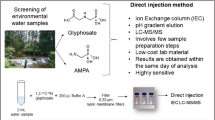
Abstract
Glyphosate, glufosinate and bialaphos are widely used worldwide as herbicides. It has been reported that they can transfer into environmental water bodies, posing health threat to human. Thus, a sensitive and rapid analysis methodology for these compounds and corresponding metabolites is highly necessary. Due to their zwitterionic nature, previously reported methods for determination of these compounds generally require derivatization. Also, preconcentration step is typically needed in order to obtain high sensitivity. Herein, we report a very convenient and sensitive assay for these herbicides as well as corresponding metabolites in surface water. With judicious tuning of chromatographic conditions, very low LODs were achieved without derivatization or preconcentration step. The LODs were 0.15 ng mL−1 for glyphosate, glufosinate, MPPA and bialaphos, 0.1 ng mL−1 for AMPA. The LOQs were 0.5 ng mL−1 for glyphosate, glufosinate, MPPA and bialaphos, 0.3 ng mL−1 for AMPA. Recoveries ranging in 90.3–102.8% were obtained. The intra-day relative standard deviations (RSDs) ranged in 4.0–5.6%, while the inter-day RSDs ranged in 4.7–6.7%. The ME (n = 6) ranged from 92.6 to 97.2%. This assay was applied to real samples of surface water. This method is very promising for application in determination and routine monitoring of these compounds in environmental water bodies.
Similar content being viewed by others
1 Introduction
Glyphosate [N-(phosphonomethyl)glycine], glufosinate [dlhomoalanine-4-yl (methyl) phosphinic acid] and bialaphos (l-2-amino-4-[(hydroxy) (methyl)-phosphinoyl] butyryl-l-alanyll-alanine) are widely used worldwide as herbicides [1, 2]. The overall use of glyphosate worldwide is 800,000 ton in 2014, establishing it as the herbicides that are used in the largest quantity [3]. They belong to the phosphorus-containing amino acid family of herbicides. These compounds can inhibit the enzymes of grasses [4, 5]. If ingested by human, these herbicides generate a series of metabolites. Aminomethylphosphonic acid (AMPA), 3-methylphosphinicopropionic acid (MPPA) and l-glufosinate are the main metabolites of these herbicides [2, 6]. Although glyphosate, glufosinate and bialaphos are generally considered as posing low risk to human, significant harm and even deaths can still occur after ingestion of glyphosate in large quantities [7,8,9].
Due to their water solubility, and the relatively long half-life in water (the half-life of glyphosate in water ranges in 7–315 days [10, 11]), the residues of glyphosate, glufosinate and bialaphos can transfer from terrestrial to aquatic environments, thus contaminating water bodies, including both surface water and ground water [6, 12]. Contamination of groundwater and sea water have been reported [13,14,15]. Glyphosate and AMPA have been detected widely in the U.S. surface water and groundwater [10, 11]. The allowed upper limits of the concentration of glyphosate for drinking water is 0.7 μg mL−1, as set by the US Environmental Protection Agency (EPA) [16]. Given the huge consumption of these herbicides worldwide, a sensitive assay allowing for convenient monitoring of these herbicides as well as corresponding metabolites in surface water is highly necessary.
Gas chromatography-mass spectrometry (GC–MS) has been used to determine glyphosate in groundwater, but the sample cleanup procedure involving derivatization was rather time-consuming [17]. Another GC–MS based method was reported with extensive optimization in the derivatization procedure [18]. Glyphosate and AMPA in seawater were determined with high performance liquid chromatography coupled with fluorescence detector, and a derivatization procedure with FMOC-Cl was used [10]. Several studies concerning the determination of these herbicides and corresponding metabolites with liquid chromatography-tandem mass spectrometry (LC–MS/MS) were proposed [19,20,21]. In general, due to their zwitterionic characteristics [22], derivatization of these compounds are typically required for the analysis, while the derivatization process is time-consuming and complicated [19, 23, 24].
In this study, with the proper choice of chromatographic columns, the time-consuming derivatization step was avoided. Additionally, the chromatographic conditions were optimized so that very low LODs were achieved without any preconcentration step. Additionally, it was found that the sensitivity obtained with basic mobile phase is much higher than that of the acidic mobile phase for these target analytes.
