Abstract
The recent outbreak of coronavirus disease (COVID-19) has challenged the survival of human existence in the last 1 year. Frontline healthcare professionals were struggling in combating the pandemic situation and were continuously supported with literature, skill set, research activities, and technologies developed by various scientists/researchers all over the world. To handle the continuously mutating severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requires amalgamation of conventional technology with emerging approaches. Nanotechnology is science, engineering, and technology dealing at the nanoscale level. It has made possible the development of nanomaterials, nano-biosensors, nanodrugs, and vaccines for diagnosis, therapy, and prevention of COVID-19. This review has elaborately highlighted the role of nanotechnology in developing various detection kits such as nanoparticle-assisted diagnostics, antibody assay, lateral flow immunoassay, nanomaterial biosensors, etc., in detection of SARS-CoV-2. Similarly, various advancements supervene through nanoparticle-based therapeutic drugs for inhibiting viral infection by blocking virus attachment/cell entry, multiplication/replication, and direct inactivation of the virus. Furthermore, information on vaccine development and the role of nanocarriers/nanoparticles were highlighted with a brief outlining of nanomaterial usage in sterilization and preventive mechanisms engineered to combat COVID-19 pandemic.
Similar content being viewed by others
1 Introduction
Severe acute respiratory syndrome-CoV-2 (SARS-CoV-2) is a coronavirus with ssRNA as genetic material and has potential to infect human beings and animals. Coronavirus is classified as alpha, beta (β-CoV), gamma, and delta-coronavirus among which SARS-CoV-2 belongs to β-CoV. This nano-sized virus is responsible for COVID-19 which was affirmed as a pandemic by the World Health Organization (WHO) [1]. COVID-19 is highly contagious and transmits from one person to another through respiratory droplets during sneezing, coughing, or talking. Primarily, this virus attaches to the angiotensin-converting enzyme 2 (ACE2) receptor of the host’s epithelial cells through its spike (S) protein and eventually infects the alveoli, trachea, and bronchi of the lungs, heart, kidney, liver, central nervous system, skeletal muscle, adrenal, and thyroid glands [2]. The major symptoms of COVID-19 include, dry cough, running nose, fever, diarrhea, fatigue, and sore throat. The severe conditions result in acute respiratory distress, coagulation dysfunction, septic shock, and death.
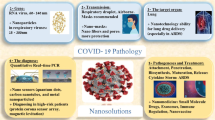
Based on the statistics received from WHO until 29 November 2020, globally, 61.8 million COVID-19 positive cases were reported in which 1.4 million people died [3]. As of 10 November 2020, there was no approved therapy for COVID-19; therefore, focus was laid upon prevention, surveillance, and containment [4]. As a part of prevention, social distancing, sanitizing hands, and wearing masks are being implemented to combat the spread of COVID-19. Several countries like the USA, Japan, China, India, Brazil, and South Korea are trying to use the latest technologies to diminish the effect of COVID-19 and reduce the losses of life as well as economy. Advanced technologies integrated with upgraded information technology improve the quality and affordability of global healthcare system, and reduce the burden of the doctors during pandemic [5,6,7]. Besides, telemedicine was developed using telecommunication technology to treat the patients residing in the remote location [8]. The emerging technologies, viz., nanotechnology, geospatial technology, big data, artificial intelligence, Internet of Medical Things, 5G technology, robotics, and smart applications, are being used for screening, diagnosing, monitoring, infection tracking, mapping, surveillance, and creating awareness [9, 10] (Fig. 1).
Generally, the virus is inanimate outside the host cell but reproduces vigorously inside the host by using its replication machinery. Thus, there is an immense need to know about the interactions between the virus and the host which can be studied by using cutting edge technology with a robust system. Nanotechnology can be an efficient solution for COVID-19 as it was already proven successful against human immunodeficiency virus, herpes simplex, human papilloma virus, and several respiratory viruses [11]. Owing to the role of nanotechnology in treating various viral diseases, the authors attempted to study the application of this technology in COVID-19. The research study has been divided into five sections that include types of nanomaterials, nanotechnology in diagnosis, treatment, and prevention, followed by the conclusion.
2 Nanomaterials
Nanotechnology-based approaches can fight against a pandemic including COVID-19 in several ways. It plays a key role in antiviral research by promoting the delivery of water-soluble drugs, enhancing the circulation time of the drug, improving the drug utilization efficiency by reducing the side effects, and protecting the mRNA and DNA vaccines. Nanomaterial can be used in the delivery of broad-spectrum antiviral drugs/vaccines, detection of infection, fabrication of face masks, surface coatings to resist viral adhesion and its inactivation, and development of tools for contact tracing. Nanomaterials exhibit unique physicochemical properties owing to their miniature size, shape, increased surface area to volume ratio, charge, functional groups, composition which distinguishes them from the bulk materials and render specificity, functionality, sensitivity, and efficiency for biomedical applications [12].
The relative difference between nanotechnology and medicine has therefore lessened and laid the foundation for nanomedicine. Some successful endeavors of nanomedicine are nanoparticles, nanocarriers, nanoemulsions, and immunostimulating complexes. These are used for the drug delivery of active pharmaceutical products which help in the treatment of COVID-19.
2.1 Nanoparticles
Small particles containing a few hundred atoms and size measured in nano-units are called nanoparticles (NPs). These polymeric particles can either originate naturally or engineered by man. These are synthesized by various methods such as spinning, laser pyrolysis, molecular condensation, biological synthesis, mechanical milling, chemical etching, sputtering, and electro-explosion which are considered under bottom-up and top-down synthesis approaches [13]. NPs find their applications in various diverse fields like engineering, medicine, biotechnology, and pharmacology [14]. These particles are composed of three layers; the surface layer is functionalized with different molecules such as metals ions, polymers, and surfactants. The intermediate layer is referred to as the shell which is quite different from the other layers chemically, whereas the innermost layer or central portion is core which is usually referred to as NP also [15]. Several organic and inorganic nanomaterials can be used in the preparation of core and shell which in combination are referred to as nanocomposites. Biological, optical, and physicochemical properties of these nanocomposites depend upon the constituents of core-shell layers. Most of the NPs are invisible to the naked eye and, therefore, exhibit more strength when compared to regular metals. This small size accounts for most of the wondrous properties of nanoparticles. In their free state, nanoparticles show rapid mobility. They absorb large amounts of solar energy and exhibit quantum effects.
NPs are available in various forms like carbon-based nanoparticles, metal nanoparticles, and lipid-based nanoparticles. Carbon nanotubes (CNTs) and fullerenes are two main classes of carbon-based NPs [16]. CNTs generally have tubular and elongated structure 1–2 nm in diameter [17]. These are similar to a graphite sheet roll but may range from single- to multi-walled carbon nanotubes. They are extensively synthesized by deposition of carbon precursors and chemical vapor and finally used in commercial applications such as gas adsorbents and fillers [18].
