Abstract
Life makes extensive use of non-covalent interactions, as they are a convenient way to build complex structures that can be assembled or disassembled quickly, with a minimum energy consumption. Among the inter-molecular interactions, hydrogen bond plays a central role, and it is the main responsible of the structure of proteins, DNA, and several other superstructures in the cell. Characterization of hydrogen bond in biologic environment is not an easy task, and several complex and imaginative techniques have been developed to circumvent the technical challenges of such studies. We present here an overview of the field of mass-resolved laser spectroscopy applied to nucleobases, peptides, and monosaccharides to demonstrate that despite the different environment the molecules encounter in the jet, such experiments yield important structural information that helps understanding the role played by hydrogen bond in biology.

Adapted from Ref.16.

Adapted from Ref.24.

Adapted from Ref. 28.








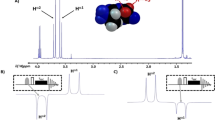
Adapted from Ref. 141.
Similar content being viewed by others
References
Crowe SA, Dossing LN, Beukes NJ, Bau M, Kruger SJ, Frei R, Canfield DE (2013) Atmospheric oxygenation three billion years ago. Nature 501:535
Hobza P, Muller-Dethlefs K (2010) Non-covalent interactions. RCS Publishing, Milan
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2011) Revealing Non-Covalent Interactions. J Am Chem Soc 132:6498–6506
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Stone AJ (2002) The theory of intermolecular forces. Oxford University Press, Oxford
Müller-Dethlefs K, Hobza P (2000) Noncovalent interactions: a challenge for experiment and theory. Chem Rev 100:143–168
Biochemistry of Lipids (2002) Lipoproteins and membranes. Elsevier Science, New York
Brown DA, London E (1998) Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol 14:111–136
Lehninger A, Nelson D, Cox M (2008) Lehninger principles of biochemistry. W. H. Freeman, New York
Poblotzki A, Gottschalk HC, Suhm MA (2017) Tipping the scales: spectroscopic tools for intermolecular energy balances. J Phys Chem Lett 8:5656–5665
Puzzarini C, Bloino J, Tasinato N, Barone V (2019) Accuracy and interpretability: the devil and the holy grail. New routes across old boundaries in computational spectroscopy. Chem Rev 119:8131–8191
Dopfer O, Fujii M (2016) Probing solvation dynamics around aromatic and biological molecules at the single-molecular level. Chem Rev 116:5432–5463
Smalley RE, Levy DH, Wharton L (1975) Molecular-jet spectroscopy. Laser Focus 11:40–43
Smalley RE, Blazy JA, Fitch PSH, Kim MS, Wharton L, Levy DH (1976) Laser spectroscopy in supersonic molecular-beams. Opt Commun 18:59–60
Zwier T (2001) Laser spectroscopy of jet-cooled biomolecules and their water-containing clusters: Water bridges and molecular conformation. J Phys Chem A 105:8827–8839
León I, Millán J, Cocinero EJ, Lesarri A, Fernández JA (2013) Magic numbers in the solvation of the propofol dimer. Chem Phys Chem 14:1558–1562
Ashfold MNR, Howe JD (1994) Multiphoton spectroscopy of molecular species. Annu Rev Phys Chem 45:57–82
Held A, Pratt DW (1992) Hydrogen bonding in the symmetry-equivalent C2h dimer of 2-pyridone in its S0 and S2 electronic states. Effect of deuterium substitution. J Chem Phys 96:4869–4876
Becucci M, Melandri S (2016) Resolution spectroscopic studies of complexes formed by medium-size organic molecules. Chem Rev 116:5014–5037
