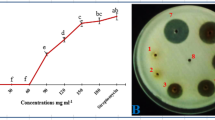
Abstract
Tomato (Solanum lycopersicum L.), one of the most widely grown vegetables worldwide, is susceptible to damping-off and root rot caused by Pythium aphanidermatum (Edson) Fitzpatrick. In in vitro assays, five Trichoderma isolates, viz., Trichoderma harzianum (Th), T. asperellum (Ta), T. virens (Tvs1), T. virens (Tvs2) and T. virens (Tvs3) were compared for their ability to suppress P. aphanidermatum. The mycelial growth of the pathogen was inhibited in vitro after placing each Trichoderma species and isolates on the opposite sides of the same Petri dish. Trichoderma isolates were able not only to arrest the spread of the pathogen but also invade the surface of its colony and sporulate over the colony. Additionally, conidia of Trichoderma isolates were able to inhibit the germination of zoospores of P. aphanidermatum in vitro. Control of tomato damping-off and root rot diseases by soil treatment with the inoculum preparations of Trichoderma isolates employed either alone or in combination was attempted. In greenhouse experiment, the combined inoculation of five Trichoderma isolates suppressed damping-off induced by P. aphanidermatum and increased the survival of tomato plants by 74.5%. In field experiment, the possibility of reducing plant death resulting from root rot disease caused by P. aphanidermatum using Trichoderma isolates, employed either alone or in combination, was investigated. The combined inoculation of five Trichoderma isolates was the most effective treatment, decreasing root rot by 57.2% and increasing the survival of tomato plants by 87.5%. The tested Trichoderma isolates stimulated systemic defence responses in tomato plants grown in the field by activating defence enzymes including peroxidase, polyphenoloxidase and chitinase. Additionally, the chlorophyll contents in the leaves of treated tomato plants were markedly increased. Moreover, the combined inoculation of the five isolates yielded the highest records of growth parameters and fruit yield compared with individual inoculation. Therefore, it was concluded that the mixture containing Trichoderma species and isolates may be used to control damping-off and root rot of tomato caused by Pythium aphanidermatum.
Similar content being viewed by others
References
Abdelzaher HMA (2004) Occurrence of damping-off of wheat caused by Pythium diclinum to kunga in El-minla, Egypt and its possible control by Gliocladium roseum and Trichoderma harzianum. Arch Phytopathol Plant Protect 37:147–159
Alhussaen K, Hussein IE, Al-Batayneh KM, Al-Khatib M, Al Khateeb W, Jocob JH, Shatnawi MA, Khashroum A, Hegazy MI (2011) Identification and controlling Pythium sp. infecting tomato seedlings cultivated in Jordan Valley using garlic extract. Asian J Plant Pathol 5(2):84–92
Altomare C, Novell WA, Bjorkman T, Harman GE (1999) Solubilization of phosphate and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl Environ Microbiol 65:2926–2933
Al-Ezerjawi N H, Kadhim Jamal H (2014) Effect of two isolates of Trichoderma harzianum on total nitrogen, chlorophyll a & b contents and yield of wheat (Triticuma estivum L.) class Eba'a-95. International. J. Sci. Res. 3(8):1078–1083
Anees M, Tronsmo A, Edel-Hermann V, Hjeljord LG, Héraud C, Steinberg C (2010) Characterization of field isolates of Trichoderma antagonistic against Rhizoctonia solani. Fungal Biol 114:691–701
Anonymous (2017) Bulletin of the agricultural statistics, 2nd edn. Ministry of Agriculture and Land Reclamation, Egypt, p 530
Arnon DI (1949) Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol 24:1–15
