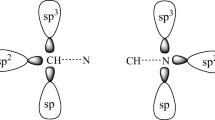
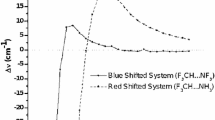
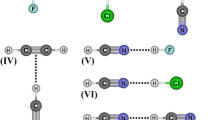
Abstract
We present a spectroscopic overview of the C–H···Y (Y = hydrogen bond acceptors) hydrogen bonded (HB or H-bond) complexes in this article. Although C–H···Y interactions have been recognized as H-bonding interactions for quite some time, they have not been investigated spectroscopically until recently. Recent results indicated that unlike the conventional hydrogen bond, C–H···Y H-bond has interesting spectroscopic characteristics, i.e. it shows both red as well as blue shift in C–H stretching frequency upon H-bond formation. This review presents examples of red, blue, and zero shifted C–H···Y H-bonds investigated in our laboratory that were characterized using laser-based IR and UV spectroscopic techniques applied to the cold isolated molecular complexes formed under supersonic expansion conditions. Along with spectroscopic information, ab initio/DFT-predicted geometry optimized structures of various conformers, harmonic frequency calculations of the optimized structures, and a number of properties such as electron densities at the bond critical points, orbital interaction energies, binding energies of the C–H···Y bound complexes are also summarized for better understanding of this type of H-bond. Not only the spectroscopic shift in C–H stretching frequency, but also the role of C–H···O H-bonds in microsolvation of several organic molecules has been highlighted. It has been found that depending upon activation of C–H moiety, C–H···Y H-bonds can provide primary or secondary stabilization for the growth of the primary solvation shell around organic molecules.




Reprinted with permission from Ref.75. Copyright © 2014 WILEY–VCH Verlag GmbH & Co. KGaA, Weinheim.

Reference92—reproduced by permission of the PCCP Owner Societies.

Reference95—reproduced by permission of the PCCP Owner Societies.

Reference95—reproduced by permission of the PCCP Owner Societies.

Reference95—reproduced by permission of the PCCP Owner Societies.

Adapted with permission from Ref.39. Copyright © 2019 American Chemical Society.

Reprinted with permission from Ref.39. Copyright © 2019 American Chemical Society.
Similar content being viewed by others
References
Desiraju GR, Steiner T (1999) The weak hydrogen bond in structural chemistry and biology. Oxford University Press, New York, pp 29–121
Moore TS, Winmill TF (1912) The state of amines an aqueous solution. J Chem Soc 101:1635–1676
Latimer WM, Rodebush WH (1920) Polarity and ionization from the standpoint of the Lewis theory of valence. J Am Chem Soc 42:1419–1433
Biswal HS, Chakraborty S, Wategaonkar S (2008) Experimental evidence of OH···S hydrogen bonding in supersonic jet. J Chem Phys 129:184311–184317
Mundlapati VR, Sahoo DK, Ghosh S, Purame UK, Pandey S, Acharya R, Pal N, Tiwari P, Biswal HS (2017) Spectroscopic evidences for strong hydrogen bonds with selenomethionine in proteins. J Phys Chem Lett 8:794–800
Sobczyk L, Grabowski SJ, Krygowski TM (2005) Interrelation between H-bond and Pi-electron delocalization. Chem Rev 105:3513–3560
Sutor DJ (1962) C–H···O hydrogen bond in crystals. Nature 195:68–69
Sutor DJ (1963) Evidence for existence of C–H···O hydrogen bonds in crystals. J Chem Soc 1105-1110
