Abstract
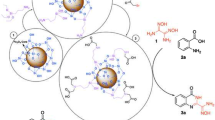
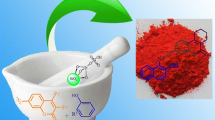
Novel water soluble triazene dyes based on glyco-conjugated pyrazolone amines were synthetized by using N-propyl-2-pyrrolidonium hydrogen sulfate supported on silica-coated magnetite nanoparticle as acid catalyst. This organic–inorganic hybrid composite showed an excellent catalytic activity toward diazotization reaction of pyrazolone amines performing triazene dyes under solvent-free conditions in high yields. The spectroscopic properties confirmed the tendency of these dyes to exist in their hydrazo-keto tautomer forms. The study of the solubility of these dyes in water showed that their water solubility requires a minimum percentage weight of 50% of the carbohydrate part.
Graphic Abstract







Similar content being viewed by others
References
Griess P, Liebiegs J (1862) Ann Chem 121:258
Zollinger H. (1994) Diazo chemistry I, aromatic and heteroaromatic compounds. Wiley VCH, Weinheim, p 391–444 (chapter 13.2)
Kimball DB, Haley MM (2002) Angew Chem Int Ed 41:3338–3351
Newlands ES, Stevens MFG, Wedge SR, Wheelhouse RT, Brock C (1997) Cancer Treat Rev 23:35–61
Wanner MJ, Koch M, Koomen GJ (2004) J Med Chem 47:6875–6883
Nicolaou KC, Boddy CNC, Li H, Koumbis AE, Hughes R, Natarajan S (1999) Chem Eur J 5:2602–2621
Lazny P, Sienkiewicz M, Brase S (2001) Tetrahedron 57:5825–5832
Gross ML, Blank DH, Welch WM (1993) J Org Chem 58:2104–2109
Chen B, Flatt AK, Jian H, Hudson JL, Tour JM (2005) Chem Mater 17:4832–4836
Tavallali H, Shafiekhani H, Rofouei KM, Payehghadra M (2014) J Braz Chem Soc 25:861–866
Gian-ya X, Wei-xia L, Shu-zhong Z, Xiao-lan W. Inorg Nano-Met Chem. https://doi.org/10.1080/24701556.2020.1723028
Shadmehr M, Davis GJ, Mehari BT, Jensen SM, Jewett JC (2018) ChemBioChem 19:2550–2552
Kimball DB, Weakley TJR, Haley MM (2002) J Org Chem 67:6395–6405
Kimball DB, Weakley TJR, Herges R (2002) MM Haley. J Am Chem Soc 124:13463–13473
Knochel P, Liu CY (2005) Org Lett 7:2543–2546
Rivera A, Gonzalez-Salas D (2010) Tetrahedron Lett 51:2500–2504
Asadollah M (2014) Novel triazene dyes based on N-phenylpiperazine. J Mol Liq 193:69–73
Isaad J, Malek F, Achari AE (2012) Dyes Pigm 92:1212–1222
Saunders KH (1949) The aromatic diazo compounds, 2nd edn. Longmans, Green and Co., New York
Kirk KL (1978) J Org Chem 43:4381–4383
Canal JP, Ramnial T, Dickie DA, Clyburne JAC (2006) Chem Commun (17):1809–1818
Crabtree RH (2005) J Organomet Chem 690:5451–5457
Arduengo AJ III (1999) Acc Chem Res 32:913–921
Bourissou D, Guerret O, Gabbaı FP, Bertrand G (2000) Chem Rev 100:39–92
Khramov DM, Belawski CW (2007) J Org Chem 72:9407–9417
Sadtchikova EV, Morkushin VS (2002) Mendeleev Commun 2:70–71
Joshi D, Adhikari N (2019) J Pharm Res Int 28(3):1–18
Isaad J, El Achari A (2013) Dyes Pigm 99:878–886
Sailor MJ, Park JH (2012) Adv Mater 24:3779–3802
Isaad J, El Achari A (2020) Int J Environ Ann Ch. https://doi.org/10.1080/03067319.2020.1790544
Isaad J, El Achari A (2013) Tetrahedron 69:4866–4874
Sepideh K, Rostami A, Khakyzadeh A, Zolfigo M, Taherpour AA, Yarieb M (2020) Mol Catal 484:110772
Riisager A, Fehrmann R, Haumann M, Wasserscheid P (2006) Top Catal 40:91–102
Watile RA, Bagal DB, Deshmukh KM, Dhake KP, Bhanage BM (2011) J Mol Catal A Chem 351:196–203
Haumann M, Jakuttis M, Franke R, Schonweiz A, Wasserscheid P (2011) ChemCatChem 3:1822–1827
Hagiwara H, Sato K, Hoshi T, Suzuki T (2011) Synlett 17:2545–2550
Isaad J (2014) RSC Adv 4:49333–49341
Isaad J, El Achari A (2018) J Mol Struct 1154:557–564
Goldani MT, Sandaroos R, Damavandi S (2014) Res Chem Intermed 40:139–147
Damavandi S, Sandaroos R (2012) Inorg Met Org Chem 42:621–627
Chrobok A, Baj S, Pudło W, Jarzebski A (2009) Appl Catal A 366:22–28
Isaad J, Perwuelz A (2010) Tetrahedron Lett 51:5328–5332
Isaad J (2013) Tetrahedron 69:2239–2250
Buruiana EC, Niculescu V, Buruiana T (2003) J Appl Polym Sci 88:1203–1210
Buruiana EC, Melinte V, Buruiana T, Lippert T, Yoshikawa H, Mashuhara H (2005) J Photochem Photobiol A Chem 171:261–267
Isaad J, Rolla M, Bianchini R (2009) Eur J Org Chem 17:2748–2764
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Isaad, J., El Achari, A. Synthesis and Spectroscopic Characterization of Water Soluble Triazene Dyes Based on Glyco-conjugated Pyrazolone Derivatives Catalyzed by an Acidic Ionic Liquid Supported on Silica-Coated Magnetite Nanoparticles. Chemistry Africa 3, 889–899 (2020). https://doi.org/10.1007/s42250-020-00186-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-020-00186-9