Abstract
Water electrolysis is a promising approach for large-scale and sustainable hydrogen production; however, its kinetics is slow and requires precious metal electrocatalysts to efficiently operate. Therefore, great efforts are being undertaken to design and prepare low-cost and highly efficient electrocatalysts to boost the hydrogen evolution reaction (HER). This is because traditional transition-metal electrocatalysts and corresponding hybrids with nonmetal atoms rely mainly on the interaction of metal–H bonds for the HER, which inevitably suffers from corrosion in extreme acidic and alkaline solutions. And as a result of all this effort, novel nanostructured electrocatalysts, such as carbon-encapsulated precious metals and non-precious metals including single metals or their alloys, transition-metal carbides, phosphides, oxides, sulfides, and selenides have all been recently reported to exhibit good catalytic activities and stabilities for hydrogen evolution. Here, the catalytic activity is thought to originate from the electron penetration effect of the inner metals to the surface carbon, which can alter the Gibbs free energy of hydrogen adsorption on the surface of materials. In this review, recent progresses of carbon-encapsulated materials for the HER are summarized, with a focus on the unique effects of carbon shells. In addition, perspectives on the future development of carbon-coated electrocatalysts for the HER are provided.
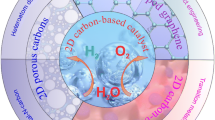
Graphical Abstract
Carbon-encapsulated electrocatalysts, such as carbon-encapsulated precious metals and non-precious metals (single metals or their alloys, metal carbides, phosphides, oxides, sulfides, and selenides), are emerging as promising candidates for water splitting. In this review, recent progresses in carbon-encapsulated electrocatalysts for hydrogen evolution are reviewed, especially the unique effects of carbon shells.



Reprinted with permission from Ref. [31]

Reprinted with permission from Ref. [35]

Reprinted with permission from Ref. [51]

Reprinted with permission from Ref. [65]

Reprinted with permission from Ref. [69]

Reprinted with permission from Ref. [71]

Reprinted with permission from Ref. [82]

Reprinted with permission from Ref. [38]

Reprinted with permission from Ref. [85]

Reprinted with permission from Ref. [90]

Reprinted with permission from Ref. [94]

Reprinted with permission from Ref. [99]

