Abstract
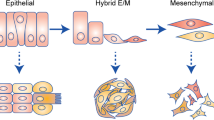
The ability of cells to assume different phenotypes without changing their genotype is referred to as cellular plasticity. It is increasingly being recognized as a fundamental and essential property of cancer cells, which enables their adaptation to the changing environmental conditions, imposed by both disease progression and therapeutic intervention. Epithelial–mesenchymal transition (EMT) is a classical well-studied example of cellular plasticity during cancer progression that aids cancer spread by metastasis. A closely associated phenomenon that entails metastatic progression is the detachment of cancer cells from the extracellular matrix (ECM) at the primary tumor site, their passage and survival in the circulation in an anchorage-independent form, and subsequent re-attachment at a distant site to establish new tumor growth. In this review, we discuss molecular and metabolic plasticity in matrix-attached and -detached states of cancer cells that aid in metastatic cancer progression. Further, cellular plasticity enables cancer cells within a population to assume different phenotypic states, thus leading to cancer heterogeneity—an emerging evil that needs to be tackled for overcoming therapy failure and achieving better treatment outcomes.






Similar content being viewed by others
References
Snell-Rood EC (2012) Selective processes in development: implications for the costs and benefits of phenotypic plasticity. Integr Comp Biol 52:31–42
Hay ED (1995) An overview of epithelio-mesenchymal transformation. Acta Anat 154:8–20
Jia D et al (2017) Distinguishing mechanisms underlying EMT tristability. Cancer Converg 1:1–19
Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science. https://doi.org/10.1126/science.1203543
Thiery JP (2009) Metastasis: alone or Together? Curr Biol 19:R1121–R1123
Senft D, Ronai ZA (2016) Adaptive stress responses during tumor metastasis and dormancy. Trends in Cancer 2:429–442
Thiery JP (2003) Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 15:740–746
Polyak K, Weinberg RA (2009) Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9:265–273
Yang X, Liang X, Zheng M, Tang Y (2018) Cellular phenotype plasticity in cancer dormancy and metastasis. Front Oncol 8:1–12
Hanahan D, Weinberg RA (2011) Review hallmarks of cancer: the next generation. Cell 144:646–674
Frisch SM, Francis H (1994) Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 124:619–626
Geiger B, Spatz JP, Bershadsky AD (2009) Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 10:21–33
Nievers MG, Schaapveld RQJ, Sonnenberg A (1999) Biology and function of hemidesmosomes. Matrix Biol 18:5–17
Barczyk M, Carracedo S (2010) At-a-glance article. Cell Tissue Sci. https://doi.org/10.1007/s00441-009-0834-6
Harburger DS, Calderwood DA (2009) Erratum: Integrin signalling at a glance (Journal of Cell Science vol. 122 (159-163)). J Cell Sci 122:1472
Frisch SM, Ruoslahti E (1997) Integrins and anoikis. Curr Opin Cell Biol 9:701–706
Zhong X, Rescorla FJ (2012) Cell surface adhesion molecules and adhesion-initiated signaling: understanding of anoikis resistance mechanisms and therapeutic opportunities. Cell Signal 24:393–401
Saha M et al (2018) AMPK–Akt double-negative feedback loop in breast cancer cells regulates their adaptation to matrix deprivation. Cancer Res 78:1497–1510
Farris JC et al (2016) Grainyhead-like 2 reverses the metabolic changes induced by the oncogenic epithelial-mesenchymal transition: effects on anoikis. Mol Cancer Res 14:528–538
Huang RYJ et al (2013) An EMT spectrum defines an anoikis-resistant and spheroidogenic intermediate mesenchymal state that is sensitive to e-cadherin restoration by a src-kinase inhibitor, saracatinib (AZD0530). Cell Death Dis 4:e915