Pérez C, Muckle MT, Zaleski DP, Seifert NA, Temelso B, Shields GC, Kisiel Z, Pate BH (2012) Structures of cage, prism, and book isomers of water hexamer from broadband rotational spectroscopy. Science 336:897–901
Seifert NA, Steber AL, Neill JL, Pérez C, Zaleski DP, Pate BH, Lesarri A (2013) The interplay of hydrogen bonding and dispersion in phenol dimer and trimer: structures from broadband rotational spectroscopy. Phys Chem Chem Phys 15:11468–11477
Pérez C, León I, Lesarri A, Pate BH, Martínez R, Millán J, Fernández JA (2018) Isomerism of the aniline trimer. Angew Chem Int Ed 57:15112–15116
Calabrese C, Li W, Prampolini G, Evangelisti L, Uriarte I, Cacelli I, Melandri S, Cocinero EJ (2019) A general treatment to study molecular complexes stabilized by Hydrogen-, Halogen-, and Carbon-Bond networks: experiment and theory of (CH2F2)n···(H2O)m. Angew Chem Int Ed 58:8437–8442
Usabiaga I, Camiruaga A, Calabrese C, Maris A, Fernández JA (2019) Exploring caffeine-phenol interactions by the inseparable duet of experimental and theoretical data. Chem Eur J 25:14230–14236
Zwier TS (1996) The spectroscopy of solvation in hydrogen-bonded aromatic clusters. Annu Rev Phys Chem 47:205–241
Fernández JA, Unamuno I, Longarte A, Castaño F (2001) S-0, S-1, and ion I-0 binding energies of the p-methoxyphenethylamine(H2O)(1-4) complexes. J Phys Chem A 105:961–968
Longarte A, Fernández JA, Unamuno I, Castaño F (2000) Isomer structures and vibrational assignment of the methyl-p-aminobenzoate(H2O)(1) complex. J Chem Phys 112:3170–3180
León I, Millan J, Cocinero E, Lesarri A, Castaño F, Fernández JA (2012) Mimicking anaesthetic-receptor interaction: a combined spectroscopic and computational study of propofol···phenol. Phys Chem Chem Phys 14:8956–8963
Siffert L, Blaser S, Ottiger P, Leutwyler S (2018) Transition from water wires to bifurcated H-bond networks in 2-Pyridone·(H2O)n, n = 1-4 Clusters. J Phys Chem A 122:9285–9297
Knochenmuss R, Sinha RK, Leutwyler S (2019) Face, Notch, or Edge? Intermolecular dissociation energies of 1-naphthol complexes with linear molecules. J Chem Phys 150:234303
Asami H, Tokugawa M, Masaki Y, Ishiuchi S, Gloaguen E, Seio K, Saigusa H, Fujii M, Sekine M, Mons M (2016) Effective strategy for conformer-selective detection of short-lived excited state species: application to the IR spectroscopy of the N1H Keto tautomer of guanine. J Phys Chem A 120:2179–2184
Habka S, Sohn WY, Vaquero-Vara V, Geleoc M, Tardivel B, Brenner V, Gloaguen E, Mons M (2018) On the turn-inducing properties of asparagine: the structuring role of the amide side chain, from isolated model peptides to crystallized proteins. Phys. Chem. Chem. Phys. 20:3411–3423
Kumar S, Mishra KK, Singh SK, Borish K, Dey S, Sarkar B, Das A (2019) Observation of a weak intra-residue C5 hydrogen-bond in a dipeptide containing Gly-Pro sequence. J Chem Phys 151:104309
Bernhard D, Fatima M, Poblotzki A, Steber AL, Pérez C, Suhm MA, Schnell M, Gerhards M (2019) Dispersion-controlled docking preference: multi-spectroscopic study on complexes of dibenzofuran with alcohols and water. Phys Chem Chem Phys 21:16032–16046
Mori H, Kugisaki H, Inokuchi Y, Nishi N, Miyoshi E, Sakota K, Ohashi K, Sekiya H (2002) LIF and IR dip spectra of jet-cooled p-aminophenol-M (M = CO, N2): hydrogen-bonded or van der waals-bonded structure?. J Phys Chem A 106:4886–4890
Dietrich F, Bernhard D, Fatima M, Pérez C, Schnell M, Gerhards M (2018) The effect of dispersion on the structure of diphenyl ether aggregates. Angew Chem Int Ed Engl 57:9534–9537