Bashan Y, Okon Y, Henis Y (1985) Peroxidase, polyphenol oxidase, and phenols in relation to resistance against 214 Pseudomonas syringae pv. tomato in tomato plants. Can J Bot 65:366–372
Bell DK, Wells HD, Markhan CR (1982) In vitro antagonism of Trichoderma species against six fungal pathogens. Phytopathology 72:379–382
Benhamou N, Chet I (1993) Hyphal interaction between Trichoderma harzianum and Rhizoctonia solani: ultrastructure and gold chemistry of the mycoparasitic process. Phytopathology 83:1062–1071
Blanchard LM, Bjorkman T (1996) The role of auxin in enhanced root growth of Trichoderma colonized sweet corn. HortScience 31(68)
Chen R, Harman GE, Afioco MI, Cheng SG (2005) Proteins related to the biocontrol of Pythium damping-off in maize with Trichoderma harzianum. J Integr Plant Biol 47(8):988–997
Christy Jeyaseelan E, Tharmila S, Niranjan K (2012) Antagonistic activity of Trichoderma spp. and Bacillus spp. against Pythium aphanidermatum isolated from tomato damping off. Arch Appl Sci Res 4(4):1623–1627
Corley DG, Miller-Wideman M, Durley RC (1994) Isolation and structure of harzianum : a new trichothecene from Trichoderma harzianum. J Nat Prod 57:422–425
Elad Y (2000) Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop Prot 19:709–714
El-Katatny MH, Gudelj M, Robra KH, Elnaghy MA, Gubitz GM (2001) Characterization of a chitinase and an endo-b-1,3- glucanase from Trichoderma harzianum Rifai T24 involved in control of the phytopathogen Sclerotium rolfsii. Appl Microbiol Biotechnol 56:137–143
Elshahawy IE, Saied N, Abd-El-Kareem F, Morsy A (2017a) Biocontrol of onion white rot by application of Trichoderma species formulated on wheat bran powder. Arch Phytopathol Plant Protect 50(3–4):150–166
Elshahawy IE, Saied N, Morsy A (2017b) Fusarium proliferatum, the main cause of clove rot during storage, reduces clove germination and causes wilt of established garlic plants. J Plant Pathol 99(1):81–89
Elshahawy I, Abouelnasr HM, Lashin SM, Darwesh OM (2018) First report of Pythium aphanidermatum infecting tomato in Egypt and its control using biogenic silver nanoparticles. J Plant Prot Res 58(2):137–151
Eziashi EI, Uma NU, Adekunle AA, Airede CE (2006) Effect of metabolites produced by Trichoderma species against Ceratocystis paradoxa in culture medium. Afr J Biotechnol 5(9):703–706
Faruk MI, Rahman ML (2015) Management of tomato seedling rot disease (Rhizoctonia solani) in seedbed with Trichoderma harzianum bio-fungicide. Agric Sci Res J 5(1):14–20
Fontenelle ADB, Guzzo SD, Lucon CMM, Harakava R (2011) Growth promotion and induction of resistance in tomato plant against Xanthomonas euvesicatoria and Alternaria solani by Trichoderma spp. Crop Prot 30:1492–1500
Gajera H, Domadiya R, Patel S, Kapopara M, Golakiya B (2013) Molecular mechanism of Trichoderma as bio-control agents against phytopathogen system-a review. Curr Res Microbiol Biotechnol 1:133–142
Gams W, Bissett J (1998) Morphology and identification of Trichoderma. In: Kubicek CP, Harman GE (eds) Trichoderma and Gliocladium. Basic biology, taxonomy and genetics. Taylor & Francis, London, pp 3–34
Ghaffer A (1969) Biological control of white rot of onion. I. Interactions of soil microorganisms with Sclerotium cepivorum Berk. Mycopathol Mycol Appl 38:101–111
Graeme-Cook KA, Faull JL (1991) Effect of ultraviolet-induced mutants of Trichoderma harzianum with altered production on selected pathogens in vivo. Can J Microbiol 37:659–664
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species: opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2:43–56
Hexon C, Macias-Rodriguez L, Cortes-Penagos C, López-Bucio J (2009) Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol 149:1579–1592