Grosse-wilde A, Fouquier A, Mcintosh E, Ertaylan G (2015) Stemness of the hybrid epithelial/mesenchymal state in breast cancer and its association with poor survival. PLoS ONE. https://doi.org/10.1371/journal.pone.0126522
Paoli P, Giannoni E, Chiarugi P (2013) Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta: Mol Cell Res 1833:3481–3498
Jessen KR, Mirsky R, Arthur-Farraj P (2015) The role of cell plasticity in tissue repair: adaptive cellular reprogramming. Dev Cell 34:613–620
Chang-Panesso M, Humphreys BD (2017) Cellular plasticity in kidney injury and repair. Nat Rev Nephrol 13:39–46
Yuan S, Norgard RJ, Stanger BZ (2019) Cellular plasticity in cancer. Cancer Discov 9:837–851
Nieto MA, Huang RYYJ, Jackson RAA, Thiery JPP (2016) Emt: 2016. Cell 166:21–45
Jolly MK et al (2019) Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas. Pharmacol Ther 194:161–184
Kröger C et al (2019) Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc Natl Acad Sci USA 116:7353–7362
Aceto N et al (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158:1110–1122
Lecharpentier A et al (2011) Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br J Cancer 105:1338–1341
Maddipati R, Stanger BZ (2015) Pancreatic cancer metastases harbor evidence of polyclonality. Cancer Discov 5:1086–1097
Mani SA et al (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133:704–715
Zhu Y, Luo M, Brooks M, Clouthier SG, Wicha MS (2014) Biological and clinical significance of cancer stem cell plasticity. Clin Transl Med 3:32
Morel AP et al (2008) Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE 3:1–7
Grosse-Wilde A et al (2015) Stemness of the hybrid epithelial/mesenchymal state in breast cancer and its association with poor survival. PLoS ONE 10:1–28
Pastushenko I et al (2018) Identification of the tumour transition states occurring during EMT. Nature 556:463–468
Boareto M et al (2016) Notch-Jagged signalling can give rise to clusters of cells exhibiting a hybrid epithelial/mesenchymal phenotype. J R Soc Interface 13:20151106
Guo W et al (2012) Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 148:1015–1028
Vesuna F, Lisok A, Kimble B, Raman V (2009) Twist modulates breast cancer stem cells by transcriptional regulation of CD24 expression. Neoplasia 11:1318–1328
Marjanovic ND, Weinberg RA, Chaffer CL (2013) Poised with purpose: cell plasticity enhances tumorigenicity. Cell Cycle 12:2713–2714
Yang G et al (2012) Dynamic equilibrium between cancer stem cells and non-stem cancer cells in human SW620 and MCF-7 cancer cell populations. Br J Cancer 106:1512–1519
Achuthan S, Santhoshkumar TR, Prabhakar J, Nair SA, Pillai MR (2011) Drug-induced senescence generates chemoresistant stemlike cells with low reactive oxygen species. J Biol Chem 286:37813–37829
Chao YL, Shepard CR, Wells A (2010) Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer 9:1–18
Karacosta LG et al (2019) Mapping lung cancer epithelial-mesenchymal transition states and trajectories with single-cell resolution. bioRxiv. https://doi.org/10.1101/570341
Ahmed F, Haass NK (2018) Microenvironment-driven dynamic heterogeneity and phenotypic plasticity as a mechanism of melanoma therapy resistance. Front. Oncol. 8:1–7
Paget S (1889) Distribution of secondary growths in cancer of the breast. Lancet 571–573. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(00)49915-0/fulltext
Watnick RS (2017) The role of the tumor microenvironment in regulating angiogenesis. Biomarkers Tumor Microenviron Basic Stud Pract Appl. https://doi.org/10.1007/978-3-319-39147-2_1
Katsuno Y et al (2019) Inhibition 12, 1–36
Mahmoud SMA et al (2012) Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol 65:159–163
Yang M, Ma B, Shao H, Clark AM, Wells A (2016) Macrophage phenotypic subtypes diametrically regulate epithelial-mesenchymal plasticity in breast cancer cells. BMC Cancer 16:1–13
Frater-Schroder M, Risau W, Hallmann R, Gautschi P, Böhlen P (1987) Tumor necrosis factor type α, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci USA 84:5277–5281