Weiler M, Bartl K, Gerhards M (2012) Infrared/ultraviolet quadruple resonance spectroscopy to investigate structures of electronically excited states. J Chem Phys 136:114202–114206
Bartl K, Funk A, Gerhards M (2008) IR/UV spectroscopy on jet cooled 3-hydroxyflavone (H2O)n (n = 1,2) clusters along proton transfer coordinates in the electronic ground and excited states. J Chem Phys 129:234306–234311
Cocinero EJ, Çarçabal P (2015) Rijs AM, Oomens J (eds) In gas-phase IR spectroscopy and structure of biological molecules. Springer, New York, pp 299–334
Barry CS, Cocinero EJ, Çarçabal P, Gamblin DP, Stanca-Kaposta E, Remmert SM, Fernández-Alonso MC, Rudic S, Simons JP, Davis BG (2013) ‘Naked’ and hydrated conformers of the conserved core pentasaccharide of N-linked glycoproteins and its building blocks. J Am Chem Soc 135:16895–16903
Stanca-Kaposta E, Çarçabal P, Cocinero EJ, Hurtado P, Simons JP (2013) Carbohydrate-aromatic interactions: vibrational spectroscopy and structural assignment of isolated monosaccharide complexes with p-hydroxy toluene and N-acetyl l-tyrosine methylamide. J Phys Chem B 117:8135–8142
Aguado E, León I, Cocinero EJ, Lesarri A, Fernández JA, Castaño F (2009) Molecular recognition in the gas phase: benzocaine-phenol as a model qof anaesthetic-receptor interaction. Phys Chem Chem Phys 11:11608–11616
Bhattacherjee A, Wategaonkar S (2016) Water bridges anchored by a C-HO hydrogen bond: the role of weak interactions in molecular solvation. Phys Chem Chem Phys 18:27745–27749
Bhattacherjee A, Wategaonkar S (2015) Conformational preferences of monohydrated clusters of imidazole derivatives revisited. Phys Chem Chem Phys 17:20080–20092
Biswal HS, Gloaguen E, Mons M, Bhattacharyya S, Shirhatti PR, Wategaonkar S (2011) Structure of the indole-benzene dimer revisited. J Phys Chem A 115:9485–9492
Rijs AM and Oomens J (eds) (2015) Gas-phase IR spectroscopy and structure of biological molecules. Springer International Publishing, Heidelberg, New York, Dordrecht, London
de Vries MS (2015) In Rijs MA, Oomens J (eds) Gas-phase ir spectroscopy and structure of biological molecules. Springer International Publishing, Cham, pp 271–297
Jaeqx S, Du WN, Meijer EJ, Oomens J, Rijs AM (2013) Conformational study of Z-Glu-OH and Z-Arg-OH: dispersion interactions versus conventional hydrogen bonding. J Phys Chem A 117:1216–1227
Rijs AM, Kabelac M, Abo-Riziq A, Hobza P, de Vries MS (2011) Isolated gramicidin peptides probed by IR spectroscopy. Chem Phys Chem 12:1816–1821
Rijs AM, Kay ER, Leigh DA, Buma WJ (2011) IR spectroscopy on jet-cooled isolated two-station rotaxanes. J Phys Chem A 115:9669–9675
León I, Cocinero EJ, Rijs AM, Millán J, Alonso E, Lesarri A, Fernández JA (2013) Formation of water polyhedrons in propofol-water clusters. Phys Chem Chem Phys 15:568–575
León I, Cocinero E, Millán J, Jaeqx S, Rijs A, Lesarri A, Castaño F, Fernández JA (2012) Exploring microsolvation of the anesthetics propofol. Phys Chem Chem Phys 14:4398
León I, Cocinero EJ, Millán J, Rijs AM, Usabiaga I, Lesarri A, Castaño F, Fernández JA (2012) A combined spectroscopic and theoretical study of propofol·(H2O)3. J Chem Phys 137:074303
Nakamura T, Schmies M, Patzer A, Miyazaki M, Ishiuchi S, Weiler M, Dopfer O, Fujii M (2014) Solvent migration in microhydrated aromatic aggregates: ionization-induced site switching in the 4-aminobenzonitrile-water cluster. Chemistry 20:2031–2039
Schmies M, Patzer A, Schuetz M, Miyazaki M, Fujii M, Dopfer O (2014) Microsolvation of the acetanilide cation (AA(+)) in a nonpolar solvent: IR spectra of AA(+)-L-n clusters (L = He, Ar, N-2; n <= 10). Phys Chem Chem Phys 16:7980–7995