Similar content being viewed by others
References
Owens-Baird, B., Kolen’ko, Y.V., Kovnir, K.: Structure-activity relationships for Pt-free metal phosphide hydrogen evolution electrocatalysts. Chem. Eur. J. 24, 7298–7311 (2018)
Morales-Guio, C.G., Stern, L.A., Hu, X.L.: Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 43, 6555–6569 (2014)
Zhang, L.S., Lu, J.J., Yin, S.B., et al.: One-pot synthesized boron-doped RhFe alloy with enhanced catalytic performance for hydrogen evolution reaction. Appl. Catal. B 230, 58–64 (2018)
Rostrup-Nielsen, J.R., Nielsen, R.: Fuels and energy for the future: the role of catalysis. Catal. Rev. Sci. Eng. 46, 247–270 (2004)
Wang, J., Xu, F., Jin, H.Y., et al.: Non-noble metal-based carbon composites in hydrogen evolution reaction: fundamentals to applications. Adv. Mater. 29, 1605838 (2017)
Conway, B.E., Jerkiewicz, G.: Relation of energies and coverages of underpotential and overpotential deposited H at Pt and other metals to the ‘volcano curve’ for cathodic H2 evolution kinetics. Electrochim. Acta 45, 4075–4083 (2000)
Miao, M., Pan, J., He, T., et al.: Molybdenum carbide-based electrocatalysts for hydrogen evolution reaction. Chem. Eur. J. 23, 10947–10961 (2017)
Liao, L., Wang, S.N., Xiao, J.J., et al.: A nanoporous molybdenum carbide nanowire as an electrocatalyst for hydrogen evolution reaction. Energy Environ. Sci. 7, 387–392 (2014)
Wan, C., Regmi, Y.N., Leonard, B.M.: Multiple phases of molybdenum carbide as electrocatalysts for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 53, 6407–6410 (2014)
Yin, J., Fan, Q.H., Li, Y.X., et al.: Ni–C–N nanosheets as catalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 138, 14546–14549 (2016)
Kou, Z.K., Wang, T.T., Cai, Y., et al.: Ultrafine molybdenum carbide nanocrystals confined in carbon foams via a colloid-confinement route for efficient hydrogen production. Small Methods 2, 1700396 (2018)
Wu, T.L., Pi, M.Y., Zhang, D.K., et al.: Three-dimensional porous structural MoP2 nanoparticles as a novel and superior catalyst for electrochemical hydrogen evolution. J. Power Sources 328, 551–557 (2016)
Zhang, X., Yu, X.L., Zhang, L.J., et al.: Molybdenum phosphide/carbon nanotube hybrids as pH-universal electrocatalysts for hydrogen evolution reaction. Adv. Funct. Mater. 28, 1706523 (2018)
Chang, J.F., Li, S.T., Li, G.Q., et al.: Monocrystalline Ni12P5 hollow spheres with ultrahigh specific surface areas as advanced electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 4, 9755–9759 (2016)
Jin, Y.S., Shen, P.K.: Nanoflower-like metallic conductive MoO2 as a high-performance non-precious metal electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 3, 20080–20085 (2015)
Phuruangrat, A., Dong, J.H., Hong, S.J., et al.: Synthesis of hexagonal WO3 nanowires by microwave-assisted hydrothermal method and their electrocatalytic activities for hydrogen evolution reaction. J. Mater. Chem. 20, 1683–1690 (2010)
Liu, Y., Yin, S.B., Shen, P.K.: Asymmetric 3d electronic structure for enhanced oxygen evolution catalysis. ACS Appl. Mater. Interfaces 10, 23131–23139 (2018)
Zhang, J.Y., Xiao, W., Xi, P.X., et al.: Activating and optimizing activity of CoS2 for hydrogen evolution reaction through the synergic effect of N dopants and S vacancies. ACS Energy Lett. 2, 1022–1028 (2017)
Benck, J.D., Chen, Z.B., Kuritzky, L.Y., et al.: Amorphous molybdenum sulfide catalysts for electrochemical hydrogen production: insights into the origin of their catalytic activity. ACS Catal. 2, 1916–1923 (2012)
Amiinu, I.S., Pu, Z.H., Liu, X.B., et al.: Multifunctional Mo–N/C@MoS2 electrocatalysts for HER, OER, ORR, and Zn–Air batteries. Adv. Funct. Mater. 27, 1702300 (2017)