Leibovich SJ et al (1987) Macrophage-induced angiogenesis is mediated by tumour necrosis factor-α. Nature 329:630–632
Baluk P et al (2009) TNF-α drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Investig 119:2954–2964
Wu Y, Zhou P (2009) Stabilization of snail by NF-κB is required for inflammation- induced cell migration and invasion. Cancer Cell 15:416–428
Sanguinetti A, Santini D, Bonafè M, Taffurelli M, Avenia N (2015) Interleukin-6 and pro inflammatory status in the breast tumor microenvironment. World J Surg Oncol 13:4–9
Ferrao P, Behren A, Anderson R, Thompson EW (2015) Editorial: cellular and phenotypic plasticity in cancer. Front Oncol 5:1–3
Hindriksen S, Bijlsma MF (2012) Cancer stem cells, EMT, and developmental pathway activation in pancreatic tumors. Cancers (Basel) 4:989–1035
Sa E et al (2012) EMT-activating transcription factors in cancer : beyond EMT and tumor invasiveness. Cell Mol Life Sci. https://doi.org/10.1007/s00018-012-1122-2
Graff JR, Gabrielson E, Fujii H, Baylin SB, Herman JG (2000) Methylation patterns of the E-cadherin 5′ CpG island are unstable and reflect the dynamic, heterogeneous loss of E-cadherin expression during metastatic progression. J Biol Chem 275:2727–2732
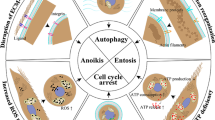
Buchheit CL, Rayavarapu RR, Schafer ZT (2012) The regulation of cancer cell death and metabolism by extracellular matrix attachment. Semin Cell Dev Biol 23:402–411
Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J (2012) Spatiotemporal regulation of EMT is essential for squamous cell carcinoma metastasis. Changes 29:997–1003
Ye X, Weinberg RA (2015) Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol 25:675–686
Shaul YD et al (2014) Dihydropyrimidine accumulation is required for the epithelial-mesenchymal transition. Cell 158:1094–1109
Sciacovelli M, Frezza C (2017) Metabolic reprogramming and epithelial-to-mesenchymal transition in cancer. FEBS J 284:3132–3144
Cufí S et al (2011) Autophagy positively regulates the CD44+ CD24−/low breast cancer stem-like phenotype. Cell Cycle 10:3871–3885
Bocci F et al (2019) NRF2 activates a partial epithelial-mesenchymal transition and is maximally present in a hybrid epithelial/mesenchymal phenotype. Integr Biol (Camb) 11:251–263
Saxena K, Subbalakshmi AR, Jolly MK (2019) Phenotypic heterogeneity in circulating tumor cells and its prognostic value in metastasis and overall survival. EBioMedicine 46:4–5
Gaude E, Frezza C (2016) Tissue-specific and convergent metabolic transformation of cancer correlates with metastatic potential and patient survival. Nat Commun 7:1–9
Papadaki MA et al (2014) Co-expression of putative stemness and epithelial-to-mesenchymal transition markers on single circulating tumour cells from patients with early and metastatic breast cancer. BMC Cancer 14:1–10
Visvader JE, Lindeman GJ (2012) Cancer stem cells: current status and evolving complexities. Cell Stem Cell 10:717–728
Krawczyk N et al (2014) Expression of stem cell and epithelial-mesenchymal transition markers in circulating tumor cells of breast cancer patients. Biomed Res Int 69. https://pubmed.ncbi.nlm.nih.gov/24895575/
De Luca A et al (2015) Mitochondrial biogenesis is required for the anchorage- independent survival and propagation of stem-like cancer cells. Oncotarget 6:14777–14795
Jia D, Park JH, Jung KH, Levine H, Kaipparettu BA (2018) Elucidating the metabolic plasticity of cancer: mitochondrial reprogramming and hybrid metabolic states. Cells. https://doi.org/10.3390/cells7030021
Gradilone A et al (2011) Circulating tumor cells (CTCs) in metastatic breast cancer (MBC): prognosis, drug resistance and phenotypic characterization. Ann Oncol 22:86–92
Agnoletto C et al (2019) Heterogeneity in circulating tumor cells: the relevance of the stem-cell subset. Cancers (Basel) 11:9–12