2 Experiment
2.1 Chemicals
Acetonitrile and methanol of chromatography grade were purchased from J.T. Baker (Phillipsburg, NJ, USA). The standards, including glyphosate, glufosinate, bialaphos sodium salt, AMPA and MPPA, were obtained from Sigma-Aldrich (St. Louis, MO, USA). Glufosinate–d3, which was used as the internal standard (IS), was supplied by Toronto Research Chemicals (Toronto, Ontario, Canada). Ultrapure water was generated by using a Millipore Direct-Q3 system (Millipore, MA, USA) and used throughout this study. Stock solutions were prepared at the concentration of 250 μg mL−1, and kept in bottles made of polypropylene (PP). The stock solutions were diluted with Milli-Q water to generate working solutions (50 μg mL−1 for the target analytes and 10 μg mL−1 for the internal standard).
2.2 LC system
A Waters ACQUITY I-Class UPLC (Milford, MA, USA) instrument was used for chromatographic separation. The sample manager temperature was 10 °C. Chromatographic separation was performed with the SeQuant ZIC-pHILIC polymeric columns (5 μm, 100 mm × 2.1 mm i.d., Torrance, CA, USA). A 7 min gradient program was used for the elution. A water solution of ammonium hydroxide with pH 10 was used as mobile phase A, while acetonitrile was used as mobile phase B. The gradient elution program was as the following: 0–1.9 min, 95% B; 1.9–2 min, from 95% B to 75% B; 2–6 min, 75% B; 6–6.1 min, from 75% B to 95% B; and 6.1–7 min, 95% B. The flow rate was 0.30 mL min−1. 2 μL filtered sample was injected.
2.3 Mass spectrometry
An AB Sciex 5500 triple quadrupole mass spectrometer (AB Sciex, Foster City, CA, USA) was used for mass spectrometry determination. The software AB Sciex Analyst (version 1.6.2) was used in data collection. Ionization was performed in negative mode. The IonSpray voltage was − 4700 V. Mass spectrometry data were collected in the multiple reaction monitoring (MRM) mode. Nitrogen was used as curtain gas (25 psi) and collision gas. The collision gas level was medium. The MRM parameters for the target analytes are exhibited in Table 1. The vaporizer temperature was 560 °C. The pressure of ion source gas 1 and gas 2 were 60 and 55 psi, respectively. The MRM chromatograms of these target analytes at the spiked concentration of 1 ng mL−1 are shown in Fig. 1.
2.4 Extraction procedures
1 mL surface water was injected into a centrifuge tube (Fisher Scientific, Pittsburgh, PA), which was made of polypropylene and has a volume of 2 mL. 5 μL IS solution (10 μg mL−1) was added to the sample. The mixture was centrifuged at 10,000 rpm for 5 min using a Beckman Coulter Allegra™ X-22 R Centrifuge (Brea, CA, USA). After centrifugation, 700 μL of the supernatants were filtered with an Oasis PRiME HLB cartridge. Then, 500 μL extract was injected into a polypropylene centrifuge tube with a volume of 2 mL. The solution was then subject to LC–MS/MS analysis.
3 Results and discussion
3.1 Sample extraction procedure
In order to separate the non-polar interferents from the extract solution, the Oasis PRiME HLB cartridge was used. Oasis PRiME HLB is a highly hydrophilic, reversed-phase polymer with a proper hydrophilic-lipophilic balance. The application of the sorbents kept the column from contamination from non-polar compounds. The standard mix solutions were passed through the Oasis PRiME HLB cartridge, and the recoveries for each compound were calculated, which were close to 100%.
3.2 Optimization of the chromatographic conditions
The target analytes are zwitterionic, thus complicated procedures of derivatization is typically needed. In the present study, hydrophilic interaction liquid chromatography (HILIC) column was used for chromatographic separation, without the derivatization procedure. SeQuant ZIC-pHILIC (Umeå, Sweden) are made of zwitterionic functional groups that are charge neutral. They are very stable in a wide pH value range (pH 2–10). Thus, SeQuant ZIC-pHILIC columns were used, and very convenient chromatographic separations were achieved without derivatization step.