Gao, M.R., Liang, J.X., Zheng, Y.R., et al.: An efficient molybdenum disulfide/cobalt diselenide hybrid catalyst for electrochemical hydrogen generation. Nat. Commun. 6, 5982 (2015)
Liang, K., Yan, Y., Guo, L.M., et al.: Strained W(SexS1−x)2 nanoporous films for highly efficient hydrogen evolution. ACS Energy Lett. 2, 1315–1320 (2017)
Yu, B., Qi, F., Chen, Y.F., et al.: Nanocrystalline Co0.85Se anchored on graphene nanosheets as a highly efficient and stable electrocatalyst for hydrogen evolution reaction. ACS Appl. Mater. Interfaces 9, 30703–30710 (2017)
Yang, L.J., Zhou, W.J., Jia, J., et al.: Nickel nanoparticles partially embedded into carbon fiber cloth via metal-mediated pitting process as flexible and efficient electrodes for hydrogen evolution reactions. Carbon 122, 710–717 (2017)
Cui, X.J., Ren, P.J., Deng, D.H., et al.: Single layer graphene encapsulating non-precious metals as high-performance electrocatalysts for water oxidation. Energy Environ. Sci. 9, 123–129 (2016)
Zhang, L.L., Xiao, J., Wang, H.Y., et al.: Carbon-based electrocatalysts for hydrogen and oxygen evolution reactions. ACS Catal. 7, 7855–7865 (2017)
Zou, X.X., Zhang, Y.: Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 44, 5148–5180 (2015)
Bockris, J.O.M., Potter, E.C.: The mechanism of the cathodic hydrogen evolution reaction. J. Electrochem. Soc. 99, 169–186 (1952)
Brad, A.J., Faulkner, L.R.: Electrochemical methods: Fundamentals and applications, vol. 60, pp. 669–676. Wiley (1980)
Yan, Y., Xia, B.Y., Xu, Z.C., et al.: Recent development of molybdenum sulfides as advanced electrocatalysts for hydrogen evolution reaction. ACS Catal. 4, 1693–1705 (2014)
Norskov, J.K., Bligaard, T., Logadottir, A., et al.: Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152, J23–J26 (2005)
Greeley, J., Jaramillo, T.F., Bonde, J., et al.: Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 5, 909–913 (2006)
Parsons, R.: The rate of electrolytic hydrogen evolution and the heat of adsorption of hydrogen. Trans. Faraday Soc. 54, 1053–1063 (1958)
Carenco, S., Portehault, D., Boissiere, C., et al.: Nanoscaled metal borides and phosphides: recent developments and perspectives. Chem. Rev. 113, 7981–8065 (2013)
Deng, J., Ren, P.J., Deng, D.H., et al.: Enhanced electron penetration through an ultrathin graphene layer for highly efficient catalysis of the hydrogen evolution reaction. Angew. Chem. Int. Ed. 54, 2100–2104 (2015)
Pu, Z.H., Amiinu, I.S., He, D.P., et al.: Activating rhodium phosphide-based catalysts for the pH-universal hydrogen evolution reaction. Nanoscale 10, 12407–12412 (2018)
Ma, Y.Y., Wu, C.X., Feng, X.J., et al.: Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C. Energy Environ. Sci. 10, 788–798 (2017)
Anjum, M.A.R., Lee, M.H., Lee, J.S.: BCN network-encapsulated multiple phases of molybdenum carbide for efficient hydrogen evolution reactions in acidic and alkaline media. J. Mater. Chem. A 5, 13122–13129 (2017)
Li, X., Yang, L., Su, T., et al.: Graphene-coated hybrid electrocatalysts derived from bimetallic metal-organic frameworks for efficient hydrogen generation. J. Mater. Chem. A 5, 5000–5006 (2017)
Gao, Y., Lang, Z.L., Yu, F.Y., et al.: A Co2P/WC nano-heterojunction covered with N-doped carbon as highly efficient electrocatalyst for hydrogen evolution reaction. ChemSusChem 11, 1082–1091 (2018)
Chen, X.L., Zheng, J., Zhong, X., et al.: Tuning the confinement space of N-carbon shell-coated ruthenium nanoparticles: highly efficient electrocatalysts for hydrogen evolution reaction. Catal. Sci. Technol. 7, 4964–4970 (2017)
Ying, J., Jiang, G.P., Cano, Z.P., et al.: Nitrogen-doped hollow porous carbon polyhedrons embedded with highly dispersed Pt nanoparticles as a highly efficient and stable hydrogen evolution electrocatalyst. Nano Energy 40, 88–94 (2017)