Charpentier M, Martin S (2013) Interplay of stem cell characteristics, EMT, and microtentacles in circulating breast tumor cells. Cancers (Basel) 5:1545–1565
Gong C et al (2013) Beclin 1 and autophagy are required for the tumorigenicity of breast cancer stem-like/progenitor cells. Oncogene 32:2261–2272
Fernandez-Zapico ME (2013) GLI1 finds a new role in cancer stem cell biology. EMBO Mol Med 5:483–485
Gupta P, Gupta N, Fofaria NM, Ranjan A, Srivastava SK (2019) HER2-mediated GLI2 stabilization promotes anoikis resistance and metastasis of breast cancer cells. Cancer Lett 442:68–81
Schafer ZT et al (2009) Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 461:109–113
Steinberg GR, Kemp BE (2009) AMPK in health and disease. Physiol Rev 89:1025–1078
Jones RG et al (2005) AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 18:283–293
Zhang H, Singh RR, Talukder AH, Kumar R (2006) Metastatic tumor antigen 3 is a direct corepressor of the Wnt4 pathway. Genes Dev 20:2943–2948
Liang J et al (2007) The energy sensing LKB1-AMPK pathway regulates p27kip1 phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol 9:218–224
He X et al (2016) Low expression of long noncoding RNA CASC2 indicates a poor prognosis and regulates cell proliferation in non-small cell lung cancer. Tumor Biol 37:9503–9510
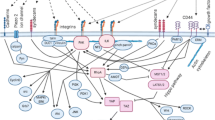
Saxena M et al (2018) AMP-activated protein kinase promotes epithelial-mesenchymal transition in cancer cells through Twist1 upregulation. J Cell Sci. https://doi.org/10.1242/jcs.208314
Gong J et al (2018) Phosphorylation of ULK1 by AMPK is essential for mouse embryonic stem cell self-renewal and pluripotency article. Cell Death Dis 9:1–8
Lahiry M, Rangarajan A (2018) AMPK promotes Notch1 stability to potentiate hypoxia-induced breast cancer aggressiveness. BioRxv. https://www.biorxiv.org/content/10.1101/458489v2
Fung C, Lock R, Gao S, Salas E, Debnath J (2008) Induction of autophagy during extracellular matrix detachment promotes cell survival christopher. Mol Biol Cell 19:797–806
Ng TL et al (2012) The AMPK stress response pathway mediates anoikis resistance through inhibition of mTOR and suppression of protein synthesis. Cell Dealth Diff. https://doi.org/10.1038/cdd.2011.119
Jeon SM, Hay N (2015) The double-edged sword of AMPK signaling in cancer and its therapeutic implications. Arch Pharm Res 38:346–357
Hindupur SK et al (2014) Identification of a novel AMPK-PEA15 axis in the anoikis-resistant growth of mammary cells. Breast Cancer Res 16:420
Sundararaman A, Amirtham U, Rangarajan A (2016) Calcium-oxidant signaling network regulates AMP-activated protein kinase (AMPK) activation upon matrix deprivation. J Biol Chem 291:14410–14429
Jeon SM, Chandel NS, Hay N (2012) AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485:661–665
Jiang P, Du W, Wu M (2014) Regulation of the pentose phosphate pathway in cancer. Protein Cell 5:1–11
Davison CA et al (2013) Antioxidant enzymes mediate survival of breast cancer cells deprived of extracellular matrix. Cancer Res 73:3704–3715
Kumar S (2019) Feedback loops involving AMPK, ERK and TFEB in matrix detachment leads to non- genetic heterogeneity, 5–10. https://www.biorxiv.org/content/10.1101/736546v1
Yeung KT, Yang J (2017) Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol 11:28–39
Acknowledgements
We acknowledge Ms. Neha Deshpande for proofreading the manuscript. The definitions were variably adapted from Encyclopaedia Britannica, Wikipedia, Khan Academy, and Genetics Home Reference.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ranganathan, S., Kumar, S., Mohanty, S.S. et al. Cellular Plasticity in Matrix-attached and -Detached Cells: Implications in Metastasis. J Indian Inst Sci 100, 525–536 (2020). https://doi.org/10.1007/s41745-020-00179-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41745-020-00179-0