Hoitink HAJ, Madden LV, Dorrance AE (2006) Systemic resistance induced by Trichoderma spp; interactions between the host, the pathogens, the biocontrol agent and soil organic matter quality. Phytopathology 96(2):186–189
Hopkins WG (1999) Introduction to plant physology. 2nd edn. John Wiley and Sons, Inc. USA
Howell CR (1998) The role of antibiosis in biocontrol. In: Harman GE, Kubicek CP (eds) Trichoderma & Gliocladium, vol. 2. Taylor & Francis, Padstow, 173–184
Howell CR (2002) Cotton seedling preemergence damping-off incited by Rhizopus oryzae and Pythium spp. and its biological control with Trichoderma spp. Phytopathology 92:177–180
Inbar J, Abramsky M, Cohen D, Chet I (1994) Plant growth enhancement and disease control by Trichoderma harzianum in vegetable seedlings growth under commercial conditions. Eur J Plant Pathol 100:337–346
Jalal MAF, Love SK, Vander-Helm D (1987) Siderophore mediated iron III uptake in Gliocladium virens (Trichoderma virens). 2. Role of ferric mono- and dihydroxamates as iron transport agent. J Inorg Biochem 29:259–267
Jayaraj J, Radhakrishnan NV, Kannan R, Sakthivel K, Suganya D, Venkatesan S, Velazhahan R (2005) Development of new formulations of Bacillus subtilis for management of tomato damping-off caused by Pythium aphanidermatum. Biocontrol Sci Tech 15(1):55–65
Jayaraj J, Radhakrishnan NV, Velazhahan R (2006) Development of formulations of Trichoderma harzianum strain M1 for control of damping-off of tomato caused by Pythium aphanidermatum. Arch Phytopathol Plant Protect 39(1):1–8
Jones SW, Donaldson SP, Deacon JW (1991) Behaviour of zoospores and zoospore cysts in relation to root infection by Pythium aphanidermatum. New Phytol 117:289–301
Khare A, Singh BK, Upadhyay RS (2010) Biological control of Pythium aphanidermatum causing damping-off of mustard by mutants of Trichoderma viride 1433. J Agric Technol 6(2):231–243
Kipngeno P, Losenge T, Maina N, Kahangi E, Juma P (2015) Efficacy of Bacillus subtilis and Trichoderma asperellum against Pythium aphanidermatum in tomatoes. Biol Control 90:92–95
Kucuk C, Kvanc M (2003) Isolation of Trichoderma spp. and determination of their antifungal, biochemical and physiological features. Turk J Biol 27(4):247–253
Kullnig-Gradinger CM, Szakacs G, Kubicek CP (2002) Phylogeny and evolution of the genus Trichoderma: a multigene approach. Mycol Res 106:757–767
Le HT, Black LL, Sikora RA (2003) Evaluation of Trichoderma species for biocontrol of tomato sudden death caused by Pythium aphanidermatum following flooding in tropical hot season. Commun Agric Appl Biol Sci 68:463–474
Lee TT (1973) On extraction and quantitation of plant peroxidase isoenzymes. Physiol Plant 29:198–203
Maketon M, Apisitsantikul J, Siriraweekul C (2008) Greenhouse evaluation of Bacillus subtilis AP-01 and Trichoderma harzianum AP-001 in controlling tobacco diseases. Braz J Microbiol 39:296–300
Małolepsza U, Nawrocka J, Szczech M (2017) Trichoderma virens 106 inoculation stimulates defence enzyme activities and enhances phenolic levels in tomato plants leading to lowered Rhizoctonia solani infection. Biocontrol Sci Tech 27(2):180–199
Monfil V O, Casas-Flores S (2014) Molecular mechanisms of biocontrol in Trichoderma spp. and their applications in agriculture. In: Gupta V K, Schmoll M, Herrera-Estrella A, Upadhyay R S, Druzhinina I, Tuohy M G (eds) Biotechnology and biology of Trichoderma. Elsevier ISBN: 978-0-444-59576-8, New York, pp 429–453
Monreal J, Reese ET (1969) The chitinase of Serratia marcescens. Can J Microbiol 15:689–696
Moran R, Porath D (1980) Chlorophyll determination in intact tissues using N,N-dimethylformamide. Plant Physiol 65(3):478–479
