrified liquor (mg-N L−1)
- \(C_{{{\text{NH}}_{4} {\text{ - N}}}}\) :
-
NH4–N concentration (mg-N L−1)
- \(C_{{{\text{in,NH}}_{4} {\text{ - N}}}}\) :
-
NH4–N concentration in the influent (mg- L−1)
- \(C_{\text{COD}}\) :
-
COD (mg L−1)
- \(C_{\text{in,COD}}\) :
-
COD in the influent (mg L−1)
- \(K_{\text{den,20}}\) :
-
Pseudo-first-order denitrification rate constant at 20 °C (1.1 × 10−2 min−1)
- \(K_{\text{den,T}}\) :
-
Pseudo-first-order denitrification rate constant at T °C (min−1)
- K nit,20 :
-
Pseudo-first-order nitrification rate constant at 20 °C (0.27 min−1)
- K COD,20 :
-
Aerobic COD removal rate constants at 20 °C (0.27 min−1)
- K COD,den,20 :
-
COD removal rate constants at 20 °C by denitrification bacteria under NO3–N rich environment 2.0 × 10−3 min−1)
- K COD,het,20 :
-
Pseudo-first-order removal rate constant of COD at 20 °C by heterotrophic bacteria (1.7 × 10−3 min−1)
- K COD,het,T :
-
Pseudo-first-order removal rate constant of COD at T °C by heterotrophic bacteria (min−1)
- θ den :
-
Temperature-activity coefficient of denitrification (1.150)
- θ nit :
-
Temperature-activity coefficient of nitrification (1.096)
- θ het :
-
Temperature-activity coefficient for COD removal by heterotrophic bacteria (1.072)
- θ COD :
-
Temperature-activity coefficient for aerobic COD removal (1.035 [9])
- T :
-
Water temperature (°C)
- \(R_{{{\text{NO}}_{3} {\text{ - N}}}}\) :
-
NO3–N removal rate under COD rich environment (mg-N L−1 min−1)
- \(R_{{{\text{NO}}_{ 3} - {\text{N}}}}^{'}\) :
-
NO3–N removal rate under NO3–N rich environment (mg-N L−1 min−1)
- R COD :
-
COD removal rate under COD rich environment (mg L−1 min−1)
- \(R_{\text{COD}}^{'}\) :
-
COD removal rate under NO3–N rich environment (mg L−1 min−1)
- α :
-
Conversion factor from COD removed to NO3–N removed (0.25
- Q :
-
Flow rate (m3 day−1)
- V :
-
Water volume of denitrification tank (2.15 × 10−3 m3)
- A :
-
Cross-sectional area of denitrification tank (6.5 × 10−3 m2)
- ε :
-
Recirculation rate of nitrified liquor
- t :
-
Hydraulic retention time of denitrification tank (h)
- t’ :
-
Time required for passing through the trickling filter (min)
- \(C_{{^{D} }}^{n}\) :
-
Constant (10.756)
- m :
-
Constant (0.68)
- q :
-
Hydraulic loading to the trickling filter (m3 m−2 day−1)
References
Borchardt JA (1966) Nitrification in the activated sludge process, the activated sludge process in sewage treatment theory and application. Univ. of Michigan, Dept. of Civil Eng., Ann Arbor, MI
Carrera J, Vicent T, Lafuente FJ (2003) Influence of temperature on denitrification of an industrial high-strength nitrogen wastewater in a two-sludge system. Water SA 29(1):11–16
ENARWATER (2015) Deliverable 2.1 study of published energy data, Standard method and online tool for assessing and improving the energy efficiency of waste water treatment plants. http://www.enerwater.eu/wp-content/uploads/2015/10/ENERWATER_D2.1-Study-of-published-energy-data-Will-contain-data-of-at-least-500-WWTPs.pdf. Accessed 22 April 2015
Henrich C-D, Marggaraff M (2013) Energy-efficient wastewater reuse—the renaissance of trickling filter technology. In: 9th International conference on water reuse, 27–31, Windhoek
Hernández-Sancho F, Molinos-Senante M, Sala-Garrido R (2011) Cost modelling for wastewater treatment processes. Desalination 1–5(1–3):268
Japanese Industrial Standards Committee (2008) Testing methods for industrial wastewater. JIS K 0102, Tokyo: Japanese Standards Association (in Japanese)
Kanda R, Kishimoto N, Hinobayashi J, Hashimoto T (2016) Effect of recirculation rate of nitrified liquor and temperature on biological nitrification-denitrification process using a trickling filter. Water Environ J. doi:10.1111/wej.12196
Kishimoto N, Ohara T, Hinobayashi J, Hashimoto T (2014) Roughness and temperature effects on the filter media of a trickling filter for nitrification. Environ Technol 35:1549–1555
Kitao T (2003) Engineering for biological wastewater treatment. Corona (in Japanese)
Logan BE, Hermanowicz SW, Parker DS (1987) A fundamental model for trickling filter process design. J Water Pollut Control Fed 59(12):1029–1042
Londong J (1992) Strategies for optimised nitrate reduction with primary denitrification. Water Sci Technol 26(5–6):1087–1096
Muslu Y (1986) Distribution of retention times in model biological filters containing packed spheres. Water Res 20(3):259–265
Okubo T, Kubota K, Yamaguchi T, Uemura S, Harada H (2016) Development of a new non-aeration-based sewage treatment technology: performance evaluation of a full-scale down-flow hanging sponge reactor employing third-generation sponge carriers. Water Res 102:138–146
Parker DS, Jacobs T, Bower E, Stowe DW, Farmer G (1997) Maximizing trickling filter nitrification rates through biofilm control: research review and full scale application. Water Sci Technol 36(1):255–262
Purtchert I, Siegrist H, Gujer W (1996) Enhanced denitrification with methanol at WWTP Zurich-Werdholzli. Water Sci Technol 33(12):126–177
Rice EW, Baird RB, Eaton AD, Clesceri LS (eds) (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association/American Water Works Association/Water Environment Federation, New York
Rieger L, Gillot S, Langergraber G, Ohtsuki T, Shaw A, Takacs I, Winkler S, IWA Task Group on Good Modelling Practice (2012) Guidelines for using activated sludge models. IWA
Saghafi S, Mehrdadi N, Hendy GNB, Rad HA (2015) Energy efficiency in wastewater treatment plant emphasizing on COD removal: a case study of Amol Industrial Zone, Iran. Can J Pure Appl Sci 9(2):3441–3448
Wang LK, Shammas NK, Hung Y-T (2009) Advanced biological treatment processes. Humana Press, Berlin
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanda, R., Kishimoto, N., Hinobayashi, J. et al. Influence of temperature and COD loading on biological nitrification–denitrification process using a trickling filter: an empirical modeling approach. Int J Environ Res 11, 71–82 (2017). https://doi.org/10.1007/s41742-017-0008-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-017-0008-4