Yang, H.Y., Tang, Z.H., Wang, K., et al.: Co@Pd core–shell nanoparticles embedded in nitrogen-doped porous carbon as dual functional electrocatalysts for both oxygen reduction and hydrogen evolution reactions. J. Colloid Interface Sci. 528, 18–26 (2018)
Li, D.L., Zong, Z., Tang, Z.H., et al.: Total water splitting catalyzed by Co@Ir core–shell nanoparticles encapsulated in nitrogen-doped porous carbon derived from metal organic frameworks. ACS Sustain. Chem. Eng. 6, 5105–5114 (2018)
Chi, J.Q., Gao, W.K., Lin, J.H., et al.: Hydrogen evolution activity of ruthenium phosphides encapsulated in nitrogen- and phosphorous-codoped hollow carbon nanospheres. ChemSusChem 11, 743–752 (2018)
Pu, Z.H., Amiinu, I.S., Kou, Z.K., et al.: RuP2-based catalysts with platinum-like activity and higher durability for the hydrogen evolution reaction at all pH values. Angew. Chem. Int. Ed. 56, 11559–11564 (2017)
Liu, X.R., Zhang, M., Yang, T.T., et al.: Carbon nanofibers as nanoreactors in the construction of PtCo alloy carbon core–shell structures for highly efficient and stable water splitting. Mater. Des. 109, 162–170 (2016)
Zhong, X., Wang, L., Zhuang, Z.Z., et al.: Double nanoporous structure with nanoporous PtFe embedded in graphene nanopores: highly efficient bifunctional electrocatalysts for hydrogen evolution and oxygen reduction. Adv. Mater. Interfaces 4, 1601029 (2017)
Su, J.W., Yang, Y., Xia, G.L., et al.: Ruthenium–cobalt nanoalloys encapsulated in nitrogen-doped graphene as active electrocatalysts for producing hydrogen in alkaline media. Nat. Commun. 8, 14969 (2017)
Xu, Y., Li, Y.H., Yin, S.L., et al.: Ultrathin nitrogen-doped graphitized carbon shell encapsulating CoRu bimetallic nanoparticles for enhanced electrocatalytic hydrogen evolution. Nanotechnology 29, 225403 (2018)
Jiang, P., Chen, J.T., Wang, C.L., et al.: Tuning the activity of carbon for electrocatalytic hydrogen evolution via an iridium–cobalt alloy core encapsulated in nitrogen-doped carbon cages. Adv. Mater. 30, 1705324 (2018)
Li, M., Liu, T.T., Bo, X.J., et al.: A novel flower-like architecture of FeCo@NC-functionalized ultra-thin carbon nanosheets as a highly efficient 3D bifunctional electrocatalyst for full water splitting. J. Mater. Chem. A 5, 5413–5425 (2017)
Zhang, S.Y., Xiao, X.X., Lv, T.T., et al.: Cobalt encapsulated N-doped defect-rich carbon nanotube as pH universal hydrogen evolution electrocatalyst. Appl. Surf. Sci. 446, 10–17 (2018)
Fei, H.L., Yang, Y., Peng, Z.W., et al.: Cobalt nanoparticles embedded in nitrogen-doped carbon for the hydrogen evolution reaction. ACS Appl. Mater. Interfaces 7, 8083–8087 (2015)
Ai, L.H., Tian, T., Jiang, J.: Ultrathin graphene layers encapsulating nickel nanoparticles derived metal-organic frameworks for highly efficient electrocatalytic hydrogen and oxygen evolution reactions. ACS Sustain. Chem. Eng. 5, 4771–4777 (2017)
Li, Y.Y., Li, Z.S., Shen, P.K.: Simultaneous formation of ultrahigh surface area and three-dimensional hierarchical porous graphene-like networks for fast and highly stable supercapacitors. Adv. Mater. 25, 2474–2480 (2013)
Chen, Z.P., Ren, W.C., Gao, L.B., et al.: Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 10, 424–428 (2011)
Zhang, H.B., Ma, Z.J., Duan, J.J., et al.: Active sites implanted carbon cages in core shell architecture: highly active and durable electrocatalyst for hydrogen evolution reaction. ACS Nano 10, 684–694 (2016)
Duan, J.J., Chen, S., Jaroniec, M., et al.: Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal. 5, 5207–5234 (2015)
Zheng, Y., Jiao, Y., Ge, L., et al.: Two-step boron and nitrogen doping in graphene for enhanced synergistic catalysis. Angew. Chem. Int. Ed. 52, 3110–3116 (2013)