Nakamura T, Miyazaki M, Ishiuchi S, Weiler M, Schmies M, Dopfer O, Fujii M (2013) IR Spectroscopy of the 4-AminobenzonitrileAr Cluster in the S0, S1 Neutral and D0 Cationic States. Chem Phys Chem 14:741–745
Beckstead AA, Zhang Y, de Vries MS, Kohler B (2016) Life in the light: nucleic acid photoproperties as a legacy of chemical evolution. Phys Chem Chem Phys 18:24228–24238
Kleinermanns K, Nachtigallová D, de Vries MS (2013) Excited state dynamics of DNA bases. Int Rev Phys Chem 32:308–342
Abo-Riziq A, Grace L, Nir E, Kabelac M, Hobza P, de Vries MS (2005) Photochemical selectivity in guanine–cytosine base-pair structures. Proc Natl Acad Sci USA 102:20–23
Shin J, Bernstein ER (2014) Vacuum ultraviolet photoionization of carbohydrates and nucleotides. J Chem Phys 140:044330
Sobolewski AL, Domcke W, Hättig C (2005) Tautomeric selectivity of the excited-state lifetime of guanine/cytosine base pairs: the role of electron-driven proton-transfer processes. Proc Natl Acad Sci USA 102:17903–17906
Zhang W, Yuan S, Wang Z, Qi Z, Zhao J, Dou Y, Lo GV (2011) A semiclassical dynamics simulation for a long-lived excimer state of π-stacked adenines. Chem Phys Lett 506:303–308
Urashima S, Asami H, Ohba M, Saigusa H (2010) Microhydration of the guanine-guanine and guanine-cytosine base pairs. J Phys Chem A 114:11231–11237
McGinty RK, Tan S (2015) Nucleosome structure and function. Chem Rev 115:2255–2273
Goldberg AD, Allis CD, Bernstein E (2007) Epigenetics: a landscape takes shape. Cell 2007(128):635–638
Mondal S, Puranik M (2017) Ultrafast structural dynamics of photoexcited adenine. Phys Chem Chem Phys 19:20224–20240
Schwell M, Hochlaf M (2015) In: Barbatti M, Borin AC, Ullrichin S (eds) Photoinduced phenomena in nucleic acids I: nucleobases in the gas phase and in solvents. Springer International Publishing, Cham, pp 155–208
Sun C, Ding F, Ding Y, Wang C (2015) The nonadditivity of stacking interactions in adenine–thymine and guanine–cytosine stacked structures: Study by MP2 and SCS-MP2 calculations. J Theor Comput Chem 14:1550037
Hunger K, Buschhaus L, Biemann L, Braun M, Kovalenko S, Improta R, Kleinermanns K (2013) UV-light-induced hydrogen transfer in guanosine–guanosine aggregates. Chem A Eur J 19:5425–5431
Mishra D, Pal S (2009) Ionization potential and structure relaxation of adenine, thymine, guanine and cytosine bases and their base pairs: A quantification of reactive sites. J Mol Struct (Thoechem) 902:96–102
Zeleny T, Hobza P, Kabelac M (2009) Microhydration of guanine···cytosine base pairs, a theoretical Study on the role of water in stability, structure and tautomeric equilibrium. Phys Chem Chem Phys 11:3430–3435
Shukla MK, Kuramshina GM, Leszczynski J (2007) Evidence of structural non-planarity in excited state: New findings provided by vibrational analysis of the guanine-cytosine base pair. Chem Phys Lett 447:330–334
Crews B, Abo-Riziq A, Grace L, Callahan M, Kabeláč M, Hobza P, de Vries MS (2005) IR-UV double resonance spectroscopy of guanine-H2O clusters. Phys Chem Chem Phys 7:3015–3020
Jurecka P, Hobza P (2003) True stabilization energies for the optimal planar hydrogen-bonded and stacked structures of guanine···cytosine, adenine···thymine, and their 9- and 1-methyl derivatives: complete basis set calculations at the MP2 and CCSD(T) levels and comparison with experiment. J Am Chem Soc 125:15608–15613
Triandafillou CG, Matsika S (2013) Excited-state tautomerization of gas-phase cytosine. J Phys Chem A 117:12165–12174