Taylor R, Kennard O (1982) Crystallographic evidence for the existence of C–H···O, C–H···N, and C–H···C1 hydrogen-bonds. J Am Chem Soc 104:5063–5070
Wahl MC, Sundaralingam M (1997) C–H···O hydrogen bonding in biology. Trends Biochem Sci 22:97–102
Senes A, Ubarretxena-Belandia I, Engelman DM (2001) The C–H···O hydrogen bond: a determinant of stability and specificity in transmembrane helix interactions. Proc Natl Acad Sci USA 98:9056–9061
Horowitz S, Trievel RC (2012) Carbon-oxygen hydrogen bonding in biological structure and function. J Biol Chem 287:41576–41582
Brovarets OO, Yurenko YP, Hovorun DM (2014) Intermolecular CH···O/N H-bonds in the biologically important pairs of natural nucleobases: a thorough quantum-chemical study. J Biomol Struct Dyn 32:993–1022
Yurenko YP, Zhurakivsky RO, Samijlenko SP, Hovorun DM (2011) Intramolecular CH···O hydrogen bonds in the AI and BI DNA-like conformers of canonical nucleosides and their Watson-Crick Pairs, quantum chemical and AIM analysis. J Biomol Struct Dyn 29:51–65
Jiang L, Lai LH (2002) CH···O hydrogen bonds at protein–protein interfaces. J Biol Chem 277:37732–37740
Thakur TS, Kirchner MT, Blaser D, Boese R, Desiraju GR (2010) C–H···F–C hydrogen bonding in 1,2,3,5-tetrafluorobenzene and other fluoroaromatic compounds and the crystal structure of alloxan revisited. CrystEngComm 12:2079–2085
Chatterjee J, Mierke DF, Kessler H (2008) Conformational preference and potential templates of N-methylated cyclic pentaalanine peptides. Chem Eur J 14:1508–1517
Erez E, Fass D, Bibi E (2009) How intramembrane proteases bury hydrolytic reactions in the membrane. Nature 459:371–378
Desiraju GR (1991) The CH···O hydrogen bond in crystals: what is it? Acc Chem Res 24:290–296
Gu Y, Kar T, Scheiner S (1999) Fundamental properties of the CH···O interaction: is it a true hydrogen bond? J Am Chem Soc 121:9411–9422
Scheiner S, Kar T, Gu Y (2001) Strength of the CαH···O hydrogen bond of amino acid residues. J Biol Chem 276:9832–9837
Steiner T, Saenger W (1993) Role of CH···O hydrogen bonds in the coordination of water molecules. Analysis of neutron diffraction data. J Am Chem Soc 115:4540–4547
Kar T, Scheiner S (2004) Comparison of cooperativity in CH···O and OH···O hydrogen bonds. J Phys Chem A 108:9161–9168
Popelier P, Bader R (1992) The existence of an intramolecular C–H···O hydrogen bond in creatine and carbamoyl sarcosine. Chem Phys Lett 189:542–548
Pierce AC, Sandretto KL, Bemis GW (2002) Kinase inhibitors and the case for CH···O hydrogen bonds in protein–ligand binding. Proteins Struct Funct Bioinform 49:567–576
Braga D, Grepioni F, Biradha K, Pedireddi V, Desiraju GR (1995) Hydrogen bonding in organometallic crystals. 2. CH···O hydrogen bonds in bridged and terminal first-row metal carbonyls. J Am Chem Soc 117:3156–3166
Samanta AK, Chakraborty T (2010) In: Chaudhuri RK, Mekkaden MV, Raveendran AV, Satya Narayanan A (eds) Recent advances in spectroscopy. Springer, pp 53–61
Mukhopadhyay A, Mukherjee M, Pandey P, Samanta AK, Bandyopadhyay B, Chakraborty T (2009) Blue shifting C − H···O hydrogen bonded complexes between chloroform and small cyclic ketones: ring-size effects on stability and spectral shifts. J Phys Chem A 113:3078–3087
Reimann B, Buchhold K, Vaupel S, Brutschy B, Havlas Z, Spirko V, Hobza P (2001) Improper, blue-shifting hydrogen bond between fluorobenzene and fluoroform. J Phys Chem A 105:5560–5566