Tavakkoli, M., Kallio, T., Reynaud, O., et al.: Single-shell carbon-encapsulated iron nanoparticles: Synthesis and high electrocatalytic activity for hydrogen evolution reaction. Angew. Chem. Int. Ed. 54, 4535–4538 (2015)
Guo, H.L., Youliwasi, N., Zhao, L., et al.: Carbon-encapsulated nickel–cobalt alloys nanoparticles fabricated via new post-treatment strategy for hydrogen evolution in alkaline media. Appl. Surf. Sci. 435, 237–246 (2018)
Feng, X.G., Bo, X.J., Guo, L.P.: CoM(M = Fe, Cu, Ni)-embedded nitrogen-enriched porous carbon framework for efficient oxygen and hydrogen evolution reactions. J. Power Sources 389, 249–259 (2018)
Noh, S.H., Seo, M.H., Kang, J., et al.: Towards a comprehensive understanding of FeCo coated with N-doped carbon as a stable bi-functional catalyst in acidic media. NPG Asia Mater. 8, 312 (2016)
Shen, Y., Zhou, Y.F., Wang, D., et al.: Nickel–copper alloy encapsulated in graphitic carbon shells as electrocatalysts for hydrogen evolution reaction. Adv. Energy Mater. 8, 1701759 (2018)
Liu, P., Rodriguez, J.A.: Catalysts for hydrogen evolution from the NiFe hydrogenase to the Ni2P(001) surface: the importance of ensemble effect. J. Am. Chem. Soc. 127, 14871–14878 (2005)
Zhuang, M.H., Ou, X.W., Dou, Y.B., et al.: Polymer-embedded fabrication of Co2P nanoparticles encapsulated in N, P-doped graphene for hydrogen generation. Nano Lett. 16, 4691–4698 (2016)
Ma, J.W., Wang, M., Lei, G.Y., et al.: Polyaniline derived N-doped carbon-coated cobalt phosphide nanoparticles deposited on N-doped graphene as an efficient electrocatalyst for hydrogen evolution reaction. Small 14, 1702895 (2018)
Yang, Y.Y., Liang, X.Y., Li, F., et al.: Encapsulating Co2P@C core–shell nanoparticles in a porous carbon sandwich as dual-doped electrocatalyst for hydrogen evolution. ChemSusChem 11, 376–388 (2018)
Li, X.Z., Fang, Y.Y., Li, F., et al.: Ultrafine Co2P nanoparticles encapsulated in nitrogen and phosphorus dual-doped porous carbon nanosheet/carbon nanotube hybrids: high-performance bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A 4, 15501–15510 (2016)
Yang, F.L., Chen, Y.T., Cheng, G.Z., et al.: Ultrathin nitrogen-doped carbon coated with CoP for efficient hydrogen evolution. ACS Catal. 7, 3824–3831 (2017)
Pan, Y., Sun, K., Liu, S.J., et al.: Core–shell ZIF-8@ZIF-67-derived CoP nanoparticle-embedded N-doped carbon nanotube hollow polyhedron for efficient overall water splitting. J. Am. Chem. Soc. 140, 2610–2618 (2018)
Wang, R., Dong, X.Y., Du, J., et al.: MOF-derived bifunctional Cu3P nanoparticles coated by a N, P-codoped carbon shell for hydrogen evolution and oxygen reduction. Adv. Mater. 30, 1703711 (2018)
Chung, D.Y., Jun, S.W., Yoon, G., et al.: Large-scale synthesis of carbon-shell-coated FeP nanoparticles for robust hydrogen evolution reaction electrocatalyst. J. Am. Chem. Soc. 139, 6669–6674 (2017)
Yang, J., Ouyang, Y., Zhang, H.J., et al.: Novel Fe2P/graphitized carbon yolk/shell octahedra for high-efficiency hydrogen production and lithium storage. J. Mater. Chem. A 4, 9923–9930 (2016)
Zhao, W.T., Lu, X.Q., Selvaraj, M., et al.: MXP(M = Co/Ni)@carbon core–shell nanoparticles embedded in 3D cross-linked graphene aerogel derived from seaweed biomass for hydrogen evolution reaction. Nanoscale 10, 9698–9706 (2018)
Pu, Z.H., Zhang, C.T., Amiinu, I.S., et al.: General strategy for the synthesis of transition-metal phosphide/N-doped carbon frameworks for hydrogen and oxygen evolution. ACS Appl. Mater. Interfaces 9, 16187–16193 (2017)
Pu, Z.H., Amiinu, I.S., Liu, X.B., et al.: Ultrastable nitrogen-doped carbon encapsulating molybdenum phosphide nanoparticles as highly efficient electrocatalyst for hydrogen generation. Nanoscale 8, 17256–17261 (2016)