Villani G (2005) Theoretical investigation of hydrogen transfer mechanism in the adenine–thymine base pair. Chem Phys 316:1–8
Nir E, Hunig I, Kleinermanns K, de Vries MS (2003) The nucleobase cytosine and the cytosine dimer investigated by double resonance laser spectroscopy and ab initio calculations. Phys Chem Chem Phys 5:4780–4785
Nir E, Janzen C, Imhof P, Kleinermanns K, de Vries MS (2002) Pairing of the nucleobases guanine and cytosine in the gas phase studied by IR-UV double-resonance spectroscopy and ab initio calculations. Phys Chem Chem Phys 4:732–739
Nir E, Janzen C, Imhof P, Kleinermanns K, de Vries MS (2001) Guanine tautomerism revealed by UV-UV and IR-UV hole burning spectroscopy. J Chem Phys 115:4604–4611
Plutzer C, Nir E, de Vries M, Kleinermanns K (2001) IR-UV double-resonance spectroscopy of the nucleobase adenine. Phys Chem Chem Phys 3:5466–5469
Nir E, Kleinermanns K, de Vries MS (2000) Pairing of isolated nucleic-acid bases in the absence of the DNA backbone. Nature 408:949–951
Florián J, Leszczynski J, Scheiner S (1995) Ab initio study of the structure of guanine-cytosine base pair conformers in gas phase and polar solvents. Mol Phys 84:469–480
Asami H, Yagi K, Ohba M, Urashima S, Saigusa H (2013) Stacked base-pair structures of adenine nucleosides stabilized by the formation of hydrogen-bonding network involving the two sugar groups. Chem Phys 419:84–89
Kabelac M, Ryjacek F, Hobza P (2000) Already two water molecules change planar H-bonded structures of the adenine···thymine base pair to the stacked ones: a molecular dynamics simulations study. Phys Chem Chem Phys 2:4906–4909
González J, Usabiaga I, Arnaiz PF, León I, Martínez R, Millán J, Fernández JA (2017) Competition between stacked and hydrogen bonded structures of cytosine aggregates. Phys Chem Chem Phys 19:8826–8834
Ligare MR, Rijs AM, Berden G, Kabeláč M, Nachtigallova D, Oomens J, de Vries MS (2015) Resonant infrared multiple photon dissociation spectroscopy of anionic nucleotide Monophosphate clusters. J Phys Chem B 119:7894–7901
González J, Baños I, León I, Contreras-García J, Cocinero EJ, Lesarri A, Fernández JA, Millán J (2016) Unravelling protein-DNA interactions at molecular level: a DFT and NCI study. J Chem Theory Comput 12:523–534
Ponomarenko EA, Poverennaya EV, Ilgisonis EV, Pyatnitskiy MA, Kopylov AT, Zgoda VG, Lisitsa AV, Archakov AI (2016) The size of the human proteome: the width and depth. Int J Anal Chem 2016:7436849
Ng SBL, Doig AJ (1997) Molecular and chemical basis of prion-related diseases. Chem Soc Rev 26:425
Dill KA, Ozkan SB, Shell MS, Weikl TR (2008) The protein folding problem. Annual Rev Biophys 37:289–316
Eisenberg D (2003) The discovery of the alpha -helix and beta -sheet, the principal structural features of proteins. Proc Natl Acad Sci 100:11207–11210
Feldmann RJ (1989) In Wittmann-Liebold B (eds) Methods in protein sequence analysis. Springer, Berlin, Heidelberg, pp 379–384
Clarke OB, Caputo AT, Hill AP, Vandenberg JI, Smith BJ, Gulbis JM (2010) Domain reorientation and rotation of an intracellular assembly regulate conduction in kir potassium channels. Cell 141:1018–1029
Matthews BW (1972) The gamma turn. evidence for a new folded conformation in proteins. Macromolecules 5:818–819
Forsting T, Gottschalk HC, Hartwig B, Mons M, Suhm MA (2017) Correcting the record: the dimers and trimers of trans-N-methylacetamide. Phys Chem Chem Phys 19:10727–10737
Ishiuchi S, Yamada K, Chakraborty S, Yagi K, Fujii M (2013) Gas-phase spectroscopy and anharmonic vibrational analysis of the 3-residue peptide Z-Pro-Leu-Gly-NH2 by the laser desorption supersonic jet technique. Chem Phys 419:145–152