Metal NPs are made of metal precursors and exhibit localized surface plasmon resonance (SPR) which imparts unique optical properties [19]. These NPs have wide applications in research: for instance, samples of scanning electron microscopy (SEM) are generally coated with gold NPs, to improve the electronic stream, for obtaining high-quality images. Various metals, viz., zinc, copper, and titanium and their oxides, are well known for their anti-viral activities. Besides, ceramic NPs are non-metallic solids synthesized through heating and successive cooling processes. They are available in various forms, hollow, polycrystalline, porous, amorphous, etc., and mostly used in photocatalysis, image processing, etc. [20].
The semiconductor NPs exhibit the properties of both metals and non-metals. These are used in photo-optics, electronic devices, and photocatalysis. Polymer nanoparticle (PNP) is spherical or capsular shaped where overall mass will be solid and the other molecules will be adsorbed onto the surface of the particles. Likewise, nanoliposomes have potential to deliver both lipophilic and hydrophilic antigens. These liposomes exhibit stability, prolonged release, mucous penetrating, and immune cell targeting abilities which make them suitable for drug delivery and cancer therapy [21, 22]. The liposomal vaccine delivery has certain limitations such as high cost, poor stability, and inactivation of phospholipid membrane; and hence, the liposomes have been formulated as bilosomes by incorporating biodegradable bile salts [23].
2.2 Nanoemulsions
Nanoemulsions (NE, 20–200 nm) encompass oil and water which are immiscible with one another. These are stabilized by incorporating required quantity of surfactant and co-surfactants. The advantages of NE lie in easy transcytosis of the lipophilic antigens across the intestinal cells, low production cost, easy manufacturing and storage, increased absorption rate, bioavailability, thermodynamic stability, and solubility of lyophilic drugs. The large surface area to volume ratio of the formulated nanoemulsion improves the antiviral activity of the drug [24].
2.3 Immuno-stimulating complexes
Immuno-stimulating complexes (ISCOMs) are used as vectors for drug delivery. These are nano-sized 30–40 nm in diameter and composed of phospholipids, saponin, cholesterol, and antigens. ISCOM acts as antigen carrier due to its nature and adjuvant effect, and elicits strong humoral and cellular immunity through MHC I and II pathways [25]. ISCOM’s capacity in providing immune response against hepatitis B and orthopneumovirus has been already proved. Oral administration of ISCOMs imparts high immune response [26]. The hydrophilic antigen inclusion into ISCOM is a tedious process but lipophilic antigens can be included easily.
Besides the abovementioned applications, several nanomaterials have already found applications in the detection, therapy, and prevention of the SARS-COV-2 that has been plaguing the general public (Fig. 2) [27]. It is also firmly believed that further advantages of nanomaterials have yet to be tapped into, which might hold the key to bring the infuriating virus under control.
3 Nanomaterials for diagnosis of COVID-19
Early diagnosis of a disease is the key factor to screen symptoms and triage in order to curb the spread of the disease and improving health conditions. Earlier, SARS CoV-2 is detected by computed tomography imaging and electron microscopy [28]. In the later stages, molecular techniques such as reverse transcription polymerase chain reaction (RT-PCR) testing are used to confirm the presence of SARS CoV-2 in the body. This test is earlier used for severe acute respiratory syndrome (SARS) in 2002. RT-PCR-based test takes a long time and has the possibility of false positive in asymptomatic/recovered patients. A recent study shows that chest CT scans are accurate in 98% of cases while RT-PCR detects 71% of cases [29]. This leads to the development of rapid, accurate, and reliable diagnostic tests based on DNA/RNA/protein from blood, stool, serum, and nasopharyngeal swabs. The rapid diagnostic test (RDT) requires 30 min to analyze the samples for interpreting the results. RDT is widely used in the detection of various infections and considered as point-of-care (POC) test. Earlier, RDTs are used for detection of Rotavirus, Adenovirus, Escherichia coli, Salmonella, Plasmodium, and other pathogens.
As the COVID-19 pandemic is vigorously spreading around the globe, refined diagnostics methods should be applied to check the spread of the disease. Nanomaterials are incorporated to produce well-designed and accurate diagnostic devices for identification of SARS-CoV-2. Materials like gold, iron oxide, fluorine-doped tin oxide (FTO), lanthanide-doped polystyrene, graphene, and carboxyl polymer at their nanoform are used in rapid test kits and biosensors for diagnosis of COVID-19 [22]. Gold-based nanomaterials are widely used in diagnosis due to their high plasmon resonance and their sensitivity towards SARS CoV-2. Incorporation of nanoparticles in diagnostic kits provides more accurate and reliable results which will be helpful in triaging the patients.
3.1 Metal and polymeric nanoparticle-assisted diagnosis
Metal nanoparticles like gold, iron oxide, graphene, tin oxide, and polymeric nanoparticles, viz., lanthanide-doped polysterene NPs, carboxyl polymer, and polymer nanoparticles coated with streptavidin dye, are being used in the diagnosis of SARS-CoV-2 [30]. Gold NPs possess high plasmon resonance due to their metallic properties and enable easy detection of RNA, DNA, antigen, and antibody with a sensitivity ranging from 89 to 100% [27]. These are extensively used in 2nd-generation biosensor and found to enhance the interaction between sensor and analyte due to high surface to volume ratios. Gold NPs were operationalized with probes altered with thiols on the surface which bind with the viral genome and also protect the NPs from aggregation. Li et al. [31] have developed a rapid IgM-IgG combined antibody kit by amalgamation of gold NPs for detection of SARS-CoV-2 and found potential screening even in asymptomatic patients. Magnetic nanoparticle-based rapid tests using iron oxide have been developed by Zhao et al. [32], where NPs bind to RNA under magnetic field and helps in rapid and reliable diagnosis.
3.2 Antibody assay kit
As an alternative to PCR, antibody assay kits act as point-of-care diagnostics which helped in earlier determination of various diseases like Rubella, allergy, autoantibodies, hepatitis, HIV, and syphilis. The main principle behind antibody assay kit is the interaction between antigenic epitopes with either IgM or IgG. These antibodies are produced during the primary immune response, wherein IgM contributes the first line of defense and IgG is responsible for the long-term immunity and immunological memory. IgG is detected 7 days after IgM appeared. Antibody testing plays an important role in controlling the spread of the disease as 20–80% of cases are asymptomatic and there is a probability of false positive in PCR. The World Nano Foundation (WNF) has developed second-generation rapid COVID-19 antibody assay kit using gold colloid with separate IgM and IgG readings. Efficacy of the kit is improved by 1000-fold after incorporation of gold NPs, and moreover requires just 3 min and 15 min for positive and negative results respectively [33]. The main drawback of this test is confirming the presence of specific virus; it only confirms infection based on which health professionals can further triage patients. To overcome this drawback, researchers from MIT developed a screening device with an ability to differentiate two similar gene sequences for accurate identification of SARS-CoV-2. This system holds thermoplasmonic heat to create surface plasmon resonance for the DNA probe immobilized on gold NPs to distinguish specific RNA sequence of SARS-CoV-2 [34].