In previous studies, formic acid and ammonium formate were often used as additives in the mobile phases, in order to improve the peak shapes and the retention capabilities [20, 22]. However, glyphosate, glufosinate and bialaphos contain the carboxyl functional groups, and the formic acid in the mobile phase would reduce the sensitivity for the target analytes under the typically used negative ESI mode for non-derivatization methods. In the present study, ammonium hydroxide was added into the mobile phase in order to improve the sensitivity in the negative ESI mode. As shown in Fig. 2, adding formic acid into the mobile phase resulted in suppressed sensitivity, whereas adding ammonium hydroxide into the mobile phase significantly improved the sensitivity. Nevertheless, the tailing of the peaks is significant at pH 8 and pH 9 (Fig. 3). When the pH was adjusted to 10 with ammonium hydroxide, the tailing essentially disappeared and the peak shape was improved. With higher pH, the interaction with the stationary phase is more uniform, more suitable for the elution by the optimized mobile phase. Also, the ionization of the target analytes are more complete. Thus, ammonium hydroxide was added so that the mobile phase reached the pH value of 10.
With aqueous phase higher than 60% in the initial mobile phase, the retention times of all the target analytes were not distinguishable, thus they were not chromatographically separated. Although in tandem triple quadrupole mass spectrometry analysis, target analytes can be detected in different MRM channels, the matrix effect generated during the ionization can reduce the ionization efficiency for the target anaytes. Therefore, the ratio of the aqueous phase was adjusted to optimize the retention times for the target analytes. As shown in Fig. 4, the retention time of glufosinate increases as the aqueous phase decreases from 60 to 25%. The retention time was 3.67 min with 25% aqueous phase. This means that as the ratio of the aqueous phase shrinks, the elution of the target analytes becomes less strong. However, as the aqueous phase component further reduces, the peak width becomes larger and the peak intensity gets weaker, which compromises the sensitivity for the target analytes. Thus, in order to optimize both the chromatographic separation and the determination sensitivity, 25% aqueous phase in the mobile phase was selected without further reducing the aqueous component. The retention times for all other target analytes were also prolonged with this aqueous phase ratio, and the chromatographic separation under this condition was reasonable.
3.3 Validation of the method
Calibration curves were constructed by analyses of the samples spiked with the target analytes in the range of 0.5–500 ng mL−1. The linear range was 0.5–500 ng mL−1, with correlation coefficients (R2) ranging in 0.9989–0.9997.
Limit of detection (LOD) and limit of quantification (LOQ) were calculated as the analyte concentration at which the signal-to-noise ratio of the chromatograms were equal to 3 and 10, respectively [25]. The LODs were 0.15 ng mL−1 for glyphosate, glufosinate, MPPA and bialaphos, 0.1 ng mL−1 for AMPA. The LOQs were 0.5 ng mL−1 for glyphosate, glufosinate, MPPA and bialaphos, 0.3 ng mL−1 for AMPA (Table 2).
The intra-day and inter-day recoveries were evaluated with spiked water samples (Table 3). The recovery was defined as the following: blank water was passed through the HLB, then spiked with standards, and the peak area of the spiked water solution is termed A; Another water sample spiked with the target analytes was passed through HLB and the peak area of this solution is termed B. Recovery was calculated as the ratio of B/A. The recoveries were assessed in sextuplets at each of the three concentrations: 0.5 ng mL−1, 5 ng mL−1 and 50 ng mL−1. Adequate recoveries ranging in 90.3–102.8% were obtained. The intra-day relative standard deviations (RSDs) were obtained by analyses in quintuplets in the same day, and ranged in 4.0–5.6%. The inter-day RSDs were obtained by analyses in quintuplets in three successive days, and ranged in 4.7–6.7%. The low values of intra-day and inter-day RSDs indicate that this method yields consistent results. ME was calculated as follows: ME (%) = (the peak area of the post-filtration spiked samples/the peak area of pure standards) *100 [26]. ME was determined at 0.5 ng mL−1 in sextuplets. The MEs (n = 6) of glyphosate, AMPA, glufosinate, MPPA and bialaphos are 97.2%, 95.2%, 93.5%, 92.6% and 94.7%, respectively, indicating that the ion suppression for the target analytes is mild.