Pu, Z.H., Ya, X., Amiinu, I.S., et al.: Ultrasmall tungsten phosphide nanoparticles embedded in nitrogen-doped carbon as a highly active and stable hydrogen-evolution electrocatalyst. J. Mater. Chem. A 4, 15327–15332 (2016)
Zhang, B.W., Lui, Y.H., Gaur, A.P.S., et al.: Hierarchical FeNiP@ultrathin carbon nanoflakes as alkaline oxygen evolution and acidic hydrogen evolution catalyst for efficient water electrolysis and organic decomposition. ACS Appl. Mater. Interfaces 10, 8739–8748 (2018)
Vrubel, H., Hu, X.L.: Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chem. Int. Ed. 51, 12703–12706 (2012)
Kitchin, J.R., Norskov, J.K., Barteau, M.A., et al.: Trends in the chemical properties of early transition metal carbide surfaces: a density functional study. Catal. Today 105, 66–73 (2005)
Ma, R.G., Zhou, Y., Chen, Y.F., et al.: Ultrafine molybdenum carbide nanoparticles composited with carbon as a highly active hydrogen-evolution electrocatalyst. Angew. Chem. Int. Ed. 54, 14723–14727 (2015)
Chi, J.Q., Gao, W.K., Lin, J.H., et al.: Porous core–shell N-doped Mo2C@C nanospheres derived from inorganic-organic hybrid precursors for highly efficient hydrogen evolution. J. Catal. 360, 9–19 (2018)
Chen, Y.Y., Zhang, Y., Jiang, W.J., et al.: Pomegranate-like N, P-doped Mo2C@C nanospheres as highly active electrocatalysts for alkaline hydrogen evolution. ACS Nano 10, 8851–8860 (2016)
Li, J.S., Wang, Y., Liu, C.H., et al.: Coupled molybdenum carbide and reduced graphene oxide electrocatalysts for efficient hydrogen evolution. Nat. Commun. 7, 11204 (2016)
Shi, Z.P., Wang, Y.X., Lin, H.L., et al.: Porous nanoMoC@graphite shell derived from a MOFs-directed strategy: an efficient electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 4, 6006–6013 (2016)
Yang, X.J., Feng, X.J., Tan, H.Q., et al.: N-doped graphene-coated molybdenum carbide nanoparticles as highly efficient electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 4, 3947–3954 (2016)
Levy, R.B., Boudart, M.: Platinum-like behavior of tungsten carbide in surface catalysis. Science 181, 547–549 (1973)
Zhou, Y., Ma, R.G., Li, P.X., et al.: Ditungsten carbide nanoparticles encapsulated by ultrathin graphitic layers with excellent hydrogen-evolution electrocatalytic properties. J. Mater. Chem. A 4, 8204–8210 (2016)
Jin, Y.S., Wang, H.T., Li, J.J., et al.: Porous MoO2 nanosheets as non-noble bifunctional electrocatalysts for overall water splitting. Adv. Mater. 28, 3785–3790 (2016)
Wu, R., Zhang, J.F., Shi, Y.M., et al.: Metallic WO2-carbon mesoporous nanowires as highly efficient electrocatalysts for hydrogen evolution reaction. J. Am. Chem. Soc. 137, 6983–6986 (2015)
Tang, Y.J., Gao, M.R., Liu, C.H., et al.: Porous molybdenum-based hybrid catalysts for highly efficient hydrogen evolution. Angew. Chem. Int. Ed. 54, 12928–12932 (2015)
Jing, S.Y., Lu, J.J., Yu, G.T., et al.: Carbon-encapsulated WOX hybrids as efficient catalysts for hydrogen evolution. Adv. Mater. 30, 1705979 (2018)
Dong, Q.C., Sun, C.C., Dai, Z.Y., et al.: Free-standing NiO@C nanobelt as an efficient catalyst for water splitting. ChemCatChem 8, 3484–3489 (2016)
Liu, Y.Y., Han, G.S., Zhang, X.Y., et al.: Co–Co3O4@carbon core–shells derived from metal-organic framework nanocrystals as efficient hydrogen evolution catalysts. Nano Res. 10, 3035–3048 (2017)
Liu, R.R., Zhang, H.M., Zhang, X., et al.: Co9S8@N, P-doped porous carbon electrocatalyst using biomass-derived carbon nanodots as a precursor for overall water splitting in alkaline media. RSC Adv. 7, 19181–19188 (2017)
Huang, S.C., Meng, Y.Y., He, S.M., et al.: N-, O-, and S-tridoped carbon-encapsulated Co9S8 nanomaterials: efficient bifunctional electrocatalysts for overall water splitting. Adv. Funct. Mater. 27, 1606585 (2017)