3.3 Lateral flow immunoassay
Lateral flow immunoassay is a diagnostic device used to detect the presence or absence of pathogens in food, water, and biological samples. Lateral flow immunoassay (LFIA) is widely used as POC diagnostics because of its low cost, ease of use, and accessibility—popularly used for pregnancy test [35]. It has shown promising results in detection of enrofloxacin residues in chicken muscle, melamine in milk, olaquindox residues in pig urine, ochratoxin A in wine and C-reactive protein, etc. Chen et al. [36] developed a lanthanide-doped polystyrene nanoparticle–based system which relies on the principle of LFIA and detects anti-SARV-CoV-2 IgG in the serum of COVID-19 patients in less than 10 min. Recombinant nucleocapsid phosphoprotein of coronavirus was immobilized on the membrane to bind with target IgG. Mouse anti-human IgG antibody coated on NPs serves as a fluorescent reporter.
3.4 Naked-eye nanodiagnosis
Naked-eye nanodiagnosis means change in color/nature of test solution in the presence of SARS-CoV-2. This visual change can be identified by any individual without any training and is more approachable as it does not require advanced lab instruments. Earlier, naked-eye diagnosis is used for detection of glutathione, lysozyme, dopamine, melamine, and ions like Ag+, Hg+2, and Mg+2 [37]. Moitra et al. [38] coated gold NPs with thiol-modified antisense oligonucleotide (ASO) with selective affinity towards N gene of SARS-CoV-2. ASO capped AuNPs with viral load is treated with RNase H at 65 °C for 5 min so that a visible precipitate was formed. RNase H cleaves the RNA from RNA and Au-ASO composite, which leads towards precipitation. The cluster of ASO-capped gold particles was studied by UV-visible absorbance spectroscopy and transmission electron microscopy (TEM). This test requires ~ 10 min for determining the total RNA. The reliability of many POC tests is less when compared to naked-eye diagnosis as viral load was treated and the identification of the result is less sophisticated. There is no chance of false positive or false negative in the naked-eye nanodiagnosis system.
3.5 Nanomaterials-based biosensors
A biosensor is an analytical device comprising biomarker, transducer, and a signal amplifier used to detect biomolecules. Biosensors are highly sensitive, cost-effective, and most suitable for diagnosis of the disease. Nanomaterial-based biosensors are more potential towards detection of viral infections and are the best alternative for the PCR-based diagnosis as it takes less time (10–100 min). Layqah et al. [39] reported an electrochemical immunosensor designed employing a group of AuNPs modified carbon electrode to detect recombinant S1 protein for recognition of coronavirus. Seo et al. [40] designed a field effect transistor (FET)–based biosensor using graphene nanosheets to detect SARS-CoV-2 in humans. This was prepared by layering graphene onto SiO2 followed by coating with specific antibodies against spike glycoprotein (S protein) of SARS-CoV-2 with a limit of 2.42 × 102 copies/mL. Nanomaterial-based biosensors have contamination due to highly sensitive bioreceptor and can be addressed through CRISPR or aerosol-mediated approach for quick response and better sensitivity [41].
3.6 Smartphone-assisted sensing
Smartphone-based sensing systems are the developing systems with semi-automated user interface and the accessibility due to less required knowledge and training. By the proper usage of the hardware and software, sensing systems can be developed with smartphones and can be used by normal people. There are numerous ways to connect a peripheral module to the phone. A sensing system can be connected to smartphones by proprietary interfaces, USB communication and power, audio headphone port, Bluetooth, and NFC [42]. Nanomaterials are employed to peripheral devices for fast and better results. Samples are analyzed by using a sensing system and the results are interpreted on the smartphone. It takes less time when compared to other diagnostic tests like PCR. Smartphone-based cloud directory provides geo-tagging. Geolocation can enable the server’s location by adding outbreak information. It helps in real-time surveillance. Smartphone-based sensing can track the spread of the disease and allows formation of a library of data [43].
4 Nanomaterials for treatment of COVID-19
Antiviral drugs are the only medications that are currently available for treatment of COVID-19. Various drugs such as remdesivir, hydrochloroquinone, lopinavir, and ritonavir are being used for treating COVID-19 patients [44]. Nanomaterials are playing a crucial role in antiviral therapy by enhancing the delivery of water-insoluble drugs and improving drug utilization efficiency. The US Food and Drug Administration (FDA)–approved nanomaterials are used in drug delivery in order to have high loading efficiency. Nanoparticle-based drugs can hinder viral infection by blocking virus attachment and its entry into the cell, inhibiting viral multiplication and direct inactivation of virus. Various nanoparticles like gold, silver, silicon, selenium, zinc oxide, silver sulfide, poly lactic acid, etc., are widely used for treatment of COVID-19 [45].
4.1 Blocking of virus attachment
The virus generally enters the host cell by binding to its receptors. In SARS-CoV-2 infection, S protein plays a key role in cell binding and entry. This protein has two subunits: S1 and S2 in which S1 helps in attachment, whereas S2 facilitates membrane fusion and entry into cell. The S1 protein binds to the human ACE2 receptor in alveoli. Besides these cells, ACE2 expression is represented in epithelial cells, esophageal cells, enterocytes, kidney proximal tubule cells, myocardial cells, bladder urothelial cells, and cholangiocytes [46]. Chloroquinone is widely used for blocking viral endocytosis which acts similar to the nanoparticles [47]. Polymeric nanoparticles like polylactic acid are generally used for encapsulation of chloroquinone which improves its delivery and cellular uptake efficacy [47]. Nanoparticles can also directly inhibit the binding of virus. Generally, AgNPs restrict the cell entry of respiratory viruses. Graphene is effective in blocking virus attachment in HIV, whereas AgNPs along with graphene oxide (GO) are effective for feline coronavirus (FCoV) and other enveloped viruses. Owing to the antiviral capacity of graphene, researches foresee that it will play a vital role in the fight against COVID-19 [45]. Gold nanoparticles are more advantageous than silver nanoparticles as they are less toxic. Among the synthetic nanoparticles, SiNPs are more approachable due to their biocompatibility. These are biodegradable and dissolve in water to form non-toxic compounds. SiNPs act as scavengers and prevent virus particles from infecting the host cells. Mesoporous-SiO2 nanoparticles have the potential to bind to enveloped viruses by hydrophobic or hydrophilic bonds and reduce the viral entry to cell [48]. A non-toxic polysaccharide, the cationic chitosan, interacts with S protein of SARS-CoV-2 and blocks the binding of spike protein to the ACE2 receptor. Thus, the nanoparticles can directly hinder the viral attachment to the host and also act as vector for drug delivery [49].