Chakraborty S, Yamada K, Ishiuchi S, Fujii M (2012) Gas phase IR spectra of tri-peptide Z-Pro-Leu-Gly: effect of C-terminal amide capping on secondary structure. Chem Phys Lett 531:41–45
Schwing K, Fricke H, Bartl K, Polkowska J, Schrader T, Gerhards M (2012) Isolated beta-turn model systems investigated by combined IR/UV spectroscopy. Chem Phys Chem 13:1576–1582
Cocinero EJ, Stanca-Kaposta EC, Gamblin DP, Davis BG, Simons JP (2009) Peptide secondary structures in the gas phase: consensus motif of N-linked glycoproteins. J Am Chem Soc 131:1282–1287
Baquero EE, James WH, Choi SH, Gellman SH, Zwier TS (2008) Single-conformation ultraviolet and infrared spectroscopy of model synthetic foldamers: beta-peptides Ac-beta(3)-hPhe-NHMe and Ac-beta(3)-hTyr-NHMe. J Am Chem Soc 130:4784–4794
Haber T, Seefeld K, Kleinermanns K (2007) Mid- and near-infrared spectra of conformers of H-Pro-Trp-OH. J Phys Chem A 111:3038–3046
Toroz D, Van Mourik T (2006) The structure of the gas-phase tyrosine-glycine dipeptide. Mol Phys 104:559–570
Gregurick SK, Fredj E, Elber R, Gerber RB (1997) Vibrational spectroscopy of peptides and peptide-water complexes: Anharmonic coupled-mode calculations. J Phys Chem B 101:8595–8606
Cable JR, Tubergen MJ, Levy DH (1989) Fluorescence spectroscopy of jet-cooled tryptophan peptides. J Am Chem Soc 111:9032–9039
Gloaguen E, De Courcy B, Piquemal J, Pilmei J, Parisel O, Pollet R, Biswal HS, Piuzzi F, Tardivel B, Broquier M, Mons M (2010) Gas-phase folding of a two-residue model peptide chain: on the importance of an interplay between experiment and theory. J Am Chem Soc 132:11860–11863
Chin W, Piuzzi F, Dognon J, Dimicoli I, Mons M (2005) Gas-phase models of gamma turns: effect of side-chain/backbone interactions investigated by IR/UV spectroscopy and quantum chemistry. J Chem Phys 123:084301
Gloaguen E, Pagliarulo F, Brenner V, Chin W, Piuzzi F, Tardivel B, Mons M (2007) Intramolecular recognition in a jet-cooled short peptide chain: γ-turn helicity probed by a neighbouring residue. Phys Chem Chem Phys 9:4491
Chin W, Dognon J, Piuzzi F, Tardivel B, Dimicoli I, Mons M (2005) Intrinsic folding of small peptide chains: spectroscopic evidence for the formation of beta-turns in the gas phase. J Am Chem Soc 127:707–712
Chin W, Dognon J, Canuel C, Piuzzi F, Dimicoli I, Mons M, Compagnon I, Von Helden G, Meijer G (2005) Secondary structures of short peptide chains in the gas phase: double resonance spectroscopy of protected dipeptides. J Chem Phys 122:054317
Nandel FS, Jaswal R (2007) New type of helix and 27 ribbon structure formation in poly (delta)leu peptides: construction of a single-handed template. Biomacromolecules 8:3093–3101
Chin W, Piuzzi F, Dognon J, Dimicoli I, Tardivel B, Mons M (2005) Gas phase formation of a 310-helix in a three-residue peptide chain: role of side chain-backbone interactions as evidenced by IR-UV double resonance experiments. J Am Chem Soc 127:11900–11901
Fricke H, Funk A, Schrader T, Gerhards M (2008) Investigation of secondary structure elements by IR/UV double resonance spectroscopy: analysis of an isolated (beta)-sheet model system. J Am Chem Soc 130:4692–4698
Gloaguen E, Loquais Y, Thomas JA, Pratt DW, Mons M (2013) Spontaneous formation of hydrophobic domains in isolated peptides. J Phys Chem B 117:4945–4955
Yoon I, Seo K, Lee S, Lee Y, Kim B (2007) Conformational study of tyramine and its water clusters by laser spectroscopy. J Phys Chem A 111:1800–1807