Park, S.K., Kang, Y.C.: MOF-templated N-doped carbon-coated CoSe2 nanorods supported on porous CNT microspheres with excellent sodium-ion storage and electrocatalytic properties. ACS Appl. Mater. Interfaces 10, 17203–17213 (2018)
Manikandan, A., Lee, L., Wang, Y.C., et al.: Graphene-coated copper nanowire networks as a highly stable transparent electrode in harsh environments toward efficient electrocatalytic hydrogen evolution reactions. J. Mater. Chem. A 5, 13320–13328 (2017)
Xue, Y.R., Guo, Y., Yi, Y.P., et al.: Self-catalyzed growth of Cu@graphdiyne core shell nanowires array for high efficient hydrogen evolution cathode. Nano Energy 30, 858–866 (2016)
Chen, Z.L., Wu, R.B., Liu, Y., et al.: Ultrafine Co nanoparticles encapsulated in carbon-nanotubes-grafted graphene sheets as advanced electrocatalysts for the hydrogen evolution reaction. Adv. Mater. 30, 1802011 (2018)
Wang, C.D., Jiang, J., Zhou, X.L., et al.: Alternative synthesis of cobalt monophosphide@C core–shell nanocables for electrochemical hydrogen production. J. Power Sources 286, 464–469 (2015)
Cai, Z.X., Xu, W., Li, F.M., et al.: Electropolymerization fabrication of Co phosphate nanoparticles encapsulated in N, P-codoped mesoporous carbon networks as a 3D integrated electrode for full water splitting. ACS Sustain. Chem. Eng. 5, 571–579 (2017)
Hu, Q., Liu, X.F., Tang, C.Y., et al.: Facile fabrication of a 3D network composed of N-doped carbon-coated core–shell metal oxides/phosphides for highly efficient water splitting. Sustain. Energy Fuels 2, 1085–1092 (2018)
Cheng, Z.H., Fu, Q., Han, Q., et al.: A Type of 1 nm molybdenum carbide confined within carbon nanomesh as highly efficient bifunctional electrocatalyst. Adv. Funct. Mater. 28, 1705967 (2018)
Jiang, J., Liu, Q.X., Zeng, C.M., et al.: Cobalt/molybdenum carbide@N-doped carbon as a bifunctional electrocatalyst for hydrogen and oxygen evolution reactions. J. Mater. Chem. A 5, 16929–16935 (2017)
Zhu, J.H., Yao, Y., Chen, Z., et al.: Controllable synthesis of ordered mesoporous Mo2C@graphitic carbon core–shell nanowire arrays for efficient electrocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 10, 18761–18770 (2018)
Wang, H., Sun, C., Cao, Y.J., et al.: Molybdenum carbide nanoparticles embedded in nitrogen-doped porous carbon nanofibers as a dual catalyst for hydrogen evolution and oxygen reduction reactions. Carbon 114, 628–634 (2017)
Hou, J.G., Wu, Y.Z., Cao, S.Y., et al.: Active sites intercalated ultrathin carbon sheath on nanowire arrays as integrated core–shell architecture: highly efficient and durable electrocatalysts for overall water splitting. Small 13, 1702018 (2017)
Zhou, W.J., Lu, J., Zhou, K., et al.: CoSe2 nanoparticles embedded defective carbon nanotubes derived from MOFs as efficient electrocatalyst for hydrogen evolution reaction. Nano Energy 28, 143–150 (2016)
Li, J., Wan, M., Li, T., et al.: NiCoSe2−x/N-doped C mushroom-like core/shell nanorods on N-doped carbon fiber for efficiently electrocatalyzed overall water splitting. Electrochim. Acta 272, 161–168 (2018)
Acknowledgements
This work was supported by the Natural Science Foundation of China (21872040), the Natural Science Foundation of Guangxi (2016GXNSFCB380002), the Major International (Regional) Joint Research Project (U1705252), the National Basic Research Program of China (2017YFB0103000), and the Guangxi Science and Technology Project (AB16380030, 20171107).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lu, J., Yin, S. & Shen, P.K. Carbon-Encapsulated Electrocatalysts for the Hydrogen Evolution Reaction. Electrochem. Energ. Rev. 2, 105–127 (2019). https://doi.org/10.1007/s41918-018-0025-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41918-018-0025-9
Keywords
- Carbon-encapsulated structure
- Hydrogen evolution reaction
- Catalytic mechanism
- Electrocatalyst
- Heteroatoms doping