4.2 Inhibition of viral multiplication
After the virus enters the cell, it uses host cell machinery to replicate and increases its number and then spreads the infection throughout the body. By inhibiting the viral multiplication, the viral count will be very low to infect the body, and the immune system can fight efficiently against the virus. Various nanoparticles are used as antivirals for inhibition of viral replication. The transmissible gastroenteritis virus (TGEV) belongs to the coronavirus family and is inhibited by silver nanoparticles and silver nanowires below the toxic level of concentration [50]. Silver nanoparticles reduce the cell death caused by the viral infection. Ag2S are potential nanoparticles that work efficiently on the coronavirus replication by preventing the budding of viral particles from host cell [51]. These nanoclusters also improve the expression of pro-inflammatory cytokines that help in reduction of viral infection. Owing to the structural similarity with coronavirus family, porcine epidemic diarrhea virus (PEDV) is used as a model [52].
PEDV is suppressed by treating with glutathione-capped Ag2S nanoclusters. The principle behind this process is inhibition of RNA synthesis [53]. There are many viruses that are similar to SARS-CoV-2, so these viruses are used as models for research. Influenza A virus (IAV) is rapidly mutated, most complicated, and more resistant to drugs [54]. Nanoparticle-based research helps out in decreasing the effect of virus. Kim and his team developed a technique by using porous gold nanoparticles (PoGNPs) to target hemagglutinin (HA) on the virus based on well-built gold-thiol interactions [55]. The results illustrate that cell viability increases to 96.8% for the cells treated with PoGNPs, whereas untreated cells have only 33.9% viability [55]. Hence, the same technique may be employed for SARS-CoV-2 virus also.
Zinc oxide nanoparticles are more efficient in inhibiting the H1N1 viral infection [56]. It is also proved that zinc (Zn) has the capability of inhibiting the replication of SARS-CoV-2 [56]. Zn helps in producing antiviral cytokines and improves the immune response to reduce inflammation. RNA-dependent RNA polymerase helps in maintaining genome accuracy which is the most important SARS-CoV-2 protein. By inhibiting this protein, the viral replication can be ceased. Earlier, the same technique was used for HIV virus by inhibiting the HIV reverse transcriptase. This technique uses two different aptamer gold nanoparticles: one is specific for polymerase and other is specific for RNaseH of HIV reverse transcriptase [57]. Silver and gold nanoparticles are the most efficient and promising nanostructures for inhibition of viral replication.
4.3 Inactivation of virus
Inactivation or destruction of virus is another approach to cease the viral infection. It is shown that AuNPs coated with 3-mercaptoethylsulfonate (MES) can efficiently inhibit the virus at concentration equal to EC90. After dilution, its viral infectivity was fully recovered which is referred to as reversible viral inhibition. When a 2:1 mixture of undecanesulfonic acid (MUS) and 1-octanethinol (OT) was used instead of MES, then MUS:OT-AuNPs can be used for irreversible inactivation of viruses like vesicular stomatitis virus (VSV), HSV, respiratory syncytial virus (RSV), and lentivirus [45]. The strong binding between virus-MUS:OT-AuNP was affirmed by electron microscopy and other techniques. The viruses that were studied under this technique are similar to coronaviruses, so this technique may be approachable for SARS-CoV-2. Kong et al. [58] illustrated that decoy virus receptor-functionalized nanodisc, self-assembled discoidal phospholipid bilayers in amphipathic membrane scaffold proteins selectively targeted the surface proteins of H1N1 and inactivated the virus irreversibly. Qin and his team showed that iron oxide nanoparticles target 12 different subtypes (H1-H12) of IAV envelope [59]. This process is known as catalytic inactivation. Iron oxide nanoparticles with 200 nm were referred to as iron oxide nanozymes (IONzymes). These IONzymes inactivate the virus by inducing lipid peroxidation which destroys the integrity of the viral envelope proteins. These nanoparticles have greater impact on H1N1, H5N1, and H7N9 and other strains of IAV. Hence, there is every probability that these nanoparticles are also potent enough to inactivate SARS-CoV-2. For instance, inhibition of measles virus is carried out by gold nanoparticles obtained by Allium sativa as a reducing agent, inhibition of hepatitis C virus by AuNP-based nanozymes, inhibition of H1N1 virus by didodecyldimethylammonium bromide-coated silica nanoparticles, inhibition of HIV by silver nanoparticles, etc. [60]. Besides the NPs that can use any one of the three approaches, few researchers used all the three approaches as a combination. Łoczechin and his team modified carbon quantum dots with functional groups, viz., amino, carboxylic, triazole for SARS-CoV-2 virus therapy [61]. These works illustrated the efficiency of various antiviral agents against several viruses which needs an in-depth investigation for their potential against SARS-CoV-2.
5 Nanomaterials for the prevention of COVID-19
5.1 Vaccines
The development of a vaccine for SARS-CoV-2 is a challenging task and various researchers in collaborations with pharma companies initiated this task after the whole genome of SARS-CoV-2 has been published. As per WHO report, until 9 June 2020, there are 136 vaccine candidates, 16 of which are nano-based vaccines under clinical trials [62]. Several studies revealed complete S protein or a region of it (N-terminal domain or receptor-binding domain) as an appropriate target for vaccine development but design of the antigen must be optimized to elicit adequate immune response [63,64,65]. Besides, nucleoproteins and non-structural proteins were found to be good candidates for cocktail vaccine development against SARS-CoV-2 [66].
During vaccine development, the antigenic part of the virus is either encapsulated in the nanocarrier or conjugated to nanoparticle surface for administration along with adjuvant [67, 68]. Various delivery systems, viz., lipid nanoparticles, virosomes, polymeric nanoparticles, virus-like particles, liposomes, emulsions, and immune-stimulating complexes, are being used as antigen carriers. The efficiency of the vaccine can be improved by modifying the size, shape, and charge of the nanoparticles. The vaccine administration could be through intramuscular/subcutaneous injection, oral/intranasal mucosa, or capillary penetration.