Rathore VRS, Mathur K, Hodha BC, Mathur K (1992) Activity of volatile and non volatile substances produced by Trichoderma viride on giner rhizone rot pathogens. Ind Phytopathol 45:253–254
Rini CR, Sulochana KK (2007) Substrate evaluation for multiplication of Trichoderma spp. J Trop Agric 45:58–60
Rudresh DL, Shivaprakash MK, Prasad RD (2005) Effect of combined application of Rhizobium, phosphate solubilizing bacterium and Trichoderma spp. on growth, nutrient uptake and yield of chickpea (Cicer aritenium L.). Appl Soil Ecol 28:139–146
Salas-Marina MA, Silva-Flores MA, Uresti-Rivera EE, Castro-Longoria E, Herrera-Estrella A, Casas-Flores S (2011) Colonization of Arabidopsis roots by Trichoderma atroviride promotes growth and enhances systemic disease resistance through jasmonic acid/ethylene and salicylic acid pathways. Eur J Plant Pathol 131:15–26
Samuels GJ, Chaverr P, Farr DF, McCray EB (2002) Trichoderma online. Systematic mycology and microbiology laboratory, ARS, USDA ; http://nt.ars-grin.gov/taxa descriptions /keys /Trichoderma Index.cfm. Accessed 10 Apr 2018
Sawant I, Mukhopadhyay AN (1990) Integration of metalaxyl with Trichoderma harzainum for the control of Pythium damping-off in sugarbeet. Ind Phytopathol 43:535–541
Schirmbock M, Lorito M, Wang YL, Hayes CK, Arisan-Atac I, Scala F, Harman GE, Kubicek CP (1994) Parallel formation and synergism of hydrolytic enzymes and peptaibol antibiotics, molecular mechanisms involved in the antagonistic action of Trichoderma harzianum against phytopathogenic fungi. Appl Environ Microbiol 60:4364–4370
Segarra G, Avilés M, Casanova E, Borrero C, Trillas I (2013) Effectiveness of biological control of Phytophthora capsici in pepper by Trichoderma asperellum strain T34. Phytopathol Mediterr 52(1):77–83
Singh SP, Singh HB (2014) Effect of mixture of Trichoderma isolates on biochemical parameters in leaf of Macrophomina phaseolina infected brinjal. J Environ Biol 35:871–876
Sivan A, Elad Y, Chet I (1984) Biological control effects of a new isolate of Trichoderma harzianum on Pythium aphanidermatum. Phytopathology 74:498–501
Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Barbetti MJ, Li H, Woo SL, Lorito M (2008) A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol Mol Plant Pathol 72:80–86
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds), PCR Protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Windham MT, Elad Y, Barker R (1986) A mechanism for increased plant growth induced by Trichoderma spp. Phytopathology 76(5):518–521
Zaghloul RA, Hanafy Ehsan A, Neweigy N A, Khalifa Neamat A (2007) Application of biofertilization and biological control for tomato production. In: Zaghloul RA, Hanafy Ehsan A, Neweigy NA, Khalifa Neamat A (eds) 12th Conference of Microbiology (18–22) March Cairo, Egypt, 198–212 pp
Zeilinger S, Omann M (2007) Trichoderma biocontrol: signal transduction pathways involved in host sensing and mycoparasitism. Gene Regul Syst Biol 1:227–234
Zeilinger S, Galhaup C, Payer K, Woo SL, Mach RL, Fekete C, Lorito M, Kubicek CP (1999) Chitinase gene expression during mycoparasitic interaction of Trichoderma harzianum with its host. Fungal Genet Biol 26:131–140
Acknowledgements
The authors thank the Affairs of Research Projects, National Research Centre, for funding this research programme under Grant no. 11030138.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elshahawy, I.E., El-Mohamedy, R.S. Biological control of Pythium damping-off and root-rot diseases of tomato using Trichoderma isolates employed alone or in combination. J Plant Pathol 101, 597–608 (2019). https://doi.org/10.1007/s42161-019-00248-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-019-00248-z