Shirhatti PR, Maity DK, Wategaonkar S (2013) C-H···Y hydrogen bonds in the complexes of p-cresol and p-cyanophenol with fluoroform and chloroform. J Phys Chem A 117:2307–2316
Shirhatti PR, Wategaonkar S (2010) Blue shifted hydrogen bond in 3-methylindole·CHX3 complexes (X = Cl, F). Phys Chem Chem Phys 12:6650–6659
Delanoye SN, Herrebout WA, van der Veken BJ (2002) Improper or classical hydrogen bonding? A comparative cryosolutions infrared study of the complexes of HCClF2, HCCl2F, and HCCl3 with dimethyl ether. J Am Chem Soc 124:7490–7498
Delanoye SN, Herrebout WA, van der Veken BJ (2002) Blue shifting hydrogen bonding in the complexes of chlorofluoro haloforms with acetone-d6 and oxirane-d4. J Am Chem Soc 124:11854–11855
Michielsen B, Dom JJJ, van der Veken BJ, Hesse S, Xue ZF, Suhm MA, Herrebout WA (2010) The complexes of halothane with benzene: the temperature dependent direction of the complexation shift of the aliphatic C–H stretching. Phys Chem Chem Phys 12:14034–14044
van der Veken BJ, Herrebout WA, Szostak R, Shchepkin DN, Havlas Z, Hobza P (2001) The nature of improper, blue-shifting hydrogen bonding verified experimentally. J Am Chem Soc 123:12290–12293
Venkatesan V, Fujii A, Ebata T, Mikami N (2004) A direct experimental evidence for an aromatic C–H···O hydrogen bond by fluorescence-detected infrared spectroscopy. Chem Phys Lett 394:45–48
Venkatesan V, Fujii A, Ebata T, Mikami N (2005) Infrared and ab initio studies on 1,2,4,5-tetrafluorobenzene clusters with methanol and 2,2,2-trifluoroethanol: presence and absence of an aromatic C–H···O hydrogen bond. J Phys Chem A 109:915–921
Venkatesan V, Fujii A, Mikami N (2005) A study on aromatic C–H···X (X = N, O) hydrogen bonds in 1,2,4,5-tetrafluorobenzene clusters using infrared spectroscopy and ab initio calculations. Chem Phys Lett 409:57–62
Ghosh S, Wategaonkar S (2019) C–H···O hydrogen bond anchored water bridge in 1,2,4,5-tetracyanobenzene-water clusters. J Phys Chem A 123:3851–3862
Samanta AK, Banerjee P, Bandyopadhyay B, Pandey P, Chakraborty T (2017) Antagonistic interplay between an intermolecular CH···O and an intramolecular OH···O hydrogen bond in a 1: 1 complex between 1, 2-cyclohexanedione and chloroform: a combined matrix isolation infrared and quantum chemistry study. J Phys Chem A 121:6012–6020
Sundararajan K, Ramanathan N (2007) Infrared and ab initio study of acetylene–acetone complex in solid argon and nitrogen matrices. J Mol Struct 833:150–160
Jemmis E, Giju K, Sundararajan K, Sankaran K, Vidya V, Viswanathan K, Leszczynski J (1999) An ab initio and matrix isolation infrared study of the 1:1 C2H2–CHCl3 adduct. J Mol Struct 510:59–68
Gopi R, Ramanathan N, Sundararajan K (2014) Experimental evidence for blue-shifted hydrogen bonding in the fluoroform–hydrogen chloride complex: a matrix-isolation infrared and ab initio study. J Phys Chem A 118:5529–5539
Gopi R, Ramanathan N, Sundararajan K (2015) Hydrogen-bonded complexes of acetylene and acetonitrile: a matrix isolation infrared and computational study. J Mol Struct 1083:364–373
Sarkar S, Ramanathan N, Gopi R, Sundararajan K (2017) Pyrrole multimers and pyrrole-acetylene hydrogen bonded complexes studied in N2 and para-H2 matrixes using matrix isolation infrared spectroscopy and ab initio computations. J Mol Struct 1149:387–403
Gopi R, Ramanathan N, Sundararajan K (2017) Experimental evidence for the blue-shifted hydrogen-bonded complexes of CHF3 with π-electron donors. Spectrochim Acta Part A Mol Biomol Spectrosc 181:137–147