Two nanoparticle-based vaccines, BNT162b2 and mRNA-1273, have completed phase III of their clinical trials and geared up for approval from the US Food and Drug Administration. BioNtech and Pfizer declared 95% efficiency of BNT162b2 on 18 November 2020. Similarly, Moderna also revealed their results and claimed 94.5% efficacy of mRNA-1273 [69]. BNT162b2 and mRNA-1273 are the mRNA-based vaccines. BNT162b2 is developed by collaboration of German startup and American pharma, whereas mRNA-1273 is developed by Cambridge-based Biotech Company with National Institutes of Health. Besides the abovementioned technology, several other technologies are being employed in nanoparticle-based vaccine development, the details of which have been summarized in Table 1 [27]. With the information available to date, it can be expected that nanotechnology will fare better than the conventional approaches in terms of quick delivery, safety, and effectiveness.
5.2 Surface decontamination and sanitization
COVID-19 is contagious and transmitted from one person to another through micro-droplets released during sneezing and coughing or by touching the contaminated surfaces. Several studies indicate that SARS-CoV-2 persists for 3 h in aerosolized form and for more than 9 days at 30 °C and above temperatures [70, 71]. In this situation, WHO recommended the use of masks; maintenance of personal hygiene; disinfection of the surfaces like door handles, chairs, switches, tables etc.; using disinfectants such as alcohol, soap, sodium hypochlorite, hydrogen peroxide, etc. [72]. Huang et al. [73] reported that under different operation conditions, deparaffination and alcohol- and water-based disinfectants may not work completely, so there is immense need to develop disinfectants that are non-toxic and persist for longer duration. Nanotechnology opens a new avenue for developing efficient disinfectant systems with antimicrobial activity and self-cleaning ability. The system releases active substance in response to electrothermal, photocatalytic, and photothermal stimuli [74]. Metallic nanoparticles are well known for their antibacterial, antiviral, and antifungal activities [75].
Vaze et al. [76] developed a nano-disinfectant “engineered water nanostructures (EWNS),” which reduced the concentration of H1N1 influenza virus. The Nanotech Surface Company formulated a disinfectant with silver nanoparticles and titanium dioxide which was used for cleaning buildings in Milan. The nanoparticle promotes oxidation reaction that is utilized by light and acts against virus and microbes, hence acting as a potential disinfectant [77]. Despite having various advantages, nano-disinfectant systems have several challenges such as production cost, scalability, toxicity, and intellectual and regulatory issues which hinder them from reaching the markets.
5.3 Air and water filtration
When the SARS-CoV-2 virus was first discovered in China, it was found lurking in air ducts. This proves that the virus can spread through recirculated air in air conditioning [78]. In these places, filters with nanomaterials render hygiene to the conditioning system. High-efficiency particulate air (HEPA) filters provide a handy solution to this. Therefore, isolation areas and hospitals or any enclosed space with air conditioning should be equipped with HEPA filters. The efficiency of HEPA filters lies in the fact that they can filter out particles with sizes of both less than or greater than 300 nm [34]. As the mean diameter of SARS-COV-2 is 50–200 nm, HEPA filters can effectively filter the virus out.
Likewise, the water also should be treated to prevent transmission of the virus. Using a nanotechnology viewpoint, a group of researchers from Rice University, Texas, have developed a multimedia treatment process for both air and water filtration/disinfection. In this, they used a few layers of graphitic carbon nitride set in motion by light which could absorb virus and deteriorate the antibiotic resistance gene of bacteria [79].
5.4 Protective gear
Nanomaterials have long been considered the key to the making of protective gears like masks, lab coats, and aprons as a significant surge in their demand presented after the COVID-19 pandemic. The physicochemical properties of fabrics used in the making of personal protective equipment (PPE) were amplified with the addition of nanomaterials. These amplifications include UV protection, antimicrobial qualities, and fire retardancy, all of which are valuable additions. Nanoengineering has found a significant place in the manufacture of PPE as it provides prevention of antimicrobial activity and hydrophobicity [29]. Despite these enhanced features, it is indeed a marvel that nanoparticles have not affected the breathability of the fabric. Since SARS-COV-2 mainly spreads through respiratory droplets, hydrophobicity lends itself to the apparent augmentation of PPE products. A collection of nanowhiskers made up of hydrocarbons escalate surface tension which intercepts the absorption of droplets.
Oxidation of microbial membrane by nanoscale biocides like polymers or peptides prevents the growth of microbes on the surface of the material. One great example of the vitality of nanoparticles in PPE is in face masks. Conventionally, face masks consist of gaps between fibers which are insufficient for protection against viruses. Moreover, these face masks lack breathability, as a result of which discomfort arises. Nanomaterials combat all these issues. Silver and copper nanomaterials have been touted for their antimicrobial and antiviral effects. Specifically, these have been shown to inactivate influenza virus and also present huge potential for the prevention of SARS-COV-2.
Even though N95 masks are proclaimed as the most effective so far, they only have an 85% efficacy for particles smaller than 300 nm. However, the COVID-19 virus has a diameter range of 60–140 nm, displaying the need for more efficient masks. Nanofibers, produced by electrospinning, offer a potential solution to this predicament [34]. Similarly, gloves infused with silver nanoparticles provide viricidal activity. There are structures coated with ACE2 receptor like nanoflowers with petals containing nanoparticles used in amplifying the enzymatic activity. Human cell membrane, the instinctive target of SARS-COV-2 virus, has been used to create nanosponges which bind to the virus further, neutralizing it in a concentration-dependent manner. Despite the functionality of these strategies, incorporation of ACE2 receptors on masks is not economically viable.
Sulfated derivatives of graphene oxide provides a more feasible alternate. Viruses typically attach to cell surface receptor sugars which can be mimicked by cell antivirals like heparin-sulfate. Recent studies have shown that interaction between S1 protein of SARS-COV-2 and heparin initiates a conformational change in protein. Hence, an amalgamation of heparin and other repurposed sulfates onto graphene oxide nanoparticles can be used to absorb SARS-COV-2 [73]. These NPs can be juxtaposed with fabrics to present another viable option for PPE. There is still a lot of potential in this field with promising developments yet to come. Despite these benefits, nanoparticles may cause allergies for some people. Moreover, when washed for reuse, they may be released as environmental waste. These potential fatalities are being studied for proper disposal of nanoparticle-induced contaminants.
6 Contribution of the study
The present study deals with the intervention of nanotechnology in the diagnosis, treatment, and prevention of COVID-19. This technology is used in the preparation of PPE, disinfectants, and surface coatings to inactivate and check the spread of the virus. Nano-based sensors are sensitive and used in quick identification of infection or immunological response. This technology also contributes to the development of new drugs with improved specificity, and enhanced activity with reduced toxicity.
7 Future scope and limitations
Over the decades, nanoparticles have been extensively studied and employed in the development of safe drugs, tissue-targeted treatments, personalized nanomedicines, early diagnosis, and prevention of diseases. Hence, in a futuristic view, this technology can be the first choice for the development of effective therapies for a range of diseases. As each and every technology has certain challenges, nanotechnology application is also accompanied with a few limitations. Since nanoparticles degrade slowly, there is a probability of their accumulation at the site of administration leading to inflammation and damage of organs. This problem arises when non-biodegradable particles are used. The issue can be overcome by using biodegradable nanoparticles which will be usually excreted from the body. Furthermore, the preparation of nanoparticles is difficult and expensive.