3.4 Comparison to previous reports
This method is highly sensitive compared to previous reports. For instance, a colorimetric method for the determination of glyphosate was developed, with LOD of 100 ng mL−1 [27]. Anion-exchange chromatography with coulometric detection was used for the determination of glyphosate and AMPA in water, and the LODs were 38 ng mL−1 for glyphosate, 240 ng mL−1 for APMA [28]. A HPLC with fluorescence detection method for glyphosate and AMPA in seawater was developed and the LODs were 0.60 ng mL−1 and 0.30 ng mL−1 for glyphosate and AMPA, respectively. There are reports with LODs far below the standard set by EPA (0.7 μg mL−1), such as the method of analyzing glyphosate, glufosinate and AMPA in water by LC–MS/MS, with LOD of 0.005 ng mL−1, but both a long derivatization step and significant preconcentration were required [20]. In contrast, the method reported herein delivers high sensitivity without any derivatization and preconcentration step.
Long derivatization procedures are typically needed for determination of glyphosate. For example, glyphosate was derivatized with 9-fluorozenylmenthylcholoroformate (FMOC-Cl), prior to the HPLC separation and fluorescence detection [10]. A GC–MS assay was used for analysis of glyphosate and AMPA in water, however, procedures including ligand-exchange, anion-exchange and derivatization were required [29]. A UPLC–MS/MS method with in situ derivatization-dispersive liquid–liquid microextraction was used for analysis of glyphosate, glufosinate and AMPA in irrigation water, while FMOC-Cl was used as the derivatization agent and preconcentration procedures of DLLME were required [30]. Overall, these methods are relatively complicated and time-consuming. Furthermore, the use of derivatization agent typically requires the non-volatile buffer of borates to adjust the pH values, and the borates can result in signal instability and formation of salt deposits [19, 20, 31, 32].
Besides avoiding the derivatizaiton step, this method also needs no preconcentration step for obtaining high sensitivity. In contrast, in previous studies time-consuming preconcentration steps, typically by solid phase extraction (SPE), were required for comparable sensitivity. For instance, a LC–MS/MS method with SPE preconcentration was used for the analysis of glyphosate and AMPA in surface water [33]. Similarly, a SPE procedure was used for pretreatment of glyphosate, glufosinate and AMPA for the LC–MS/MS detection [20]. Sample pretreatment with SPE needs complicated procedures and is rather time-consuming, as it requires multiple steps including column conditioning, loading of sample, washing and the elution of the target analytes [14]. The fact that the present method involves no preconcentration provides great feasibility for application in monitoring water quality, particularly for handling a large number of samples. A LC–UV and LC–MS/MS method for determination of glyphosate and glufosinate were reported, with LOD as low as 0.01 and 0.008 mg kg−1 [34]. Compared to this method, the present method using HLB consumes less organic solvent, and is relatively rapid. Additionally, the present method allows simultaneous determination of glyphosate, glufosinate, bialaphos, MPPA and AMPA.
Notably, previous methods focused on the determination of glyphosate and AMPA. Although glyphosate is the herbicide that is used in the largest quantity, the use of glufosinate and bialaphos is also appreciable, and the simultaneous determination of these compounds as well as their metabolites is desired.
3.5 Application to real samples
To evaluate the practicality of this method, the five target analytes were determined in surface water collected near a farm. Six samples were acquired from different locations. These samples were kept in plastic containers at 5 °C. The samples were analyzed the same day they were collected. These water samples collected near the farm were pretreated and analyzed with the method discussed above. In these water samples, glyphosate was detected at 10.7, 5.1, 1.4, 3.5, 11.5 and 5.7 ng mL−1, while AMPA was detected at 6.1, 3.4, 1.0, 2.2, 8.5 and 2.9 ng mL−1. Bialaphos, glufosinate and MPPA were not detected with this method.
4 Conclusion
A convenient and sensitive assay for glyphosate, glufosinate, bialaphos and corresponding metabolites is developed. The sample preparation is exceptionally convenient and rapid, with the target analytes simply passing through HLB catridges. The application of HILIC columns avoided the complexity of derivatization. The target analytes were determined with tandem mass spectrometry, and the LODs range in 0.10–0.15 ng mL−1. The judicious choice of chromatographic conditions, particularly the introduction of ammonium hydroxide into the mobile phase, resulted in very high sensitivity without the need of preconcentration step. Compared to previous method, the present approach is rapid, convenient and sensitive. Thus, this method possesses high potential for monitoring of these herbicides and corresponding metabolites in environmental water bodies.