Hobza P, Spirko V, Selzle HL, Schlag EW (1998) Anti-hydrogen bond in the benzene dimer and other carbon proton donor complexes. J Phys Chem A 102:2501–2504
Hobza P, Spirko V, Havlas Z, Buchhold K, Reimann B, Barth HD, Brutschy B (1999) Anti-hydrogen bond between chloroform and fluorobenzene. Chem Phys Lett 299:180–186
Yamamoto R, Ebata T, Mikami N (2001) Mode dependent intracluster vibrational energy redistribution rate in size-selected benzonitrile-(CHCl3)n=1–3 clusters. J Chem Phys 114:7866–7876
Hobza P, Havlas Z (2000) Blue-shifting hydrogen bonds. Chem Rev 100:4253–4264
Hobza P, Havlas Z (2002) Improper, blue-shifting hydrogen bond. Theor Chem Acc 108:325–334
Hobza P (2001) The H-index unambiguously discriminates between hydrogen bonding and improper blue-shifting hydrogen bonding. Phys Chem Chem Phys 3:2555–2556
Hermansson K (2002) Blue-shifting hydrogen bonds. J Phys Chem A 106:4695–4702
Pejov L, Hermansson K (2003) On the nature of blue shifting hydrogen bonds: ab initio and density functional studies of several fluoroform complexes. J Chem Phys 119:313–324
Alabugin IV, Manoharan M, Peabody S, Weinhold F (2003) Electronic basis of improper hydrogen bonding: a subtle balance of hyperconjugation and rehybridization. J Am Chem Soc 125:5973–5987
Joseph J, Jemmis ED (2007) Red-, blue-, or no-shift in hydrogen bonds: a unified explanation. J Am Chem Soc 129:4620–4632
Levy DH (1980) Laser Spectroscopy of cold gas-phase molecules. Annu Rev Phys Chem 31:197–225
Levy DH (1981) The spectroscopy of very cold gases. Science 214:263–269
Zare RN (2012) My life with LIF: a personal account of developing laser-induced fluorescence. Annu Rev Anal Chem 5:1–14
Friedrich DM, Mcclain WM (1980) 2-photon molecular electronic spectroscopy. Annu Rev Phys Chem 31:559–577
Ashfold MNR, Howe JD (1994) Multiphoton spectroscopy of molecular-species. Annu Rev Phys Chem 45:57–82
Brutschy B (2000) The structure of microsolvated benzene derivatives and the role of aromatic substituents. Chem Rev 100:3891–3920
Wiley WC, McLaren IH (1955) Time-of-flight mass spectrometer with improved resolution. Rev Sci Instrum 26:1150–1157
Rothman LS, Jacquemart D, Barbe A, Benner DC, Birk M, Brown L, Carleer M, Chackerian C Jr, Chance K, Coudert L et al (2005) The HITRAN 2004 molecular spectroscopic database. J Quant Spectrosc Radiat Transf 96:139–204
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, Revision C.01, Gaussian, Inc. Wallingford CT
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91:893–928
Koch U, Popelier PLA (1995) Characterization of C–H···O hydrogen bonds on the basis of the charge density. J Phys Chem 99:9747–9754
Glendening ED, Landis CR, Weinhold F (2013) NBO 6.0: natural bond orbital analysis program. J Comput Chem 34:1429–1437
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic-structure system. J Comput Chem 14:1347–1363
Morita S, Fujii A, Mikami N, Tsuzuki S (2006) Origin of the attraction in aliphatic C–H/π interactions: infrared spectroscopic and theoretical characterization of gas-phase clusters of aromatics with methane. J Phys Chem A 110:10583–10590
Biswal HS, Wategaonkar S (2009) Sulfur, not too far behind O, N, and C: SH···π hydrogen bond. J Phys Chem A 113:12774–12782