Blom MN, Compagnon I, Polfer NC, von Helden G, Meijer G, Suhai S, Paizs B, Oomens J (2007) Stepwise solvation of an amino acid: the appearance of zwitterionic structures. J Phys Chem A 111:7309–7316
Sakota K, Kouno Y, Harada S, Miyazaki M, Fujii M, Sekiya H (2012) IR spectroscopy of monohydrated tryptamine cation: rearrangement of the intermolecular hydrogen bond induced by photoionization. J Chem Phys 137:224311
Biswal HS, Loquais Y, Tardivel B, Gloaguen E, Mons M (2011) Isolated monohydrates of a model peptide chain: effect of a first water molecule on the secondary structure of a capped phenylalanine. J Am Chem Soc 133:3931–3942
Fricke H, Schwing K, Gerlach A, Unterberg C, Gerhards M (2010) Investigations of the water clusters of the protected amino acid Ac-Phe-OMe by applying IR/UV double resonance spectroscopy: microsolvation of the backbone. Phys Chem Chem Phys 12:3511–3521
Solís D, Bovin NV, Davis AP, Jiménez-Barbero J, Romero A, Roy R, SmetanaJr K, Gabius H (2015) A guide into glycosciences: how chemistry, biochemistry and biology cooperate to crack the sugar code. Biochim Biophys Acta (BBA) General Subj 1850:186–235
Gabius H, André S, Jiménez-Barbero J, Romero A, Solís D (2011) From lectin structure to functional glycomics: principles of the sugar code. Trends Biochem Sci 36:298–313
Grindley TB (2008) In: Fraser-Reid B, Tatsuta K, Thiem J (eds) Springer, Berlin Heidelberg, pp 3–55
Ghazarian H, Idoni B, Oppenheimer SB (2011) A glycobiology review: Carbohydrates, lectins and implications in cancer therapeutics. Acta Histochem 113:236–247
Stanca-Kaposta EC, Gamblin DP, Cocinero EJ, Frey J, Kroemer RT, Fairbanks AJ, Davis BG, Simons JP (2008) Solvent interactions and conformational choice in a core N-glycan segment: gas phase conformation of the central, branching trimannose unit and its singly hydrated complex. J Am Chem Soc 130:10691–10696
Gabius HJ (2000) Biological information transfer beyond the genetic code: the sugar code. Naturwissenschaften 87:108–121
Cocinero EJ, Carcabal P, Vaden TD, Simons JP, Davis BG (2011) Sensing the anomeric effect in a solvent-free environment. Nature 469:76-U1400
Cocinero EJ, Çarçabal P, Vaden TD, Davis BG, Simons JP (2011) Exploring carbohydrate–peptide interactions in the gas phase: structure and selectivity in complexes of pyranosides with N-acetylphenylalanine methylamide. J Am Chem Soc 133:4548–4557
Cocinero EJ, Gamblin DP, Davis BG, Simons JP (2009) The building blocks of cellulose: the intrinsic conformational structures of cellobiose, its epimer, lactose, and their singly hydrated complexes. J Am Chem Soc 131:11117–11123
Cocinero EJ, Stanca-Kaposta EC, Scanlan EM, Gamblin DP, Davis BG, Simons JP (2008) Conformational choice and selectivity in singly and multiply hydrated monosaccharides in the gas phase. Chem A Eur J 14:8947–8955
Simons JP (2009) CARB 24-conformational landscapes of polysaccharides and glycopeptides: spectroscopy and modeling of the building blocks. Mol Phys 107:2435–2458
Jockusch RA, Kroemer RT, Talbot FO, Simons JP (2003) Hydrated sugars in the gas phase: spectroscopy and conformation of singly hydrated phenyl b-d-glucopyranoside. J Phys Chem A 107:10725–10732
Jockusch RA, Talbot FO, Simons JP (2003) Sugars in the gas phase Part 2: the spectroscopy and structure of jet-cooled phenyl small beta]-D-galactopyranoside. Phys Chem Chem Phys 5:1502–1507
Talbot FO, Simons JP (2002) Sugars in the gas phase: the spectroscopy and structure of jet-cooled phenyl small beta]-D-glucopyranoside. Phys Chem Chem Phys 4:3562–3565