References
Glass R (1983) Liquid chromatographic determination of glyphosate in fortified soil and water samples. J Agric Food Chem 31:280–282
Yoshioka N, Asano M, Kuse A, Mitsuhashi T, Nagasaki Y, Ueno Y (2011) Rapid determination of glyphosate, glufosinate, bialaphos, and their major metabolites in serum by liquid chromatography-tandem mass spectrometry using hydrophilic interaction chromatography. J Chromatogr A 1218:3675–3680
Benbrook CM (2016) Trends in glyphosate herbicide use in the United States and globally. Environ Sci Europe 28:3
Samsel A, Seneff S (2013) Glyphosate’s suppression of cytochrome P450 enzymes and amino acid biosynthesis by the gut microbiome: pathways to modern diseases. Entropy 15:1416–1463
Duke SO, Powles SB (2008) Glyphosate: a once-in-a-century herbicide. Pest Manag Sci 64:319–325
Popp M, Hann S, Mentler A, Fuerhacker M, Stingeder G, Koellensperger G (2008) Determination of glyphosate and AMPA in surface and waste water using high-performance ion chromatography coupled to inductively coupled plasma dynamic reaction cell mass spectrometry (HPIC-ICP-DRC-MS). Anal Bioanal Chem 391:695–699
Talbot AR, Shiaw M-H, Huang J-S, Yang S-F, Goo T-S, Wang S-H, Chen C-L, Sanford TR (1991) Acute poisoning with a glyphosate-surfactant herbicide (‘Roundup’): a review of 93 cases. Hum Exp Toxicol 10:1–8
Roberts DM, Buckley NA, Mohamed F, Eddleston M, Goldstein DA, Mehrsheikh A, Bleeke MS, Dawson AH (2010) A prospective observational study of the clinical toxicology of glyphosate-containing herbicides in adults with acute self-poisoning. Clin Toxicol 48:129–136
Zouaoui K, Dulaurent S, Gaulier JM, Moesch C, Lachâtre G (2013) Determination of glyphosate and AMPA in blood and urine from humans: about 13 cases of acute intoxication. Forensic Sci Int 226:e20–e25
Wang S, Liu B, Yuan D, Ma J (2016) A simple method for the determination of glyphosate and aminomethylphosphonic acid in seawater matrix with high performance liquid chromatography and fluorescence detection. Talanta 161:700–706
Annett R, Habibi HR, Hontela A (2014) Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J Appl Toxicol 34:458–479
Battaglin WA, Meyer MT, Kuivila KM, Dietze JE (2014) Glyphosate and its degradation product AMPA occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation. J Am Water Resour Assoc 50:275–290
Botta F, Lavison G, Couturier G, Alliot F, Moreau-Guigon E, Fauchon N, Guery B, Chevreuil M, Blanchoud H (2009) Transfer of glyphosate and its degradate AMPA to surface waters through urban sewerage systems. Chemosphere 77:133–139
Pinto E, Soares AG, Ferreira IMPLVO (2018) Quantitative analysis of glyphosate, glufosinate and AMPA in irrigation water by in situ derivatization–dispersive liquid–liquid microextraction combined with UPLC-MS/MS. Anal Methods 10:554–561
Mercurio P, Flores F, Mueller JF, Carter S, Negri AP (2014) Glyphosate persistence in seawater. Mar Pollut Bull 85:385–390
Borggaard OK, Gimsing AL (2008) Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review. Pest Manag Sci 64:441–456
Börjesson E, Torstensson L (2000) New methods for determination of glyphosate and (aminomethyl)phosphonic acid in water and soil. J Chromatogr A 886:207–216
Stalikas CD, Pilidis GA (2000) Development of a method for the simultaneous determination of phosphoric and amino acid group containing pesticides by gas chromatography with mass-selective detection: Optimization of the derivatization procedure using an experimental design approach. J Chromatogr A 872:215–225
Hanke I, Singer H, Hollender J (2008) Ultratrace-level determination of glyphosate, aminomethylphosphonic acid and glufosinate in natural waters by solid-phase extraction followed by liquid chromatography-tandem mass spectrometry: Performance tuning of derivatization, enrichment and detection. Anal Bioanal Chem 391:2265–2276