Tsuzuki S, Honda K, Uchimaru T, Mikami M, Tanabe K (2002) The interaction of benzene with chloro- and fluoromethanes: effects of halogenation on CH/π interaction. J Phys Chem A 106:4423–4428
Li X, Liu L, Schlegel HB (2002) On the physical origin of blue-shifted hydrogen bonds. J Am Chem Soc 124:9639–9647
Shirhatti PR, Maity DK, Bhattacharyya S, Wategaonkar S (2014) C–H···N hydrogen-bonding interaction in 7-Azaindole:CHX3 (X = F, Cl) complexes. ChemPhysChem 15:109–117
Qian WL, Krimm S (2002) Vibrational spectroscopy of hydrogen bonding: origin of the different behavior of the C–H···O hydrogen bond. J Phys Chem A 106:6628–6636
Krajewska M, Olbert-Majkut A, Mielke Z (2002) Matrix infrared spectra and ab initio calculations of the acetylene complexes with nitric and nitrous acids. Phys Chem Chem Phys 4:4305–4313
Tatamitani Y, Liu BX, Shimada J, Ogata T, Ottaviani P, Maris A, Caminati W, Alonso JL (2002) Weak, improper, C-O···H-C hydrogen bonds in the dimethyl ether dimer. J Am Chem Soc 124:2739–2743
Lockwood SP, Fuller TG, Newby JJ (2018) Structure and spectroscopy of Furan:H2O complexes. J Phys Chem A 122:7160–7170
Hussein MA, Millen DJ (1976) Hydrogen-bonding in gas-phase. 4. Infrared spectroscopic investigation of O-H···O and C–H···N complexes–alcohol + ether and trichloromethane + amine systems. J Chem Soc Farad Trans II 72:693–699
Rutkowski KS, Karpfen A, Melikova SM, Herrebout WA, Koll A, Wolschann P, van der Veken BJ (2009) Cryospectroscopic and ab initio studies of haloform-trimethylamine H-bonded complexes. Phys Chem Chem Phys 11:1551–1563
Herrebout WA, Melikova SM, Delanoye SN, Rutkowski KS, Shchepkin DN, van der Veken BJ (2005) A cryosolution infrared study of the complexes of fluoroform with ammonia and pyridine: evidence for a C–H···N pseudo blue-shifting hydrogen bond. J Phys Chem A 109:3038–3044
Guin M, Patwari GN, Karthikeyan S, Kim KS (2011) Do N-heterocyclic aromatic rings prefer π-stacking? Phys Chem Chem Phys 13:5514–5525
Hippler M (2007) Quantum chemical study and infrared spectroscopy of hydrogen-bonded CHCl3–NH3 in the gas phase. J Chem Phys 127:084306
Hippler M, Hesse S, Suhm MA (2010) Quantum-chemical study and FTIR jet spectroscopy of CHCl3–NH3 association in the gas phase. Phys Chem Chem Phys 12:13555–13565
Paulson SL, Barnes AJ (1982) Trihalogenomethane—base complexes studied by vibrational spectroscopy in low-temperature matrices. J Mol Struct 80:151–158
Gord JR, Garrett AW, Bandy RE, Zwier TS (1990) Rempi fragmentation as a probe of hydrogen-bonding in aromatic-X clusters. Chem Phys Lett 171:443–450
Fujii A, Shibasaki K, Kazama T, Itaya R, Mikami N, Tsuzuki S (2008) Experimental and theoretical determination of the accurate interaction energies in benzene–halomethane: the unique nature of the activated CH/π interaction of haloalkanes. Phys Chem Chem Phys 10:2836–2843
Jin Z (2006) Imidazole, oxazole and thiazole alkaloids. Nat Prod Rep 23:464–496
Ghosh S, Reddy CM (2012) Elastic and bendable caffeine cocrystals: implications for the design of flexible organic materials. Angew Chem Int Ed 51:10319–10323
Garist IV, Verevkin SP, Bara JE, Hindman MS, Danielsen SP (2012) Building blocks for ionic liquids: vapor pressures and vaporization enthalpies of 1-(n-alkyl)-benzimidazoles. J Chem Eng Data 57:1803–1809
Bhattacherjee A, Wategaonkar S (2015) Conformational preferences of monohydrated clusters of imidazole derivatives revisited. Phys Chem Chem Phys 17:20080–20092