Robertson EG, Simons JP (2001) Getting into shape: conformational and supramolecular landscapes in small biomolecules and their hydrated clusters. Phys Chem Chem Phys 3:1–18
Peña I, Cabezas C, Alonso JL (2015) Unveiling epimerization effects: a rotational study of α-d-galactose. Chem Commun 51:10115–10118
Bermúdez C, Peña I, Mata S, Alonso JL (2016) Sweet structural signatures unveiled in ketohexoses. Chem Eur J 22:16829–16837
Alonso ER, Peña I, Cabezas C, Alonso JL (2016) Structural expression of exo-anomeric effect. J Phys Chem Lett 7:845–850
Alonso JL, Lozoya MA, Pena I, Lopez JC, Cabezas C, Mata S, Blanco S (2014) The conformational behaviour of free d-glucose-at last. Chem Sci 5:515–522
Cocinero EJ, Lesarri A, Écija P, Cimas A, Davis BG, Basterretxea FJ, Fernández JA, Castaño F (2013) Free fructose is conformationally locked. J Am Chem Soc 135:2845–2852
Cocinero EJ, Lesarri A, Écija P, Basterretxea FJ, Grabow J, Fernández JA, Castaño F (2012) Ribose found in the gas phase. Angew Chem Int Ed 51:3119–3124
Peña I, Kolesnikova L, Cabezas C, Bermúdez C, Berdakin M, Simao A, Alonso JL (2014) The shape of d -glucosamine. Phys Chem Chem Phys 16:23244–23250
Camiruaga A, Usabiaga I, Insausti A, Cocinero EJ, León I, Fernández JA (2017) Understanding the role of tyrosine in glycogenin. Mol BioSyst 2017(13):1709–1712
Usabiaga I, González J, León I, Arnaiz PF, Cocinero EJ, Fernández JA (2017) Influence of the anomeric effect in the intermolecular interactions of sugars. J Phys Chem Lett 8:1147–1151
Usabiaga I, González J, Arnaiz PF, León I, Cocinero EJ, Fernández JA (2016) Modeling the tyrosine-sugar interactions in supersonic expansions: the glucopyranose-phenol clusters. Phys Chem Chem Phys 18:12457–12465
Su Z, Cocinero EJ, Stanca-Kaposta EC, Davis BG, Simons JP (2009) Carbohydrate-aromatic interactions: a computational and IR spectroscopic investigation of the complex, methyl alpha-L-fucopyranoside center dot toluene, isolated in the gas phase. Chem Phys Lett 471:17–21
Fernández-Alonso del Carmen M, Cañada FJ, Jiménez-Barbero J, Cuevas G (2005) Molecular recognition of saccharides by proteins. insights on the origin of the carbohydrate-aromatic interactions. J Am Chem Soc 127:7379–7386
Simons JP, Davis BG, Cocinero EJ, Gamblin DP, Stanca-Kaposta EC (2009) Conformational change and selectivity in explicitly hydrated carbohydrates. Tetrahedron-Asymmetry 20:718–722
Cocinero EJ, Stanca-Kaposta EC, Dethlefsen M, Liu B, Gamblin DP, Davis BG, Simons JP (2009) Hydration of sugars in the gas phase: regioselectivity and conformational in N-acetyl glucosamine and glucose. Chem A Eur J 15:13427–13434
Usabiaga I, Camiruaga A, Insausti A, Çarçabal P, Cocinero EJ, León I, Fernández JA (2018) Phenyl-(beta)-D-glucopyranoside and phenyl-(beta)-D-galactopyranoside dimers: small structural differences but very different interactions. Front Phys 6:3
Acknowledgements
This work has received funds from MINECO/FEDER (CTQ2015-68148-C2-1-P), MICIU(PGC2018-098561-B-C21) and Basque Government (IT1162-19). Most of the results presented in this work have been possible thanks to the support of the computing infrastructure of the i2BASQUE academic network and the SGI/IZO-SGIker network. I would like to thank the technical support from the personnel from the UPV/EHU laser facility.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fernández, J.A. Exploring Hydrogen Bond in Biological Molecules. J Indian Inst Sci 100, 135–154 (2020). https://doi.org/10.1007/s41745-019-00146-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-019-00146-4