8 Conclusion
The global demand for efficient technology to combat COVID-19 has motivated the researchers and industries to shift from conventional methods towards emerging and smart technology, referred to as nanotechnology. Nano-based approaches are efficient in promoting the drug delivery to a specific target site, improving the residence time and utilizing efficiency of the drug. The nanoparticles, viz., metallic NPs, were found to exhibit antiviral, antibacterial, and antifungal activities, and hence employed in diagnosis, therapy, and designing the preventive aids for SARS-CoV-2. Despite numerous advantages, the toxicity and side effects of the nanomaterials are yet to be fully explored in human health.
References
S. Matsuyama, N. Nao, K. Shirato, M. Kawase, S. Saito, I. Takayama, N. Nagata, T. Sekizuka, H. Katoh, F. Kato, M. Sakata, Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. PNAS 117(13), 7001–7003 (2020)
X. Ou, Y. Liu, X. Lei, P. Li, D. Mi, L. Ren, L. Guo, R. Guo, T. Chen, J. Hu, Z. Xiang, Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 11(1), 1–12 (2020)
WHO, COVID-19 weekly epidemiological update (2020a)
E. Mbunge, Effects of COVID-19 in South African health system and society: an explanatory study. Diab Metab Syndr. 14(6), 1809–1814 (2020)
A. Haleem, M. Javaid, Medical 4.0 and its role in healthcare during COVID-19 pandemic: a review. J. Ind. Integr. Manag 5(4), 531–545 (2020)
M.I.U. Haq, S. Khuroo, A. Raina, S. Khajuria, M. Javaid, M.F.U. Haq, A. Haleem, 3D printing for development of medical equipment amidst coronavirus (COVID-19) pandemic-review and advancements. Res. Biomed. Eng, 1–11 (2020)
M. Javaid, A. Haleem, R.P. Singh, M.I.U. Haq, A. Raina, R. Suman, Industry 5.0: potential applications in COVID-19. J. Ind. Integr. Manag 5(04), 507–530 (2020)
S. Bahl, R.P. Singh, M. Javaid, I.H. Khan, R. Vaishya, R. Suman, Telemedicine technologies for confronting COVID-19 pandemic: a review. J. Ind. Integr. Manag. 5(4), 547–561 (2020)
R.P. Singh, M. Javaid, A. Haleem, R. Suman, Internet of Things (IoT) applications to fight against COVID-19 pandemic. Diabetes Metab. Syndr. 14(4), 521–524 (2020)
R.P. Singh, M. Javaid, A. Haleem, R. Vaishya, S. Al, Internet of Medical Things (IoMT) for orthopaedic in COVID-19 pandemic: roles, challenges, and applications. J. Clin. Orthop. Trauma. 11(4), 713–717 (2020)
T. Yadavalli, D. Shukla, Role of metal and metal oxide nanoparticles as diagnostic and therapeutic tools for highly prevalent viral infections. Nanomedicine 13(1), 219–230 (2017)
S.R. Guntur, N.S. Sampath Kumar, M. Manasa Hegde, R.D. Vijaya, In vitro studies of the antimicrobial and free-radical scavenging potentials of silver nanoparticles biosynthesized from the extract of Desmostachya bipinnata. Anal. Chem. Insights 13, 1177390118782877 (2018)
I. Khan, K. Saeed, I. Khan, Nanoparticles: properties, applications and toxicities. Arab. J. Chem. 12(7), 908–931 (2019)
A.D. Chintagunta, A. Kumar, S.J. Kumar, M.L. Verma, in Metal and metal oxides for energy and electronics. Contribution of metallic nanomaterials in algal biofuel production (Springer, Cham, 2020), pp. 331–353
W.K. Shin, J. Cho, A.G. Kannan, Y.S. Lee, D.W. Kim, Cross-linked composite gel polymer electrolyte using mesoporous methacrylate-functionalized SiO2 nanoparticles for lithium-ion polymer batteries. Sci. Rep. 6, 26332 (2016)
A. Astefanei, O. Núñez, M.T. Galceran, Characterisation and determination of fullerenes: a critical review. Anal. Chim. Acta 882, 1–21 (2015)
K.S. Ibrahim, Carbon nanotubes-properties and applications: a review. Carbon lett. 14(3), 131–144 (2013)
J.M. Ngoy, N. Wagner, L. Riboldiand, O. Bolland, A CO2 capture technology using multi-walled carbon nanotubes with polyaspartamide surfactant. Energy Procedia 63, 2230–2248 (2014)
E.C. Dreaden, A.M. Alkilany, X. Huang, C.J. Murphy, M.A. El-Sayed, The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 41(7), 2740–2779 (2012)
C.S. Thomas, P. Kumar Mishra, S. Talegaonkar, Ceramic nanoparticles: fabrication methods and applications in drug delivery. Curr. Pharm. Des. 21(42), 6165–6188 (2015)
M.K. Rawat, A. Jain, S. Singh, Studies on binary lipid matrix based solid lipid nanoparticles of repaglinide: in vitro and in vivo evaluation. J. Pharm. Sci. 100(6), 2366–2378 (2011)
M. Gujrati, A. Malamas, T. Shin, E. Jin, Y. Sun, Z.R. Lu, Multifunctional cationic lipid-based nanoparticles facilitate endosomal escape and reduction-triggered cytosolic siRNA release. Mol. Pharm. 11(8), 2734–2744 (2014)
H. Choudhury, B. Gorain, B. Chatterjee, U.K. Mandal, P. Sengupta, R.K. Tekade, Pharmacokinetic and pharmacodynamic features of nanoemulsion following oral, intravenous, topical and nasal route. Curr. Pharm. Des. 23(17), 2504–2531 (2017)
F.D. Cojocaru, D. Botezat, I. Gardikiotis, C.M. Uritu, G. Dodi, L. Trandafir, C. Rezus, E. Rezus, B.I. Tamba, C.T. Mihai, Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics 12(2), 171 (2020)
W.T. McBurney, D.G. Lendemans, J. Myschik, T. Hennessy, T. Rades, S. Hook, In vivo activity of cationic immune stimulating complexes (PLUSCOMs). Vaccine 26(35), 4549–4556 (2008)
E.M. Pridgen, F. Alexis, O.C. Farokhzad, Polymeric nanoparticle technologies for oral drug delivery. Clin. Gastroenterol. Hepatol. 12(10), 1605–1610 (2014)
E.V. Campos, A.E. Pereira, J.L. De Oliveira, L.B. Carvalho, M. Guilger-Casagrande, R. De Lima, L.F. Fraceto, How can nanotechnology help to combat COVID-19? Opportunities and urgent need. J. Nanobiotechnology 18(1), 1–23 (2020)
B. Udugama, P. Kadhiresan, H.N. Kozlowski, A. Malekjahani, M. Osborne, V.Y. Li, H. Chen, S. Mubareka, J.B. Gubbay, W.C. Chan, Diagnosing COVID-19: the disease and tools for detection. ACS Nano 14(4), 3822–3835 (2020)
Y. Fang, H. Zhang, J. Xie, M. Lin, L. Ying, P. Pang, W. Ji, Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology 2004, 32 (2020)
M. Srivastava, N. Srivastava, P.K. Mishra, B.D. Malhotra, Prospects of nanomaterials-enabled biosensors for COVID-19 detection. Sci. Total Environ. 754, 142363 (2021)