Ibáñez M, Pozo ÓJ, Sancho JV, López FJ, Hernández F (2005) Residue determination of glyphosate, glufosinate and aminomethylphosphonic acid in water and soil samples by liquid chromatography coupled to electrospray tandem mass spectrometry. J Chromatogr A 1081:145–155
Stalikas CD, Konidari CN (2001) Analytical methods to determine phosphonic and amino acid group-containing pesticides. J Chromatogr A 907:1–19
Guo H, Wang H, Zheng J, Liu W, Zhong J, Zhao Q (2018) Sensitive and rapid determination of glyphosate, glufosinate, bialaphos and metabolites by UPLC–MS/MS using a modified Quick Polar Pesticides Extraction method. Forensic Sci Int 283:111–117
Qian K, Tang T, Shi T, Wang F, Li J, Cao Y (2009) Residue determination of glyphosate in environmental water samples with high-performance liquid chromatography and UV detection after derivatization with 4-chloro-3,5-dinitrobenzotrifluoride. Anal Chim Acta 635:222–226
Sun L, Kong D, Gu W, Guo X, Tao W, Shan Z, Wang Y, Wang N (2017) Determination of glyphosate in soil/sludge by high performance liquid chromatography. J Chromatogr A 1502:8–13
Vial J, Jardy A (1999) Experimental comparison of the different approaches to estimate LOD and LOQ of an HPLC method. Anal Chem 71:2672–2677
Matuszewski BK, Constanzer AML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC–MS/MS. Anal Chem 75:3019–3030
De Almeida LKS, Chigome S, Torto N, Frost CL, Pletschke BI (2015) A novel colorimetric sensor strip for the detection of glyphosate in water. Sens Actuators B Chem 206:357–363
Coutinho CFB, Coutinho LFM, Mazo LH, Nixdorf SL, Camara CAP (2008) Rapid and direct determination of glyphosate and aminomethylphosphonic acid in water using anion-exchange chromatography with coulometric detection. J Chromatogr A 1208:246–249
Börjesson E, Torstensson L (2000) New methods for determination of glyphosate and (aminomethyl)phosphonic acid in water and soil. J Chromatogr A 886:207–216
Pinto E, Soares AG, Ferreira IMPLVO (2018) Quantitative analysis of glyphosate, glufosinate and AMPA in irrigation water by: in situ derivatization-dispersive liquid–liquid microextraction combined with UPLC-MS/MS. Anal Methods 10:554–561
Ehling S, Reddy TM (2015) Analysis of glyphosate and aminomethylphosphonic acid in nutritional ingredients and milk by derivatization with fluorenylmethyloxycarbonyl chloride and liquid chromatography-mass spectrometry. J Agric Food Chem 63:10562–10568
Goscinny S, Unterluggauer H, Aldrian J, Hanot V, Masselter S (2012) Determination of glyphosate and its metabolite AMPA (aminomethylphosphonic acid) in cereals after derivatization by isotope dilution and UPLC-MS/MS. Food Anal Methods 5:1177–1185
Poiger T, Buerge IJ, Bächli A, Müller MD, Balmer ME (2017) Occurrence of the herbicide glyphosate and its metabolite AMPA in surface waters in Switzerland determined with on-line solid phase extraction LC-MS/MS. Environ Sci Pollut Res 24:1588–1596
Li X, Xu J, Jiang Y, Chen L, Xu Y, Pan C (2009) Hydrophilic-interaction liquid chromatography (HILIC) with dad and mass spectroscopic detection for direct analysis of glyphosate and glufosinate residues and for product quality control. Acta Chromatogr 21:559–576
Funding
This work was supported by East China Normal University through startup funding (11200-120215-10363).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, H., Gao, Y., Guo, D. et al. Sensitive, rapid and non-derivatized determination of glyphosate, glufosinate, bialaphos and metabolites in surface water by LC–MS/MS. SN Appl. Sci. 1, 305 (2019). https://doi.org/10.1007/s42452-019-0306-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0306-x