Abstract
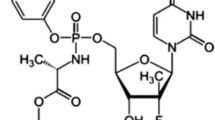
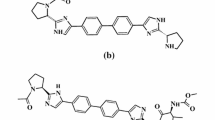
Elbasvir (ELB) and grazoprevir (GZP) were two new approved drugs and their co-formulation presents one of the more recently approved combinations for treatment of hepatitis C virus. A stability indicating reversed phase-high performance liquid chromatography (RP-HPLC) method was developed for simultaneous determination of ELB and GZP and their degradation products under hydrolysis and oxidative stress conditions as per International Conference on Harmonization [ICH Q1A (R2) guidelines]. Adequate chromatographic separation with well-defined peaks was achieved using a Waters Spherisorb phenyl Column (150 mm × 4.6 mm I.D, 5 µm particle size) with a temperature maintained at 40 °C ± 2 °C. The mobile phase consists of acetonitrile: 5 mM ammonium formate buffer (+ 0.1% v/v of trimethylamin, pH was adjusted to 3.2 by formic acid) (60:40 v/v) at a flow rate of 0.8 mL min−1. A validation study was performed according to the accuracy profile methodology. Specificity, suitability, robustness, precision and trueness of the developed method were confirmed. The proposed method was also successfully compatible to identification of molecular structure of degradation products by liquid chromatography-mass spectrometry (LC–MS) coupling. A quadrupole-time of flight mass analyzer equipped with an electrospray ionization source was used to characterizing degradation products based on the MS spectra and accurate mass measurements.












Similar content being viewed by others
References
Min GK, Min JK, Eunhee J, Bong KY (2017) Meta-analysis of the efficacy and safety of grazoprevir and elbasvir for the treatment of Hepatitis C Virus infection. Korean J Clin Pharm 5:4
Hussien A, Abdelrahman IA, Amr M, Attia A, Arwa M, Ahmed N, Mohamed MAD (2018) Meta-analysis of grazoprevir plus elbasvir for treatment of Hepatitis C virus genotype 1 infection. Ann Hepatol. https://doi.org/10.5604/01.3001.0010.7532
Debbeche R, Said Y, Temime HB, El Jery K, Bouzaïdi S, Salem M (2013) Taoufik Najjar Epidémiologie de l’hépatite C en Tunisie. La Tunisie Medicale 91(02):86–91
Mohamed EK, Tamer E, Yasmeen AEL, Gamal E (2016) Elbasvir and grazoprevir for chronic hepatitis C genotypes 1 and 4. Expert Rev Clin Pharmacol. https://doi.org/10.1080/17512433.2016.1233813
Thierno D, Delphine C, Françoise D, Anne A, Loïc G (2016) Infection par le virus de l’hépatite C: prise en charge thérapeutique. Presse Med. https://doi.org/10.1016/j.lpm.2016.02.006
Laurent A, Isabelle OH, Emilie B, Sophie H, Maeva G, Anais VP, Brigitte BC, Veronique LR, Marc B, Victor L, Isabelle FH, Valerie C, Anne M, Eric NK, Vincent L, David S, Dominique T, Stanislas P, Nassim K (2018) Grazoprevir plus elbasvir in HCV genotype1 or 4 infected patients with stage 4/5 severe chronic kidney disease is safe and effective. Kidney Int. https://doi.org/10.1016/j.kint.2018.02.019
Jan S, Gabor H, Waldemar H, Juan ART, Anca SC, Ligita J, Klara W, Hege K, Seyfettin K, Jan G, Petr U, Robert F, Rafael L, Edita K, Renate P, Sushma PMS, Jingjun Q, Ernest AA, Janice W, Bach-Yen N, Barr E, Heather LP (2016) Efficacy and safety of elbasvir/grazoprevir and sofosbuvir/pegylated interferon/ribavirin: a phase III randomized controlled trial. J Hepatol. https://doi.org/10.1016/j.jhep.2016.07.050
Kwo P, Gane E, Peng C-Y, Pearlman B, Vierling JM, Serfaty L, Buti M, Shafran S, Stryszak P, Lin L, Gress J, Black S, Dutko FJ, Robertson M, Wahl J, Lupinacci L, Barr E, Haber B (2016) Effectiveness of elbasvir and grazoprevir combination, with or without ribavirin, for treatment-experienced patients with chronic hepatitis C infection. Gastroenterology. https://doi.org/10.1053/j.gastro.2016.09.045
Nimisha S, Syed-Mohammed J, Stuart CG (2016) Pharmacodynamics and pharmacokinetics of elbasvir and grazoprevir in the treatment of hepatitis C. Expert Opin Drug Metab Toxicol. https://doi.org/10.1517/17425255.2016.1148685
Celia C, Di Marzio L, Locatelli M, Ramundo P, D’Ambrosio F, Tartaglia A (2020) Current trends in simultaneous determination of Co-administered drugs. Separations 7:29
Padmavathi S, Durga BP, Sai SG, Naga VT, Vani T (2018) RP-HPLC method development and validation for the simultaneous estimation of grazoprevir and elbasvir in bulk and pharmaceutical dosage form. Int J Curr Adv Res. https://doi.org/10.24327/ijcar.2018.13783.2474