Z. Li, Y. Yi, X. Luo, N. Xiong, Y. Liu, S. Li, R. Sun, Y. Wang, B. Hu, W. Chen, Y. Zhang, Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. (2020). https://doi.org/10.1002/jmv.25727
Z. Zhao, H. Cui, W. Song, X. Ru, W. Zhou, X. Yu, A simple magnetic nanoparticles-based viral RNA extraction method for efficient detection of SARS-CoV-2. Mol. Biol. 8, 96126 (2020). https://doi.org/10.1101/2020.02.22.96126
The World Nano Foundation (TWNF). Use of gold nanoparticles is the key advantage of 2nd generation Covid-19 rapid antibody Tests. 2020. Available from: https://static1.squarespace.com/static/5ad8857175f9ee9687e844b6/t/5ea6a8f1c522de16ff69b5ad/1587980529949/White+Paper+Gen+2+Nano+particles+explanation+v4.4.pdf
S. Adhikari, U. Adhikari, A. Mishra, B.S. Guragain, Nanomaterials for diagnostic, treatment and prevention of COVID-19. Appl. Sci. Technol. Annals 1(1), 155–164 (2020)
H. Liu, E. Dai, R. Xiao, Z. Zhou, M. Zhang, Z. Bai, Y. Shao, K. Qi, J. Tu, C. Wang, S. Wang, Development of a SERS-based lateral flow immunoassay for rapid and ultra-sensitive detection of anti-SARS-CoV-2 IgM/IgG in clinical samples. Sensors Actuators B Chem. 2020, 129196 (2020)
Z. Chen, Z. Zhang, X. Zhai, Y. Li, L. Lin, H. Zhao, L. Bian, P. Li, L. Yu, Y. Wu, G. Lin, Rapid and sensitive detection of anti-SARS-CoV-2 IgG, using lanthanide-doped nanoparticles-based lateral flow immunoassay. Anal. Chem. 92, 7226–7231 (2020)
M.R. Awual, M.M. Hasan, J. Iqbal, A. Islam, M.A. Islam, A.M. Asiri, M. Rahman, Naked-eye lead (II) capturing from contaminated water using innovative large-pore facial composite materials. Microchem. J. 154, 104585 (2020)
P. Moitra, M. Alafeef, K. Dighe, M.B. Frieman, D. Pan, Selective nakedeye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano 14, 7617–7627 (2020)
L.A. Layqah, S. Eissa, An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchim. Acta 186, 224 (2019)
G. Seo, G. Lee, M.J. Kim, S.H. Baek, M. Choi, K.B. Ku, C.S. Lee, S. Jun, D. Park, H.G. Kim, S.J. Kim, Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano 14(5), 135–142 (2020)
T.M. Nguyen, Y. Zhang, P.P. Pandolfi, Virus against virus: a potential treatment for 2019-nCov (SARS-CoV-2) and other RNA viruses. Cell Res. 30, 189–190 (2020)
P. Chandra, Miniaturized label-free smartphone assisted electrochemical sensing approach for personalized COVID-19 diagnosis. Sens. Int. 1, 100019 (2020)
C. Lellis-Santos, F. Abdulkader, Smartphone-assisted experimentation as a didactic strategy to maintain practical lessons in remote education: alternatives for physiology education during the COVID-19 pandemic. Adv. Physiol. Educ. 44(4), 579–586 (2020)
K. Uzunova, E. Filipova, V. Pavlova, T. Vekov, Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2. Biomed. Pharmacother. 2020, 110668 (2020)
V. Palmieri, M.J.N.T. Papi, Can graphene take part in the fight against COVID-19? Nano Today 33, 100883 (2020)
H. Xu, L. Zhong, J. Deng, J. Peng, H. Dan, X. Zeng, T. Li, Q. Chen, High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 12(1), 1–5 (2020)
A. Sánchez, S.P. Mejía, J. Orozco, Recent Advances in polymeric nanoparticle-encapsulated drugs against intracellular infections. Molecules 25(16), 3760 (2020)
J. Jampílek, K. Kráľová, in Nanotheranostics. Nanoformulations: a valuable tool in the therapy of viral diseases attacking humans and animals (Springer, Cham, 2019), pp. 137–178
M. Chakravarty, A. Vora, Nanotechnology-based antiviral therapeutics. Drug Deliv. Transl. Res. 2020, 1–40 (2020)
E. Alphandéry, The potential of various nanotechnologies for coronavirus diagnosis/treatment highlighted through a literature analysis. Bioconjug. Chem. 31(8), 1873–1882 (2020)
S. Gurunathan, M. Qasim, Y. Choi, J.T. Do, C. Park, K. Hong, J.H. Kim, H. Song, Antiviral potential of nanoparticles-can nanoparticles fight against coronaviruses? Nanomaterials 10(9), 1645 (2020)
K. Jung, L.J. Saif, Q. Wang, Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 198045 (2020)
J. Du, J. Luo, J. Yu, X. Mao, Y. Luo, P. Zheng, J. He, B. Yu, D. Chen, Manipulation of intestinal antiviral innate immunity and immune evasion strategies of porcine epidemic diarrhea virus. Biomed. Res. Int. 2019, 9 (2019)
W. Shao, X. Li, M.U. Goraya, S. Wang, J.L. Chen, Evolution of influenza a virus by mutation and re-assortment. Int. J. Mol. Sci. 18(8), 1650 (2017)
J. Kim, M. Yeom, T. Lee, H.O. Kim, W. Na, A. Kang, J.W. Lim, G. Park, C. Park, D. Song, S. Haam, Porous gold nanoparticles for attenuating infectivity of influenza A virus. J. Nanobiotechnol. 18(1), 1–11 (2020)
M.T. Rahman, S.Z. Idid, Can Zn Be a Critical Element in COVID-19 Treatment? Biol. Trace Elem. Res. 2020, 1–9 (2020)
S. Giorgi-Coll, M.J. Marín, O. Sule, P.J. Hutchinson, K.L. Carpenter, Aptamer-modified gold nanoparticles for rapid aggregation-based detection of inflammation: an optical assay for interleukin-6. Microchim. Acta 187(1), 13 (2020)
B. Kong, S. Moon, Y. Kim, P. Heo, Y. Jung, S.H. Yu, J. Chung, C. Ban, Y.H. Kim, P. Kim, B.J. Hwang, Virucidal nano-perforator of viral membrane trapping viral RNAs in the endosome. Nat. Commun. 10(1), 1–10 (2019)
T. Qin, R. Ma, Y. Yin, X. Miao, S. Chen, K. Fan, J. Xi, Q. Liu, Y. Gu, Y. Yin, J. Hu, Catalytic inactivation of influenza virus by iron oxide nanozyme. Theranostics 9(23), 6920 (2019)
M.A. Meléndez-Villanueva, K. Morán-Santibañez, J.J. Martínez-Sanmiguel, R. Rangel-López, M.A. Garza-Navarro, C. Rodríguez-Padilla, D.G. Zarate-Triviño, L.M. Trejo-Ávila, Virucidal activity of gold nanoparticles synthesized by green chemistry using garlic extract. Viruses 11(12), 1111 (2019)
A. Łoczechin, K. Séron, A. Barras, E. Giovanelli, S. Belouzard, Y.T. Chen, N. Metzler-Nolte, R. Boukherroub, J. Dubuisson, S. Szunerits, Functional carbon quantum dots as medical countermeasures to human coronavirus. ACS Appl. Mater. Interfaces 11(46), 42964–42974 (2019)
WHO. Draft landscape of COVID-19 candidate vaccines (2020b) https://www.who.int/who-documents-detail-redirect/draft-landscape-of-covid-19-candidate-vaccines.