Bhattacherjee A, Wategaonkar S (2017) Role of the C (2)–H hydrogen bond donor in gas-phase microsolvation of imidazole derivatives with ROH (R = CH3, C2H5). J Phys Chem A 121:4283–4295
Bhattacherjee A, Wategaonkar S (2017) Conformational heterogeneity and the role of the C(2)–H donor in mono- and dihydrated clusters of benzoxazole. J Phys Chem A 121:5420–5427
Bhattacherjee A, Wategaonkar S (2016) Water bridges anchored by a C–H···O hydrogen bond: the role of weak interactions in molecular solvation. Phys Chem Chem Phys 18:27745–27749
Talbot F, Simons J (2002) Infrared ion dip and ultraviolet spectroscopy of 4-phenyl imidazole, its tautomer, 5-phenyl imidazole, and its multiply hydrated clusters. Eur Phys J D At Mol Opt Plasma Phys 20:389–398
Coussan S, Manca C, Tanner C, Bach A, Leutwyler S (2003) Ammonia-chain clusters: vibronic spectra of 7-hydroxyquinoline·(NH3)2. J Chem Phys 119:3774–3784
Matsumoto Y, Ebata T, Mikami N (2001) OH stretching vibrations and hydrogen-bonded structures of 7-hydroxyquinoline-(H2O)1–3 investigated by IR–UV double-resonance spectroscopy. Chem Phys Lett 338:52–60
Snoek LC, Kroemer RT, Simons JP (2002) A spectroscopic and computational exploration of tryptophan–water cluster structures in the gas phase. Phys Chem Chem Phys 4:2130–2139
Carney JR, Dian BC, Florio GM, Zwier TS (2001) The role of water bridges in directing the conformational preferences of 3-indole-propionic acid and tryptamine. J Am Chem Soc 123:5596–5597
Nakajima A, Hirano M, Hasumi R, Kaya K, Watanabe H, Carter C, Williamson J, Miller TA (1997) High-resolution laser-induced fluorescence spectra of 7-Azaindole–water complexes and 7-azaindole dimer. J Phys Chem A 101:392–398
Tanner C, Manca C, Leutwyler S (2003) Probing the threshold to H atom transfer along a hydrogen-bonded ammonia wire. Science 302:1736–1739
Garczarek F, Gerwert K (2006) Functional waters in intraprotein proton transfer monitored by FTIR difference spectroscopy. Nature 439:109–112
Freier E, Wolf S, Gerwert K (2011) Proton transfer via a transient linear water-molecule chain in a membrane protein. Proc Natl Acad Sci USA 108:11435–11439
Kaila VRI, Hummer G (2011) Energetics and dynamics of proton transfer reactions along short water wires. Phys Chem Chem Phys 13:13207–13215
Park SY, Kim B, Lee YS, Kwon OH, Jang DJ (2009) Triple proton transfer of excited 7-hydroxyquinoline along a hydrogen-bonded water chain in ethers: secondary solvent effect on the reaction rate. Photochem Photobiol Sci 8:1611–1617
Folmer DE, Wisniewski ES, Stairs JR, Castleman AW (2000) Water-assisted proton transfer in the monomer of 7-azaindole. J Phys Chem A 104:10545–10549
Acknowledgements
SJW would like to acknowledge the exemplary hard work and dedication shown by his students Drs. Pranav Shirhatti, Aditi Bhattacherjee, and Sanat Ghosh whose work is presented in the review. The technical support provided by Mr Ajay Patil and Mr Sachin Temkar is also gratefully acknowledged. The work was supported by the Tata Institute of Fundamental Research, Mumbai via project no. 12-R&D-TFR-5.10-0100.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghosh, S., Wategaonkar, S. C–H···Y (Y=N, O, π) Hydrogen Bond: A Unique Unconventional Hydrogen Bond. J Indian Inst Sci 100, 101–125 (2020). https://doi.org/10.1007/s41745-019-00145-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-019-00145-5