Manoja B, Abhishiktha V, Madhavi LB, Satyavati D, Lalitha T (2018) Development and validation of a RP-HPLC method for simultaneous determination of grazoprevir and elbasvir in pure and pharmaceutical dosage form. J Sci Res Pharm. https://doi.org/10.5281/zenodo.1326526
Minou VS, Marga JAGT, Nielka PVE, David MB (2019) Quantification of second generation direct-acting antivirals daclatasvir, elbasvir, grazoprevir, ledipasvir, simeprevir, sofosbuvir and velpatasvir in human plasma by UPLC MS/MS. J Chromatogr B. https://doi.org/10.1016/j.jchromb.2019.01.024
Haritha P, Sreenivasa RB, Sunandamma Y (2016) Picogram level quantification of grazoprevir and elbasvir with deuterated internal standards in human plasma samples by LC–ESI-MS/MS. Indian J Pharm Educ Res. https://doi.org/10.5530/ijper.50.4.14
Khalid AMA, Nasr MEA, Ahmed EO, Ahmed HA (2017) Application of different spectrophotometric methods for simultaneous determination of elbasvir and grazoprevir in pharmaceutical preparation. Spectrochim Acta Part A Mol Biomol Spectrosc. https://doi.org/10.1016/j.saa.2017.08.026
Khalid AMA, Nasr MEA, Ahmed EO, Ahmed HA, Mohammed ED (2018) Simultaneous determination of elbasvir and grazoprevir in their pharmaceutical preparation using high-performance liquid chromatographic method. J Chromatogr Sci. https://doi.org/10.1093/chromsci/bmy049
Chen L, Chen J, Lu M, Stämpfli A (2019) Simultaneous determination of Elbasvir and Grazoprevir in fixed-dose combination and mass spectral characterization of each degradation product by UHPLC-ESI-QTOF-MS/MS. J Pharm Biomed Anal. https://doi.org/10.1016/j.jpba.2019.112964
Zeid AM, Abdelazim AH, Shahin M (2021) Simultaneous spectrophotometric quantitative analysis of elbasvir and grazoprevir using assisted chemometric models. Spectrochim Acta Part A Mol Biomol Spectrosc 252:119505
Attia KA, El-Olemy A, Ramzy S, Abdelazim AH, Hasan MA, Abdel-Kareem RF (2021) Simultaneous determination of elbasvir and grazoprevir in their pharmaceutical formulation by synchronous fluorescence spectroscopy coupled to dual wavelength method. Spectrochim Acta Part A Mol Biomol Spectrosc 248:119157
Swapna G, Abdul RSK, Prameelarani A (2017) RP-HPLC forced degradation studies development and validation by RP-HPLC method for the simultaneous estimation of combination drugs elbasvir and grazoprevir in bulk and pharmaceutical dosage forms. Indo Am J Pharm Sci. https://doi.org/10.5281/zenodo.848507
Madana GN, Sridhar C (2017) Stability indicating validated RP-UPLC method for simultaneous determination of elbasvir and grazoprevir in bulk and pharmaceutical dosage form. Int J Pharm Sci Rev Res 44(2):43–48
ICH Harmonised Tripartite Guideline (2005) Validation of Analytical Procedures: Text and Methodology Q2(R1). In: International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, pp 1–13
Aymen L, El Atrache LL (2019) Chemometrically assisted development of ultra-high-performance liquid chromatography method for the simultaneous quantification of sofosbuvir, daclatasvir and ledipasvir in pharmaceutical dosage forms. J Chromatogr Sci. https://doi.org/10.1093/chromsci/bmz076
ICH Topic Q 1A (R2) (2003) Stability Testing of new Drug Substances and Products. European Medicines Agency. CPMP/ICH/2736/99
Azim MdS, Mitra M, Bhasin PS (2013) HPLC method development and validation: a review. Int Res J Pharm. https://doi.org/10.7897/2230-8407.04407
United States Pharmacopeia (2011) USP 34-NF 29
Md SJ, Md JI, Rehana B, Ruhul K, Asma R (2014) A study of method development, validation, and forced degradation for simultaneous quantification of paracetamol and ibuprofen in pharmaceutical dosage form by RP-HPLC method. Anal Chem Insights. https://doi.org/10.4137/ACI.S18651
Max F (2007) Validation of analytical methods based on accuracy profiles. J Chromatogr A. https://doi.org/10.1016/j.chroma.2007.02.021.JChromatogrBAnalytTechnolBiomedLifeSci
Hibbert DB (2012) Experimental design in chromatography: a tutorial review. J Chromatogr. https://doi.org/10.1016/j.jchromb.2012.01.020
Goupy J, Creighton L (2006) Introduction aux plans d’experiences, 3rd edn. Dunod, Paris
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
About this article
Cite this article
Labidi, A., Jebali, S., Safta, M. et al. RP-HPLC Stability Indicating Method Development and Validation for Simultaneous Determination of Grazoprevir and Elbasvir. Chemistry Africa 4, 607–619 (2021). https://doi.org/10.1007/s42250-021-00253-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-021-00253-9