N. Lurie, M. Saville, R. Hatchett, J. Halton, Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med. 382(21), 1969–1973 (2020)
F. Amanat, F. Krammer, SARS-CoV-2 vaccines: status report. Immunity 52, 583–589 (2020)
L. Du, Y. He, Y. Zhou, S. Liu, B.J. Zheng, S. Jiang, The spike protein of SARS-CoV-a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 7(3), 226–236 (2009)
E. Ong, M.U. Wong, A. Huffman, Y. He, COVID-19 coronavirus vaccine design using reverse vaccinology and machine learning. BioRxiv (2020). https://doi.org/10.1101/2020.03.20.000141
A.D. Chintagunta, M. Kumar, N.S. Kumar, S.J. Kumar, in Diagnostic strategies for COVID-19 and other coronaviruses. Differential diagnosis and possible therapeutics for coronavirus disease 2019 (Springer, Singapore, 2020b), pp. 51–71
N.S.S. Kumar, A.D. Chintagunta, S.J. Kumar, S. Roy, M. Kumar, Immunotherapeutics for Covid-19 and post vaccination surveillance. 3 Biotech 10(12), 1–11 (2020)
Nanomedicine and the COVID-19 vaccines. Nat. Nanotechnol. 15, 963 (2020). https://doi.org/10.1038/s41565-020-00820-0
N. Van Doremalen, T. Bushmaker, D.H. Morris, M.G. Holbrook, A. Gamble, B.N. Williamson, A. Tamin, J.L. Harcourt, N.J. Thornburg, S.I. Gerber, J.O. Lloyd-Smith, Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 382(16), 1564–1567 (2020)
G. Kampf, Potential role of inanimate surfaces for the spread of coronaviruses and their inactivation with disinfectant agents. Infect Prev Pract. 2, 100044 (2020)
A. Kapoor, R. Saha, Hand washing agents and surface disinfectants in times of coronavirus (COVID-19) outbreak. Indian J. Community Health 32, 225–227 (2020)
H. Huang, C. Fan, M. Li, H.L. Nie, F.B. Wang, H. Wang, R. Wang, J. Xia, X. Zheng, X. Zuo, J. Huang, COVID-19: a call for physical scientists and engineers. ACS Nano 14(4), 3747–3754 (2020)
S.P. Dalawai, M.A. Saad Aly, S.S. Latthe, R. Xing, R.S. Sutar, S. Nagappan, C.S. Ha, K.K. Sadasivuni, S. Liu, Recent advances in durability of superhydrophobic self-cleaning technology: a critical review. Prog. Org. Coat. 138, 105381 (2020)
M. Rai, S.D. Deshmukh, A.P. Ingle, I.R. Gupta, M. Galdiero, S. Galdiero, Metal nanoparticles: the protective nanoshield against virus infection. Crit. Rev. Microbiol. 42, 46–56 (2016)
N. Vaze, G. Pyrgiotakis, J. McDevitt, L. Mena, A. Melo, A. Bedugnis, L. Kobzik, M. Eleftheriadou, P. Demokritou, Inactivation of common hospital acquired pathogens on surfaces and in air utilizing engineered water nanostructures (EWNS) based nano-sanitizers. Nanomed. Nanotechnol. Biol. Med. 18, 234–242 (2019)
StatNano. Mineral nanocrystal-based coating activated by light kills coronavirus | STATNANO (2020) https://statnano.com/news/67583/Mineral-Nanocrystal-based-Coating-Activated-by-Light-Kills-Coronavirus.
J. Lu, J. Gu, K. Li, C. Xu, W. Su, Z. Lai, D. Zhou, C. Yu, B. Xu, Z. Yang, COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. 26(7), 1628 (2020)
Q. Yuan, D. Zhang, P. Yu, R. Sun, H. Javed, G. Wu, P.J. Alvarez, Selective adsorption and photocatalytic degradation of extracellular antibiotic resistance genes by molecularly-imprinted graphitic carbon nitride. Environ. Sci. Technol. 54(7), 4621–4630 (2020)
Acknowledgements
The authors are grateful to Management, VFSTR, India, for extending their kind support.
Author information
Authors and Affiliations
Contributions
Anjani Devi Chintagunta: resources, writing-original draft. N.S. Sampath Kumar: conceptualization, reviewing, editing. M. Sai Krishna: writing-original draft. Sanjana Nalluru: writing-original draft
Corresponding author
Ethics declarations
The authors express their consent for communicating this article to your journal and, moreover, confirm that this article has not been communicated to any other journal for publication.
Ethics approval
This article does not contain any studies with human or animal subjects
Conflict of interest
The authors have no competing interests.
Rights and permissions
About this article
Cite this article
Chintagunta, A.D., M, S., Nalluru, S. et al. Nanotechnology: an emerging approach to combat COVID-19. emergent mater. 4, 119–130 (2021). https://doi.org/10.1007/s42247-021-00178-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-021-00178-6